Cardiomyocytes within aged mouse hearts exhibited impaired sarcomere lengthening during left ventricular filling, and cardiomyocytes isolated from aged hearts demonstrated increased stiffness as measured with atomic force microscopy. Impaired sarcomere lengthening with ventricular filling will contribute to cardiac dysfunction with advancing age.
Keywords: aging, cardiomyocyte, stiffness
Abstract
The Frank-Starling mechanism, whereby increased diastolic filling leads to increased cardiac output, depends on increasing the sarcomere length (Ls) of cardiomyocytes. Ventricular stiffness increases with advancing age, yet it remains unclear how such changes in compliance impact sarcomere dynamics in the intact heart. We developed an isolated murine heart preparation to monitor Ls as a function of left ventricular pressure and tested the hypothesis that sarcomere lengthening in response to ventricular filling is impaired with advanced age. Mouse hearts isolated from young (3–6 mo) and aged (24–28 mo) C57BL/6 mice were perfused via the aorta under Ca2+-free conditions with the left ventricle cannulated to control filling pressure. Two-photon imaging of 4-{2-[6-(dioctylamino)-2-naphthalenyl]ethenyl}1-(3-sulfopropyl)-pyridinium fluorescence was used to monitor t-tubule striations and obtain passive Ls between pressures of 0 and 40 mmHg. Ls values (in μm, aged vs. young, respectively) were 2.02 ± 0.04 versus 2.01 ± 0.02 at 0 mmHg, 2.13 ± 0.04 versus 2.23 ± 0.02 at 5 mmHg, 2.21 ± 0.03 versus 2.27 ± 0.03 at 10 mmHg, and 2.28 ± 0.02 versus 2.36 ± 0.01 at 40 mmHg, indicative of impaired sarcomere lengthening in aged hearts. Atomic force microscopy nanoindentation revealed that intact cardiomyocytes enzymatically isolated from aged hearts had increased stiffness compared with those of young hearts (elastic modulus: aged, 41.9 ± 5.8 kPa vs. young, 18.6 ± 3.3 kPa; P = 0.006). Impaired sarcomere lengthening during left ventricular filling may contribute to cardiac dysfunction with advancing age by attenuating the Frank-Starling mechanism and reducing stroke volume.
NEW & NOTEWORTHY
Cardiomyocytes within aged mouse hearts exhibited impaired sarcomere lengthening during left ventricular filling, and cardiomyocytes isolated from aged hearts demonstrated increased stiffness as measured with atomic force microscopy. Impaired sarcomere lengthening with ventricular filling will contribute to cardiac dysfunction with advancing age.
the frank-starling mechanism describes the heart's intrinsic response to diastolic filling, where an increase in filling associates with an increase in stroke volume [for historical review, see Katz (18)]. At the cellular level, the Frank-Starling mechanism occurs via increases in sarcomere length (Ls) of cardiomyocytes within the chamber wall, with subsequent length-dependent activation of myofilament force production (2, 8, 10, 26). For example, during submaximal Ca2+ activation of isolated cardiomyocytes (7, 9, 13) or trabecular/papillary muscle preparations (17, 19, 32), contractile force can increase severalfold through the range in Ls from ∼1.70 to ∼2.20 μm. While force production as a function of Ls has been well defined in isolated cardiac muscle preparations, there is a paucity of information concerned with how left ventricular filling pressure alters Ls of cardiomyocytes in the intact living heart. Fluorescent labeling of the t-tubule network gives accurate indication of z-line spacing and therefore Ls in cardiomyocytes of the rat heart (5, 6). However, these latter studies examined Ls in the absence of ventricular filling pressure. Thus the relationship between ventricular filling and Ls in the living heart remains to be elucidated.
The aged heart exhibits complex chamber remodeling as manifested by cardiomyocyte hypertrophy, cellular disorganization, and stiffening of both cardiomyocytes (23) and extracellular matrix components (20). A decrease in ventricular compliance is evident in the pressure-volume relationship of the aged compared with young heart (3, 27), yet it remains unclear how age-associated ultrastructural changes impact the functional relationship between ventricular filling pressure and cardiomyocyte Ls in the intact heart. For the present study in mice, we developed an isolated heart preparation to monitor the relationship between left ventricular pressure and cardiomyocyte Ls. We tested the hypothesis that sarcomere lengthening in response to ventricular filling is impaired with advanced age.
MATERIALS AND METHODS
Solutions and Chemicals
Chemicals and reagents were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Nominally Ca2+-free physiological saline solution (Ca2+-free PSS) contained (in mM) 135 NaCl, 5 KCl, 1 MgCl2, 10 d-glucose, and 10 HEPES (pH 7.4) with NaOH. Experiments using isolated cardiomyocytes were performed in nominally Ca2+-free PSS or PSS containing 2 mM CaCl2. Caffeine (10 mM) was prepared daily in 2 mM CaCl2 PSS. Ca2+-free cardioplegia solution consisted of Ca2+-free PSS, supplemented with 100 μM EGTA (to chelate contaminating Ca2+) and 6% dextran (∼500,000 mol wt, to maintain colloid osmotic pressure and limit tissue edema).
Animals and Cardiac Excision
Procedures were approved by the Animal Care and Use Committee at the University of Missouri and complied with all United States regulations involving animal experimentation. Male C57BL/6 mice were obtained from the colony of the National Institute on Aging (housed at Charles River Laboratories, Wilmington, MA) and housed at the University of Missouri for 1–4 wk before experimentation. Age groups studied were 3–6 mo (defined as young, n = 25) and 24–28 mo (defined as aged, n = 16). Each mouse was anesthetized with intraperitoneal injection of pentobarbital sodium (60 mg/kg). Following confirmation of deep anesthesia, the heart was rapidly (∼30 s) excised and immersed in 4°C Ca2+-free PSS, and the mouse was euthanized by exsanguination.
Cardiomyocyte Isolation, Dye Loading, and Confocal Imaging
Fluorescent markers of cardiomyocyte Ls were initially characterized and compared with sarcomere striations evaluated using transmitted light in enzymatically isolated cardiomyocytes. Following cardiac excision, the aorta was cannulated for retrograde perfusion of the myocardium via the coronary arteries. Perfusion began with Ca2+-free PSS containing 2 U/ml heparin for 10 min, followed by 15 to 16 min with a MEM-based enzymatic isolation solution, supplemented with (in mM) 10 NaHCO3, 2 Na-pyruvate, 10 Na-HEPES, 10 HEPES, 8 taurine in addition to 20 μM CaCl2, 50,000 U/l penicillin-streptomycin (PenStrep, Life Technologies, Grand Island, NY), and 22.5 μg/ml−Liberase Blendzyme TH (Roche Applied Science, Indianapolis, IN). Perfusion solutions were maintained at 37°C (pH, 7.35) while oxygenated with 95% O2-5% CO2. The left ventricle and septum were then isolated, minced, agitated, and filtered at 22–25°C. Isolated cardiomyocytes were plated on laminin-coated 25 × 25-mm glass coverslips and loaded with 5 μM 4-{2-[6-(dioctylamino)-2-naphthalenyl]ethenyl}1-(3-sulfopropyl)-pyridinium (di-8-ANEPPS; Molecular Probes/Life Technologies, Grand Island, NY) and 5 μM MitoTracker Deep Red (Molecular Probes/Life Technologies) for 10–20 min followed by a 30–60-min wash. Ventricular cardiomyocytes were studied using two-dimensional imaging using the galvano scanhead of the Leica SP5/MP (×40 oil objective; HCX PL APA, numerical aperture, 1.25; 512 × 128 pixels; bidirectional scanning/2× line averaging; pixel size, 0.24 μm). Excitation was at 488 and 633 nm with emission recorded at 500–560 and 650–800 nm for respective dyes. Transmitted light images were simultaneously acquired to monitor Ls. To deplete sarcoplasmic reticulum Ca2+ stores and induce cardiomyocyte contracture (producing short Ls), 10 mM caffeine was applied via a gravity-fed pipette. Imaging was performed at 22–25°C.
Isolated-Perfused Heart Preparation with Left Ventricular Cannulation
Imaging platform.
A custom imaging platform was fabricated for use with a Leica SP5/MP inverted confocal microscope. The platform secured an imaging chamber and two cannulae for concurrent aortic and ventricular cannulation. The imaging chamber was sealed to 24 × 50-mm coverslips using vacuum grease and placed within a PH-1 heating element (Warner Instruments, Hamden, CT). The aortic cannula [polyethylene (PE)-90 tubing, coupled to a SH-27B inline solution heater (Warner Instruments)] and ventricular cannula (PE-100 tubing) were positioned at angles to 1) facilitate cannulation procedures beneath the stereomicroscope; 2) permit cannulation of both the left ventricle (via the left atrium) and the ascending aorta (superior to the aortic valve) while avoiding bending or twisting of the aorta; 3) allow the left ventricular free wall to rest gently against the coverglass; 4) prevent physical contact between platform components and the Leica SP5/MP.
Procedures.
Excised hearts were placed in 4°C Ca2+-free PSS within the imaging platform, and the aorta was cannulated and then perfused retrogradely with room temperature Ca2+-free PSS containing 2 U/ml heparin for ∼3 min at a pressure of 41 cm·H2O to flush blood from the coronary vasculature, which coincided with loss of spontaneous contractile activity. Note that all pressures refer to a column of PSS above the heart, where 0, 7, 14, 27, 41, and 54 cm of PSS equate to pressures of 0, 5, 10, 20, 30, and 40 mmHg. The aortic cannula was then removed, and the cannula for the ventricle was inserted into the left atrium (via an incision) and guided through the mitral valve into the left ventricle. To secure the tip of cannula within the ventricle, the atrium and pulmonary vessels were ligated with 6-0 silk suture. Ca2+-free cardioplegia solution was perfused into the ventricular compartment via the cannula (∼10 mmHg for ∼10 s) to flush the chamber, and the heart was rotated to position the left ventricular free wall against the coverslip (see Fig. 2, A and B). The aorta was then recannulated, and coronary perfusion was reintroduced at 30 mmHg, with a ∼1-mm incision made in the right atrium to drain coronary venous effluent. Coronary flow draining into the left ventricular chamber via the Thebesian circulation was removed by opening the ventricular cannula, set at 0 mmHg. Elevation of the ventricular solution column above the aortic solution column resulted in aortic valve opening and equilibration of the columns and confirmed that the ventricular compartment filled at the pressure set by the ventricular column. The time required for ventricular cannulation (following perfusion of the myocardium with Ca2+-free cardioplegia solution) was typically 10–15 min.
Fig. 2.
Ls measurements in an isolated, perfused heart preparation. A and B: photographs of an isolated perfused heart preparation held at left ventricular (LV) pressures of 0 (A) and 40 (B) mmHg. The aortic and LV cannulae are marked aortic and LV, respectively. In this configuration, the LV free wall gently rests against the coverslip for imaging. The superfusion pipette, vacuum suction pipette, and superfusion solution were removed for clarity. C–E: 2-photon fluorescence images (×20 objective) of subepicardial (10–25 μm beneath the pericardium) areas of a heart loaded with di-8-ANEPPS. Variability included highly vascularized regions (C), complex cardiomyocyte arrangements (D), and parallel sheets of cardiomyocytes interspersed between capillaries (E).
Dye Loading and Two-Photon Imaging of Perfused Heart Preparation
Following dual-cannulation procedures, the heart was retrogradely perfused at 30 mmHg with solutions oxygenated with 95% O2-5% CO2 at 22–25°C. Di-8-ANEPPS was loaded into cells of the heart by perfusing 10 ml of Ca2+-free PSS containing 2 U/ml heparin and 10 μM di-8-ANEPPS, followed by 10 ml of Ca2+-free PSS with 2 U/ml heparin (perfusion rate was typically 1.5–2 ml/min). The perfusion solution was then switched to Ca2+-free cardioplegia solution (perfusion rate, ∼0.5 ml/min), and the preparation was moved to an inverted Leica SP5/MP microscope for frame scan (xy) two-photon fluorescence imaging (×40 oil objective; HCX PL APA, excitation at 840 nm; emission at 500–700 nm, 1,024 × 1,024 pixels at 0.19 μm/pixel). Laser and photomultiplier gain settings were optimized to pixel intensity histograms obtained from fluorescence signal within cardiomyocyte regions. These settings associated with saturated pixel intensities within the coronary vasculature (Fig. 2, C–E). Subepicardial cardiomyocytes located 10–25 μm beneath the surface of the heart were imaged while ventricular pressure was maintained at 0, 5, 10, 20, 30, and 40 mmHg. Two minutes before each set of measurements, the hydrostatic column perfusing the aorta was repositioned to achieve an aortic pressure of 1 mmHg greater than ventricular pressure. This approach was used to maintain aortic valve closure and ensure that in the case of aortic valve incompetence (associated with advancing age), the ventricular pressure would be within 1 mmHg of the desired pressure. Angle of the longitudinal cell plane relative to the imaging plane, particularly with respect to rotation around the middle (width) axis of the cell, affects apparent Ls as described in detail by Bub et al. (6) and Botcherby et al. (5). To determine this angular component, we performed optical sectioning (z sectioning) of subepicardial cardiomyocytes over a distance of 20–25 μm (section thickness of 1 or 2 μm) to determine the number of optical sections separating longitudinal edges of cardiomyocytes. With the assumption of rectangular prism cardiomyocyte geometry, the edges on opposite sides of the prism will be confined within a z distance of (n sections +1) × section thickness. Maximum angular rotation (around the middle axis) of the longitudinal cell plane relative to the imaging plane (θmax) was calculated according to the equation: Tan(θmax) = z distance/length between cell edges. The ratio of true Ls to measured Ls was then calculated as Cosθmax. In subepicardial cardiomyocytes, θmax ranged from 0.3 to 4.3° (mean, 1.6 ± 0.2°, n = 24); thus measured Ls values are within 1% of true Ls values.
Atomic Force Microscopy Nanoindentation of Intact Cardiomyocytes
Atomic force microscopy (AFM) nanoindentation was performed as previously described to estimate the elastic modulus (stiffness) of individual myocytes (15, 35, 36). In brief, cardiomyocytes were applied to laminin-coated dishes for 40 min and then washed 3 times in nominally Ca2+-free PSS at 22–25°C. Cell elasticity was monitored in contact mode using silicon nitride microlevers (model MLCT with spring constants 10–30 pN/nm; Veeco Mertrology, Santa Barbara, CA) and a Bioscope AFM System (Veeco Mertrology) mounted on an Olympus IX81 Microscope (Olympus, NY). Probes repeatedly approached and retracted the surface of the cardiomyocyte at a frequency of 0.5 Hz (tip speed 800 nm/s). Indentation depth of the AFM probe was ∼150–250 nm. Approach curves were used to calculate cell elasticity, where E is elastic modulus, according to the following equation: F = [Tan(α)] × [(2 × E × δ2)/π(1 − ν2)], where α represents probe shape (conical with an approximate half-angle of 21.25°); δ is indentation depth, calculated as the difference in the AFM piezo z-movement and the measured deflection of the probe; and ν is the Poisson ratio (0.5 for the cell). Indentation force (F) was calculated using Hooke's law (F = kΔx), where k is the AFM probe spring constant and Δx is the measured deflection of the probe.
Data Analysis and Statistics
Measurements of Ls were obtained from cellular regions (>20 μm) with well-defined intensity profiles. Analyses were performed by three separate observers using three independent approaches: 1) measurement of the distance between the first and last sarcomere within a defined series (distance/number of sarcomeres); 2) Fast Fourier transform analysis using ImageJ software (average number of input cycles was 26); and 3) wavelength of damped sinusoid fitted to the autocorrelation of the intensity profile. All three approaches yielded similar values for Ls. Analysis of Ls for young versus aged was performed blinded to experimental pressure or group, with values from 4–19 cardiomyocytes averaged to determine the mean Ls value at a defined pressure for each heart. Resulting values were used to determine the relationship between pressure and Ls for each heart (Fig. 4, A and B) and to compile summary data (Fig. 4, C and D). Two-parameter fits were used for nonlinear regression according to the equations Pressure = [expk*(Ls-Ls,0)] − 1 (Fig. 4, A and B) and Ls = [k−1·ln(1 + pressure)] + Ls,0 (Fig. 4C), where adjustable parameters k and Ls,0 correspond to the stiffness constant and Ls at zero pressure, respectively (31). For AFM studies, a single elastic modulus per heart was obtained by averaging values from 1–5 individually sampled cardiomyocytes (n = 15 cardiomyocytes from N = 6 young mice; n = 15 cardiomyocytes from N = 6 aged mice). Data are presented as individual observations or as means ± SE. Statistical comparisons were made using paired t-tests or two-way ANOVA (Bonferroni post hoc) with SigmaPlot 10/SigmaStat 3.5 (Systat, San Jose, CA), with statistical significance set at P < 0.05.
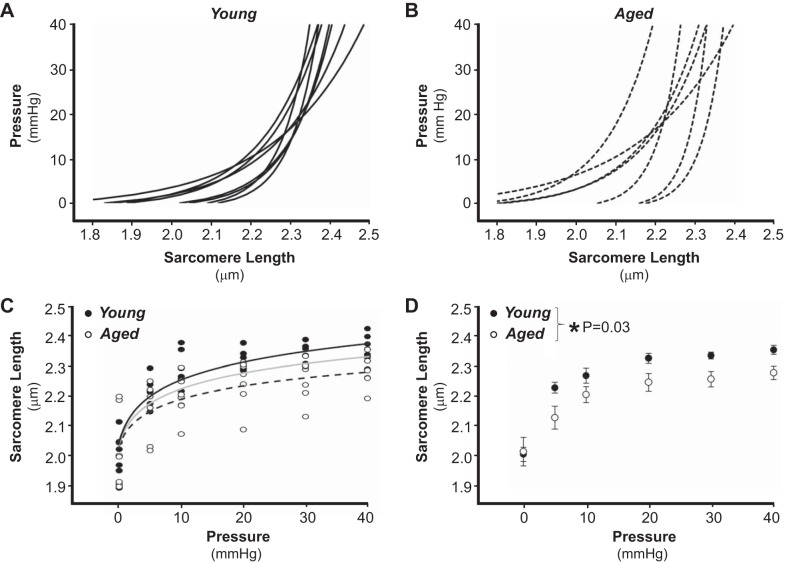
Fig. 4.
Ls and LV pressure in hearts of young and aged mice. A and B: LV pressure vs. Ls curves in hearts from young (A, solid lines, n = 8) and aged (B, dashed lines, n = 7). At each pressure (0, 5, 10, 20, 30, and 40 mmHg), Ls values from 4–19 cardiomyocytes were averaged to obtain a single Ls value per heart, and data were fit according to Pressure = [expk·(Ls-Ls,0)] − 1. Note that in A and B, data are presented with pressure (independent variable) on the y-axis to correlate with typical presentation of pressure-volume relationships. C: raw data of Ls vs. LV pressure of young (●, n = 8) and aged (○, n = 7), with separate 2 parameter fits for young (black line) and aged (dashed line). Intermediate gray line corresponds to 2 parameter fit of pooled data across age groups. Data from respective age groups were fit best by separate (vs. pooled) analyses indicative of samples representing separate populations. D: summary data of Ls vs. LV pressure in hearts of young (●, n = 8) and aged (○, n = 7). *P = 0.03 young vs. aged (repeated-measures ANOVA).
RESULTS
The aims of this investigation were to 1) determine the relationship between left ventricular filling pressure and Ls in the intact murine heart and 2) test the hypothesis that sarcomere lengthening in response to ventricular filling is impaired with advanced age. First, to determine fluorescent markers of Ls in cardiac cells, ventricular cardiomyocytes were enzymatically isolated from mouse hearts and then loaded with spectrally distinct markers of the plasma membrane/t-tubule network (di-8-ANEPPS) and mitochondria (MitoTracker Deep Red). Figure 1 illustrates that di-8-ANEPPS and MitoTracker Deep Red, while giving reciprocal fluorescence profiles (Fig. 1B, b and c), resulted in Ls measurements that were well correlated with each other (r2 = 0.94) and with Ls monitored using transmitted light (r2 = 0.97 and 0.95, respectively; Fig. 1C). In many cases MitoTracker Deep Red fluorescence signal was low at both the cellular Z line and M line, yielding two cycles per sarcomere (Fig. 1B,c). Conversely, di-8-ANEPPS consistently produced a single, distinct peak for each sarcomere (at the Z line) and did so over extended distances. Thus di-8-ANEPPS was used for subsequent Ls measurements in whole heart experiments.
Fig. 1.
Fluorescent measurement of sarcomere length (Ls) in isolated cardiomyocytes. A: transmitted light (a), 4-{2-[6-(dioctylamino)-2-naphthalenyl]ethenyl}1-(3-sulfopropyl)-pyridinium (di-8-ANEPPS) fluorescence (b), and MitoTracker Deep Red fluorescence (c) images of an isolated mouse cardiomyocyte. B: high-magnification transmitted light (a), di-8-ANEPPS (b), and MitoTracker Deep Red (c) images (left) and corresponding intensity profiles (right) of a separate cardiomyocyte. C: 3-dimensional scatter plot (n = 43 measurements from 29 cardiomyocytes from 3 mice) comparing Ls obtained simultaneously with transmitted light (x-axis), di-8-ANEPPS (y-axis), and MitoTracker Deep Red (z-axis).
Two-photon fluorescence imaging of hearts loaded with di-8-ANEPPS primarily revealed indicator accumulation in the endothelial cell membrane of the coronary microvasculature and the sarcolemmal/t-tubular membrane of cardiomyocytes (Fig. 2, C–E). Measurements were typically obtained from sheets of subepicardial cardiomyocytes with well-defined sarcomere striations between capillary networks (Fig. 2E), avoiding regions with larger blood vessels (Fig. 2C) or areas with less-organized cardiomyocyte arrangements (Fig. 2D). Upon left ventricular pressurization, the heart freely slid against the coverslip (Fig. 2, A and B), and in some cases it was possible to relocate tissue regions following pressurization and thus obtain the pressure versus Ls relationship from the same series of sarcomeres within the same cardiomyocyte within the heart (Fig. 3).
Fig. 3.
Ls measurements from the same cardiomyocyte in the intact heart at 3 LV filling pressures. A–C: images of cardiomyocytes within an aged heart at 0 (A), 20 (B), and 40 (C) mmHg. Fluorescence profiles, obtained from regions marked by brackets at left of images, are presented below images. Fast-Fourier transform (FFT) analysis of fluorescence profiles resulted in Ls values of 2.02 (A), 2.25 (B), and 2.28 (C) μm. AU, arbitrary units.
Ventricular stiffness increases with advancing age, yet it remains unknown how this relates to the filling pressure versus Ls relationship. We therefore used our experimental approach to monitor this relationship under passive conditions (Ca2+-free cardioplegia) in hearts of young and aged mice. Shown in Fig. 4 are pressure versus Ls relationships from eight young (Fig. 4A) and seven aged (Fig. 4B) hearts. Ls increased with pressure and was more dynamic over the pressure range of 0–20 mmHg than the pressure range of 20–40 mmHg. In young hearts from different mice, there was significant overlap in the pressure-Ls relationship and each heart operated over a similar Ls range (Fig. 4A). In contrast, aged hearts from different mice exhibited greater variability in the shapes of their respective pressure-Ls relationship as well as in their Ls range (Fig. 4B). Summary data illustrate that across pressures, Ls was significantly shorter in aged hearts than in young hearts (Fig. 4, C and D).
Increased passive ventricular stiffness arising from alterations in the extracellular matrix (e.g., fibrosis) is well established in humans and animals of advanced age. In addition, changes in the passive stiffness of individual cardiomyocytes may also contribute to passive stiffness of the heart. AFM nanoindentation was used to assess intrinsic cortical stiffness of intact enzymatically isolated cardiomyocytes under Ca2+-free conditions (Fig. 5A). The apparent elastic modulus of cardiomyocytes of aged hearts was approximately twofold greater than that of cardiomyocytes of young hearts (Fig. 5B), indicative of increased cell stiffness with advanced age.
Fig. 5.

Passive stiffness of cardiomyocytes from hearts of young and aged mice. A: example traces of dynamic measurement of the elastic modulus of intact LV cardiomyocytes of young (●) and aged (○) with atomic force microscopy nanoindentation. Note difference in dynamic stiffness properties between young and aged, with cardiomyocytes of aged exhibiting high-amplitude oscillations in elastic modulus. B: elastic modulus of young (black bar, n = 15 cardiomyocytes from 6 mice) and aged (white bar, n = 15 cardiomyocytes from 6 mice).*P = 0.006 aged vs. young (unpaired t-test).
DISCUSSION
Cardiomyocyte Ls is the critical determinant of the Frank-Starling mechanism of the heart (2, 8, 10). The operating range of Ls in cardiomyocytes within the left ventricular wall has been determined using hearts arrested and chemically fixed at experimentally controlled pressures, with spacing between electron dense Z lines measured using electron microscopy (30) or transmitted light microscopy (11). These approaches require careful tissue preparation to avoid fixation artifacts associated with shrinkage resulting in underestimation of Ls [e.g., see Huang et al. (16)]. Furthermore, fixed preparations limit Ls evaluation to a single filling pressure in each heart, effectively preventing evaluation of the pressure versus Ls relationship in a single heart. Fluorescent labeling of the t-tubule network (and by anatomical constraints the cardiac Z lines) has previously enabled Ls measurements in living (unfixed) tissue in perfused rat hearts (6). Here we use fluorescent labeling in freshly excised mouse hearts during ventricular pressurization under Ca2+-free conditions to define the pressure versus Ls relationship and quantify passive properties of the intact heart at the level of the sarcomere.
Comparison with Previous Ls Measurements in the Heart
The sarcomere is the highly organized, functional unit of contractile protein organization within striated muscle. Despite such ultrastructural organization, Ls varies considerably within the heart because of complex anatomical arrangements (e.g., see Fig. 2D) and endocardial to epicardial regional differences (11, 28, 30). The mean Ls values determined for subepicardial cardiomyocytes in our preparation at 0 mmHg (young, 2.01 μm; and aged, 2.02 μm) fit well within the range (1.93 to 2.09 μm) determined in unpressurized intact rat hearts (5, 6) or fixed hearts maintained at 0 mmHg filling pressure (11, 28, 30). Upon left ventricular filling, the pressure versus Ls relationship of hearts from young mice studied here (0–40 mmHg Ls range, 2.01 to 2.36 μm) was also similar to that reported in classic measurements of dog hearts fixed ex vivo (0–30 mmHg Ls range, 1.93 to 2.33 μm) (30) and from reconstructions of Ls dynamics in vivo at physiological filling pressures (28). Contrasting with these data are measurements of closed-chest fixed rat hearts where the pressure versus Ls relationship was shifted toward a shorter Ls range (0–35 mmHg Ls range, 1.94 to 2.26 μm). However, the latter studies acknowledge a ∼4% Ls shrinkage due to formaldehyde (11). Mathematical correction of their data increases this range to 2.02 to 2.35 μm, which is in agreement with the present findings. The passive pressure versus Ls relationship steepens at Ls values > 2.3 μm (Fig. 4A), which is consistent with data in isolated cardiomyocyte preparations where passive force increases substantially between 2.3 and 2.4 μm (13). Such myocardial properties help prevent sarcomere overextension and allow cardiomyocytes to operate along the ascending limb or near the plateau of the Ls-tension relationship (9).
Ventricular Stiffness with Advancing Age
Cardiac stiffness increases with advancing age (3) and cardiac disease as a result of alterations in both active (i.e., Ca2+ dependent) and passive mechanical properties of the myocardium (20). Our experiments used Ca2+-free conditions to prevent cardiomyocyte excitation-contraction coupling (i.e., active stiffness) and to thereby monitor passive physical properties of the ventricular myocardium. In hearts of aged mice, we observed a shift in the relationship between Ls and pressure, reflecting impaired ability of the myocardium to stretch in response to a defined increase in pressure. Increased passive stiffness of the aged heart has been attributed to multiple factors including hypertrophy and wall thickening, cellular disorganization, changes in relative expression of extracellular matrix components (collagen, fibronectin, laminin) and fibrosis, or modification of the extracellular matrix via cross-linking or advanced glycation end-products (25). Passive stiffness of individual cardiomyocytes has also been shown to increase with advancing age and may contribute to altered ventricular compliance. Our data in mice are consistent with data in rats where nanoindentation with AFM revealed a significant increase in cortical stiffness in aged compared with young (23). At the level of the enzymatically isolated cardiomyocyte (i.e., a cell largely devoid of extracellular matrix components), increases in passive cardiomyocyte stiffness often occur concurrent with increases in the stiffness properties of the large sarcomeric protein titin (22, 24), and age-associated augmentation of titin-based cardiomyocyte stiffness has been observed in dog and human subjects (4, 12). Passive cardiomyocyte stiffness may also arise from subsarcolemma-associated proteins (e.g., ankyrin, cadherin, catenin, dystroglycans and dystrophin, integrins, spectrin, talin, vinculin) or cytoskeletal proteins (e.g., actin, desmin, tubulin), and changes in expression or macromolecular architecture of many of these proteins has been reported to occur with cardiac disease (14, 33). Such changes, in addition to established alterations in extracellular matrix components, may underlie the decrease in chamber compliance observed with advancing age.
Study Limitations and Future Directions
Crystalloid-perfused heart preparations associate with tissue edema, which may artificially extend Ls (6). The experimental perfusion solution used for this study included 6% dextran to increase colloid osmotic pressure of the perfusate and limit tissue edema. Nevertheless, initial Ls measurements occurred >40 min post-cardiac excision and time-dependent sarcomere lengthening may have occurred during that interval. However, resulting measurements of Ls were consistent with those in previous investigations using fixed tissue, suggesting that this approach resulted in accurate measurements of Ls. This study used nonphysiological temperatures under passive (Ca2+ free) conditions to reduce tissue movement and facilitate acquisition of fluorescence images. Diastolic Ls in vivo is likely to be shorter than that observed in the present investigation because of Ca2+-dependent diastolic contracture (i.e., active diastolic stiffness), and this effect may be particularly pronounced in the murine heart where high heart rates lead to diastolic elevation of intracellular Ca2+ with incomplete myofilament relaxation. Indeed, a recent report using t-tubule labeling in vivo revealed a diastolic Ls of ∼1.9 μm (1), which is less than the lowest value observed in our investigation under passive conditions (2.01 μm at 0 mmHg). Diastolic Ca2+ removal is typically impaired with advancing age (20, 21), leading to excessive active diastolic contracture. Therefore, sarcomere lengthening may be further impaired in aged hearts in vivo because of the combined influence of both active and passive myocardial properties. Data acquisition and analysis focused primarily on the subepicardium as this region of the heart exhibited well-organized cardiomyocyte arrangements that were parallel to the focal plane. Although not applied in the present investigation, two-photon microscopy can be used to image into greater depths (>100 μm) of the heart and examine additional aspects of myocardial architecture including cardiomyocyte dimensions, angle, and organization with respect to vascular networks. In subepicardial cardiomyocytes of young and aged mice, the structure of the t-tubule network was largely maintained permitting assessment of Ls. However, with cardiac disease significant loss or disorganization of t-tubules may occur (29, 34), which may limit the accuracy of Ls measurements using this approach. In aged hearts we observed variability in the pressure versus Ls relationship, which suggests variability in cardiac remodeling processes. Future studies are needed for correlation of extracellular matrix properties (fibrosis) or the stiffness of isolated cardiomyocytes with sarcomere lengthening on an animal-to-animal basis to afford additional insight into the variability in cardiac remodeling and ventricular stiffness associated with advancing age.
Conclusions
The novel approach developed for these experiments provides evaluation of the relationship between ventricular filling pressure and Ls in the intact mouse heart. Our studies reveal an aging-associated shift in the pressure versus Ls relationship, consistent with an increase in passive ventricular stiffness. The aged heart operates at elevated diastolic pressures (27), which under resting (i.e., nonstressed) conditions may extend Ls to maintain stroke volume according to the Frank-Starling relationship. However, this form of compensation will associate with a depressed capability to further increase Ls to augment stroke volume and may limit cardiac reserve.
GRANTS
This work was supported by National Institutes of Health Grants R01HL090749 (to K. S. Campbell), UL1TR000117 (to K. S. Campbell), P01HL095486 (to G. A. Meininger), R01HL057852 (to K. S. McDonald), R01HL086483 and R37HL041026 (to S. S. Segal), and K01AG041208 (to T. L. Domeier).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.E.N., Y.Z., and T.L.D. performed experiments; M.E.N., J.T.W., Y.Z., A.K.G., L.M.H., K.S.C., G.A.M., K.S.M., S.S.S., and T.L.D. analyzed data; M.E.N., J.T.W., Y.Z., L.M.H., K.S.C., G.A.M., K.S.M., S.S.S., and T.L.D. interpreted results of experiments; M.E.N., K.S.C., and T.L.D. prepared figures; M.E.N. and T.L.D. drafted manuscript; M.E.N., J.T.W., Y.Z., A.K.G., L.M.H., K.S.C., G.A.M., K.S.M., S.S.S., and T.L.D. edited and revised manuscript; M.E.N., J.T.W., Y.Z., A.K.G., L.M.H., K.S.C., G.A.M., K.S.M., S.S.S., and T.L.D. approved final version of manuscript; T.L.D. conception and design of research.
ACKNOWLEDGMENTS
T. L. Domeier acknowledges the expert guidance of Drs. Lothar A. Blatter, Michael J. Davis, Michael A. Hill, Kerry S. McDonald, Gerry A. Meininger, Michael W. Rich, and Steven S. Segal as part of the Career Development of K01AG041208.
REFERENCES
- 1.Aguirre AD, Vinegoni C, Sebas M, Weissleder R. Intravital imaging of cardiac function at the single-cell level. Proc Natl Acad Sci USA 111: 11257–11262, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen DG, Kentish JC. The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol 17: 821–840, 1985. [DOI] [PubMed] [Google Scholar]
- 3.Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation 110: 1799–1805, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Borbely A, Falcao-Pires I, van Heerebeek L, Hamdani N, Edes I, Gavina C, Leite-Moreira AF, Bronzwaer JG, Papp Z, van der Velden J, Stienen GJ, Paulus WJ. Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res 104: 780–786, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Botcherby EJ, Corbett A, Burton RA, Smith CW, Bollensdorff C, Booth MJ, Kohl P, Wilson T, Bub G. Fast measurement of sarcomere length and cell orientation in Langendorff-perfused hearts using remote focusing microscopy. Circ Res 113: 863–870, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Bub G, Camelliti P, Bollensdorff C, Stuckey DJ, Picton G, Burton RA, Clarke K, Kohl P. Measurement and analysis of sarcomere length in rat cardiomyocytes in situ and in vitro. Am J Physiol Heart Circ Physiol 298: H1616–H1625, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cazorla O, Le Guennec JY, White E. Length-tension relationships of sub-epicardial and sub-endocardial single ventricular myocytes from rat and ferret hearts. J Mol Cell Cardiol 32: 735–744, 2000. [DOI] [PubMed] [Google Scholar]
- 8.de Tombe PP, Mateja RD, Tachampa K, Ait Mou Y, Farman GP, Irving TC. Myofilament length dependent activation. J Mol Cell Cardiol 48: 851–858, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabiato A, Fabiato F. Dependence of the contractile activation of skinned cardiac cells on the sarcomere length. Nature 256: 54–56, 1975. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs F, Martyn DA. Length-dependent Ca2+ activation in cardiac muscle: some remaining questions. J Muscle Res Cell Motil 26: 199–212, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Grimm AF, Lin HL, Grimm BR. Left ventricular free wall and intraventricular pressure-sarcomere length distributions. Am J Physiol Heart Circ Physiol 239: H101–H107, 1980. [DOI] [PubMed] [Google Scholar]
- 12.Hamdani N, Bishu KG, von Frieling-Salewsky M, Redfield MM, Linke WA. Deranged myofilament phosphorylation and function in experimental heart failure with preserved ejection fraction. Cardiovasc Res 97: 464–471, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Hanft LM, McDonald KS. Length dependence of force generation exhibit similarities between rat cardiac myocytes and skeletal muscle fibres. J Physiol 588: 2891–2903, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heling A, Zimmermann R, Kostin S, Maeno Y, Hein S, Devaux B, Bauer E, Klovekorn WP, Schlepper M, Schaper W, Schaper J. Increased expression of cytoskeletal, linkage, and extracellular proteins in failing human myocardium. Circ Res 86: 846–853, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Hong Z, Sun Z, Li M, Li Z, Bunyak F, Ersoy I, Trzeciakowski JP, Staiculescu MC, Jin M, Martinez-Lemus L, Hill MA, Palaniappan K, Meininger GA. Vasoactive agonists exert dynamic and coordinated effects on vascular smooth muscle cell elasticity, cytoskeletal remodelling and adhesion. J Physiol 592: 1249–1266, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang SH, Hsiao CD, Lin DS, Chow CY, Chang CJ, Liau I. Imaging of zebrafish in vivo with second-harmonic generation reveals shortened sarcomeres associated with myopathy induced by statin. PloS One 6: e24764, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Julian FJ, Sollins MR. Sarcomere length-tension relations in living rat papillary muscle. Circ Res 37: 299–308, 1975. [DOI] [PubMed] [Google Scholar]
- 18.Katz AM. Ernest Henry Starling, his predecessors, and the “Law of the Heart”. Circulation 106: 2986–2992, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Kentish JC, ter Keurs HE, Ricciardi L, Bucx JJ, Noble MI. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circ Res 58: 755–768, 1986. [DOI] [PubMed] [Google Scholar]
- 20.Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev 73: 413–467, 1993. [DOI] [PubMed] [Google Scholar]
- 21.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation 107: 346–354, 2003. [DOI] [PubMed] [Google Scholar]
- 22.LeWinter MM, Granzier HL. Titin is a major human disease gene. Circulation 127: 938–944, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lieber SC, Aubry N, Pain J, Diaz G, Kim SJ, Vatner SF. Aging increases stiffness of cardiac myocytes measured by atomic force microscopy nanoindentation. Am J Physiol Heart Circ Physiol 287: H645–H651, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Linke WA, Hamdani N. Gigantic business: titin properties and function through thick and thin. Circ Res 114: 1052–1068, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Loffredo FS, Nikolova AP, Pancoast JR, Lee RT. Heart failure with preserved ejection fraction: molecular pathways of the aging myocardium. Circ Res 115: 97–107, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonald KS. The interdependence of Ca2+ activation, sarcomere length, and power output in the heart. Pflügers Arch 462: 61–67, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pacher P, Mabley JG, Liaudet L, Evgenov OV, Marton A, Hasko G, Kollai M, Szabo C. Left ventricular pressure-volume relationship in a rat model of advanced aging-associated heart failure. Am J Physiol Heart Circ Physiol 287: H2132–H2137, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez EK, Hunter WC, Royce MJ, Leppo MK, Douglas AS, Weisman HF. A method to reconstruct myocardial sarcomere lengths and orientations at transmural sites in beating canine hearts. Am J Physiol Heart Circ Physiol 263: H293–H306, 1992. [DOI] [PubMed] [Google Scholar]
- 29.Shah SJ, Aistrup GL, Gupta DK, O'Toole MJ, Nahhas AF, Schuster D, Chirayil N, Bassi N, Ramakrishna S, Beussink L, Misener S, Kane B, Wang D, Randolph B, Ito A, Wu M, Akintilo L, Mongkolrattanothai T, Reddy M, Kumar M, Arora R, Ng J, Wasserstrom JA. Ultrastructural and cellular basis for the development of abnormal myocardial mechanics during the transition from hypertension to heart failure. Am J Physiol Heart Circ Physiol 306: H88–H100, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spotnitz HM, Sonnenblick EH, Spiro D. Relation of ultrastructure to function in the intact heart: sarcomere structure relative to pressure volume curves of intact left ventricles of dog and cat. Circ Res 18: 49–66, 1966. [DOI] [PubMed] [Google Scholar]
- 31.Stuyvers BD, McCulloch AD, Guo J, Duff HJ, ter Keurs HE. Effect of stimulation rate, sarcomere length and Ca2+ on force generation by mouse cardiac muscle. J Physiol 544: 817–830, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ter Keurs HE, Rijnsburger WH, van Heuningen R, Nagelsmit MJ. Tension development and sarcomere length in rat cardiac trabeculae. Evidence of length-dependent activation. Circ Res 46: 703–714, 1980. [DOI] [PubMed] [Google Scholar]
- 33.Tsutsui H, Ishihara K, Cooper GT. Cytoskeletal role in the contractile dysfunction of hypertrophied myocardium. Science 260: 682–687, 1993. [DOI] [PubMed] [Google Scholar]
- 34.Wei S, Guo A, Chen B, Kutschke W, Xie YP, Zimmerman K, Weiss RM, Anderson ME, Cheng H, Song LS. T-tubule remodeling during transition from hypertrophy to heart failure. Circ Res 107: 520–531, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu X, Sun Z, Foskett A, Trzeciakowski JP, Meininger GA, Muthuchamy M. Cardiomyocyte contractile status is associated with differences in fibronectin and integrin interactions. Am J Physiol Heart Circ Physiol 298: H2071–H2081, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Y, Qiu H, Trzeciakowski JP, Sun Z, Li Z, Hong Z, Hill MA, Hunter WC, Vatner DE, Vatner SF, Meininger GA. Temporal analysis of vascular smooth muscle cell elasticity and adhesion reveals oscillation waveforms that differ with aging. Aging Cell 11: 741–750, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]