This study highlights the relationship between type 2 angiotensin-converting enzyme and metalloprotease 17 (ADAM17) in hypertension, emphasizing the role of oxidative stress as a mediator between the type 1 ANG II receptor and ADAM17 in the mouse brain. Furthermore, our study shows the benefits of lipoic acid treatment in preventing ADAM17 upregulation and thus the development of hypertension.
Keywords: metalloprotease 17, deoxycorticosterone acetate-salt hypertension, antioxidant, type 2 angiotensin-converting enzyme
Abstract
We previously reported that type 2 angiotensin-converting enzyme (ACE2) compensatory activity is impaired by the disintegrin and metalloprotease 17 (ADAM17), and lack of ACE2 is associated with oxidative stress in neurogenic hypertension. To investigate the relationship between ADAM17 and oxidative stress, Neuro2A cells were treated with ANG II (100 nM) 24 h after vehicle or α-lipoic acid (LA, 500 μM). ADAM17 expression was increased by ANG II (120.5 ± 9.1 vs. 100.2 ± 0.8%, P < 0.05) and decreased after LA (69.0 ± 0.3 vs. 120.5 ± 9.1%, P < 0.05). In another set of experiments, LA reduced ADAM17 (92.9 ± 5.3 vs. 100.0 ± 11.2%, P < 0.05) following its overexpression. Moreover, ADAM17 activity was reduced by LA in ADAM17-overexpressing cells [109.5 ± 19.8 vs. 158.0 ± 20.0 fluorescence units (FU)·min−1·μg protein−1, P < 0.05], in which ADAM17 overexpression increased oxidative stress (114.1 ± 2.5 vs. 101.0 ± 1.0%, P < 0.05). Conversely, LA-treated cells attenuated ADAM17 overexpression-induced oxidative stress (76.0 ± 9.1 vs. 114.1 ± 2.5%, P < 0.05). In deoxycorticosterone acetate (DOCA)-salt hypertensive mice, a model in which ADAM17 expression and activity are increased, hypertension was blunted by pretreatment with LA (119.0 ± 2.4 vs. 131.4 ± 2.2 mmHg, P < 0.05). In addition, LA improved dysautonomia and baroreflex sensitivity. Furthermore, LA blunted the increase in NADPH oxidase subunit expression, as well as the increase in ADAM17 and decrease in ACE2 activity in the hypothalamus of DOCA-salt hypertensive mice. Taken together, these data suggest that LA might preserve ACE2 compensatory activity by breaking the feedforward cycle between ADAM17 and oxidative stress, resulting in a reduction of neurogenic hypertension.
NEW & NOTEWORTHY
This study highlights the relationship between type 2 angiotensin-converting enzyme and metalloprotease 17 (ADAM17) in hypertension, emphasizing the role of oxidative stress as a mediator between the type 1 ANG II receptor and ADAM17 in the mouse brain. Furthermore, our study shows the benefits of lipoic acid treatment in preventing ADAM17 upregulation and thus the development of hypertension.
the renin-angiotensin system (RAS) is an important regulator of arterial blood pressure (BP). Angiotensin II (ANG II) is the major effector molecule of the RAS, which exerts its biological effects mainly via the type 1 ANG II receptor (AT1R). Therefore, ANG II acts as a potent vasoconstrictor, regulates water intake and salt metabolism, and increases sympathetic outflow and BP (27).
Abundant evidence suggests that a key mechanism by which ANG II contributes to hypertension is increasing oxidative stress (48). Oxidative stress is a condition of increased production of ROS and/or reduction of scavenging mechanisms (38). Previous studies from our laboratory and others have shown that ANG II administration in the central nervous system, as well as in the periphery, induces hypertension via NAD(P)H oxidase (Nox)-mediated ROS production in different cardiovascular regulatory brain regions (38, 48). Therefore, ROS are important mediators in ANG II-dependent hypertension.
A new important component of the RAS is the type 2 angiotensin-converting enzyme (ACE2). ACE2, a homolog of ACE, can cleave ANG I and ANG II to ANG-(1–9) and ANG-(1–7), respectively (43). ANG-(1–7), the product of ANG II degradation by ACE2, has opposite properties to that of ANG II, by acting on the Mas receptor (MasR). The ACE2-ANG-(1–7)-MasR axis of the RAS promotes vasodilation, antiproliferation, and reduction of heart failure (39).
ACE2 is a membrane protein that can undergo shedding to release a catalytically active ectodomain from the cell surface in the extracellular milieu (23). The proteases involved in this process are called sheddases, and they control the biological activity of membrane proteins (10). A well-known sheddase is a disintegrin and metalloproteinase 17 (ADAM17), also called tumor necrosis factor-(TNF-α)-converting enzyme (1).
Studies have shown that ANG II induces ADAM17-mediated transactivation of the epidermal growth factor receptor (EGFR) and hypertrophy of vascular smooth muscle cells (26). Other in vitro studies demonstrated that ROS are also able to activate ADAM17 in platelets, and this effect could characterize a limiting mechanism for platelet function (6). Furthermore, Nox4-mediated ROS production is required to increase ADAM17 expression and subsequent induction of cardiac hypertrophy (46).
Therefore, we hypothesized that chronic RAS activation enhances oxidative stress and ADAM17 activity thus promoting ACE2 shedding. To test this hypothesis, we used an antioxidant, α-lipoic acid (LA), to decrease oxidative stress and downregulate ADAM17 expression. LA, also known as 1,2-dithiolane-3-pentanoic acid or thioctic acid, is a disulfide antioxidant and exists in both R- and S-enantiomeric forms. However, only (R)-LA is conjugated to conserved lysine residues in an amide linkage, thus making this isoform essential as a cofactor in biological systems (31). We recently documented that LA reduces BP and improves baroreflex sensitivity in renovascular hypertensive rats (29). However, the mechanisms underlying these effects remain unknown. To address this, we used Neuro2A cells (neuroblastoma cell line) and an experimental model of neurogenic hypertension combining chronic administration of deoxycorticosterone acetate (DOCA), reduced renal mass, and a high-salt diet (DOCA-salt model) (37).
MATERIALS AND METHODS
Animals.
Experiments were performed in male C57BL/6J mice (25–30 g) (Jackson Laboratories). Animals were housed in a temperature- and humidity-controlled facility under a 12:12-h dark-light cycle and fed standard mouse chow and water ad libitum. All procedures were approved by the Louisiana State University Health Sciences Center-New Orleans Animal Care and Use Committee and are in agreement with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. BP measurements using radiotelemetry and DOCA-salt treatments in mice were performed as previously described (37).
DOCA-salt treatment and physiological recordings.
For each surgery, mice were anesthetized with isoflurane (2%) in an oxygen flow (1 l/min) and placed on a heating pad to maintain body temperature. Postoperative care included a buprenorphine injection to relieve pain at the end of the surgery and after 12 h (0.05 mg/kg sc).
Baseline BP was recorded by telemetry in uninephrectomized mice for 3 days, and then mice were randomly divided into four groups (n = 6/group), all implanted subcutaneously with a DOCA-silicone (DOCA-silicone = 1:3; DOCA: 1 mg/g body wt) or an empty silicone (sham surgery) sheet. Drinking water for DOCA-implanted mice was replaced by 1% NaCl solution. From the day of DOCA implantation, mice were treated with tempol (258 mg·kg·−1day−1) or LA (600 mg·kg·−1day−1) for 21 days orally. Drugs were mixed with a banana-flavored gel pudding (LabGel; ClearH2O) to form dispensable pellets according to body weight. Gel pellets were provided daily with the chow to the following groups: sham (n = 6), DOCA (n = 6), DOCA + tempol (n = 6), and DOCA + LA (n = 6). BP was continuously recorded for three additional weeks. Spontaneous baroreceptor reflex sensitivity (SBRS), reflecting the baroreflex control of heart rate, was calculated using the sequence method, as previously described (14). Autonomic function was assessed in conscious freely moving mice before and 3 wk after DOCA-salt and antioxidant treatment, using a pharmacological method involving ip injections of propranolol (β-blocker, 4 mg/kg), atropine (muscarinic receptor blocker, 1 mg/kg), and chlorisondamine (ganglionic blocker, 5 mg/kg) (36, 38). Each injection was separated by a 3-h recovery period. Maximum changes in heart rate or mean arterial pressure (MAP) were calculated following administration of these blockers. At the end of the protocol, mice were killed, and the brain was collected and stored at −80°C until used in the biochemical assays.
Cell culture.
Neuro2A mouse neuroblastoma cells (ATCC, Manassas, VA) were grown in minimum essential medium (GIBCO, Invitrogen, Carlsbad, CA) with l-glutamine (2 mM) and Eagle's balanced salt solution adjusted to contain sodium bicarbonate (1.5 g/l), nonessential amino acids (0.1 mM), sodium pyruvate (1.0 mM), and fetal bovine serum (10%; GIBCO) at 37°C under a humidified 95% and 5% (vol/vol) mixture of air and CO2. Neuro2A cells were pretreated with either tempol (10 μM in PBS) or LA (500 μM in PBS + 0.03% NaOH) and 24 h later treated with ANG II (100 nmol/l in PBS) for 24 h. Cells were grown to chamber slides at a density of 1 × 105 cells/chamber for dihydroethidium (DHE) fluorescence measurement or 10-cm dishes at a density of 106 cells/dish for Western blot, ADAM17 assay, and QRT-PCR experiments.
For experiments using ADAM17 overexpression, cells were treated with mADAM17 in pcDNA3.1 plasmid [1.25 μg/ml, from Addgene (Cambridge, MA), catalog no. 19141], and green fluorescence protein (GFP) plasmid (1.25 μg/ml, pEGFP-C3, catalog no. 6082-1; Clontech) was used as a control of successful transfection. Both plasmids are cytomegalovirus promoter driven. For both plasmids we used Lipofectamine transfection reagents (Invitrogen, Life Technologies). After 24 h, LA (500 μM in PBS + 0.03% NaOH) was added to the cells for 24 h in serum-free medium. Cells were then harvested for Western blotting analysis, DHE staining, ADAM17 activity assay, and QRT-PCR, as described above.
Western blot.
Cell pellets were incubated in 300 μl lysis buffer [in mmol/l: 10 HEPES, 150 NaCl, 5 MgCl2, 1 EGTA, 0.02% (wt/vol) NaN3, pH 7.4] containing a protease inhibitor cocktail (Sigma, St. Louis, MO). Protein concentration was measured using a BCA assay kit (Pierce, Rockford, IL). Cell lysates were mixed with SDS-PAGE sample buffer, heated, and loaded on a 4–15% SDS-polyacrylamide gel for electrophoresis (Life Technologies, Carlsbad, CA). Proteins were transferred to a nitrocellulose membrane (Fisher Scientific, Houston, TX). Membranes were blocked with 5% nonfat milk in 1.47 mM NaH2PO4, 8.09 mM Na2HPO4, 145 mM NaCl, 0.05% vol/vol Tween 20H, and 0.01% wt/vol thimerosal, pH 7.4 (PBST) for 1 h at room temperature and incubated with anti-ADAM17 (1:1,000, ab2051; Abcam), at 4°C, overnight. Membranes were washed with PBST three times for 5 min and then incubated with goat antirabbit immunoglobulin G (IgG)-horseradish peroxidase (HRP) (1:5,000; Perkin Elmer) for 1 h at room temperature. Specific bands were detected by chemiluminescence according to the manufacturer's instructions (ECLH; Perkin Elmer) at room temperature and reprobed with mouse anti-γ-tubulin (1:10,000, no. 5192; Sigma) and goat anti-mouse IgG-HRP. Bands corresponding to specific antibodies were quantified by densitometry (ImageJ version 1.45 K) and normalized to γ-tubulin.
Proteins extracted from hypothalamus (20 μg) were processed for Western blotting as previously described (14). Briefly, after death, brains were quickly removed from the animals and immediately frozen on dry ice. Total protein was extracted from hypothalamus tissue, and equal amounts were loaded and separated by SDS-PAGE and electrophoretically transferred to the membranes. The membranes were washed and incubated with following primary antibodies: anti-ADAM17 (1:1,000, ab2051; Abcam), anti-Nox4 (1:1,000, ab109225; Abcam), and mouse anti-Nox2 (1:2,000, catalog no. 611415; BD Transduction Laboratories).
Fluorogenic monitoring of superoxide production.
Neuro2A cells were incubated in serum-free medium in the presence of ADAM17 and GFP plasmids for 24 h. On the next day posttransfection, GFP expression was observed using a fluorescence microscope (excitation/emission wavelengths: 488/509 nm, IX81; Olympus) as an index of successful transfection, and cells were treated for 24 h with LA (500 μM). The oxidant-sensitive fluorogenic probe DHE (2 μmol/l) was loaded for 30 min. Cells were washed three times in the dark with PBS and examined on a fluorescence microscope (excitation/emission wavelength: 518/605 nm, U-TB190; Olympus). Each experiment was performed in triplicate. DHE fluorescence was quantified using Image J software.
ADAM17 activity assay.
ADAM17 activity was measured in Neuro2A cells or hypothalamus (6 μg protein/well) using a TACE activity kit (Sensolyte 520 TACE activity assay; ANASPEC), following the manufacturer's instructions. Fluorescence emission was monitored using a SpectraMax M2 Fluorescence Reader (excitation/emission wavelengths: 490/520 nm; Molecular Devices, Sunnyvale, CA) during 2 h. Data are presented as amount of enzymatic reaction normalized for total protein and expressed as arbitrary fluorescence units (AFU).
ACE2 activity assay.
ACE2 activity was measured as previously described (28). Tissue from brain hypothalamus was homogenized with ACE2 activity reaction buffer and centrifuged, and the supernatant was transferred to a clean tube. ACE2 activity measurement was carried out in the presence of captopril to eliminate any contribution by endogenous ACE and based on the use of the fluorogenic peptide substrate VI [FPSVI, 7Mca-Y-V-A-D-A-P-K(Dnp)-OH] (R&D Systems, Minneapolis, MN). Nonspecific enzyme activity was measured by including DX600 (1 μmol/l), a specific ACE2 inhibitor (Phoenix Pharmaceutical, Belmont, CA). Fluorescence emission was monitored using a SpectraMax M2 Fluorescence Reader (Molecular Devices). Data are presented as amounts of substrate FPSVI converted to product per minute, normalized for total protein and expressed as AFU.
Measurement of mRNA expression (QRT-PCR).
Total RNA was isolated from Neuro2A cells using an RNeasy Mini Kit (catalog no. 74104; Qiagen), and then we performed the DNase digestion using RNeasy-free DNase set (catalog no. 79254; Qiagen). A cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad). Real-time RT-PCR amplification reactions were performed with iQ SYBR green super mix with ROX (Bio-Rad) using a Bio-Rad iQ5 real-time PCR machine (Bio-Rad). Primer sequences used for mADAM17 (Integrated DNA Technologies) were (554F) forward 5′-CGT GGT TGG TGA GCC TGA CT-3′ and (643R) reverse 5′-TTA TAT TCT GCC CCA TCT GTG TTG-3′. Data were analyzed by the ΔΔCT comparative method and expressed as fold changes compared with the control group.
Statistical analysis.
Data are expressed as means ± SE. Data were analyzed by Student's t-test or one-way ANOVA, followed by Newman-Keuls correction for multiple comparisons between means as appropriate. Statistical comparisons were performed using Prism 5 (GraphPad Software, San Diego, CA). Difference was considered significant when P < 0.05.
RESULTS
LA prevents the increase in ANG II-induced ADAM17 expression in Neuro2A cells.
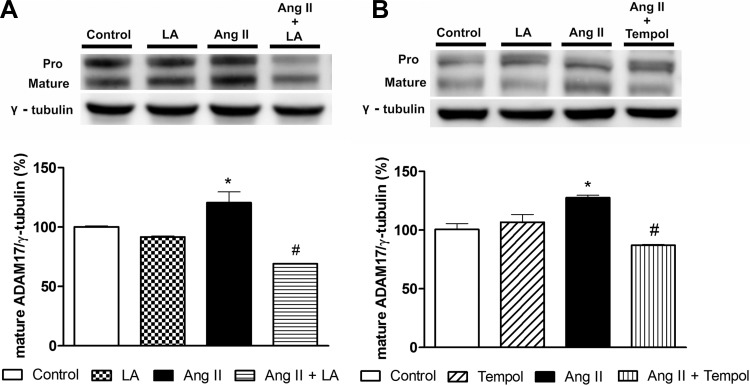
We previously reported that ADAM17 expression is increased after ANG II treatment in Neuro2A cells (37). Here, we investigated whether oxidative stress is involved in ADAM17 modulation. Neuro2A cells incubated with ANG II (100 nmol/l, 24 h) exhibited an increase in ADAM17 protein expression compared with control (120.5 ± 9.1 vs. 100.2 ± 0.8%, P < 0.05, Fig. 1A). This increase was reduced by pretreatment with either LA (69.0 ± 0.3 vs. 120.5 ± 9.1%, P < 0.05, Fig. 1A) or tempol (87.0 ± 0.7 vs. 118.4 ± 7.0%, P < 0.05, Fig. 1B). These data confirm the ANG II-mediated upregulation of neuronal ADAM17 and suggest a critical role for oxidative stress in this process.
Fig. 1.
Metalloprotease 17 (ADAM17) expression induced by angiotensin II (ANG II) in Neuro2A cells. Representative Western blots showing the pro and mature forms of ADAM17 and group data (mature ADAM17) showing the reduction of ADAM17 expression induced by lipoic acid (LA, A) or tempol (B). *P < 0.05 vs. control and #P < 0.05 vs. ANG II. All data are means ± SE.
LA reduces ADAM17 overexpression and activity in Neuro2A cells.
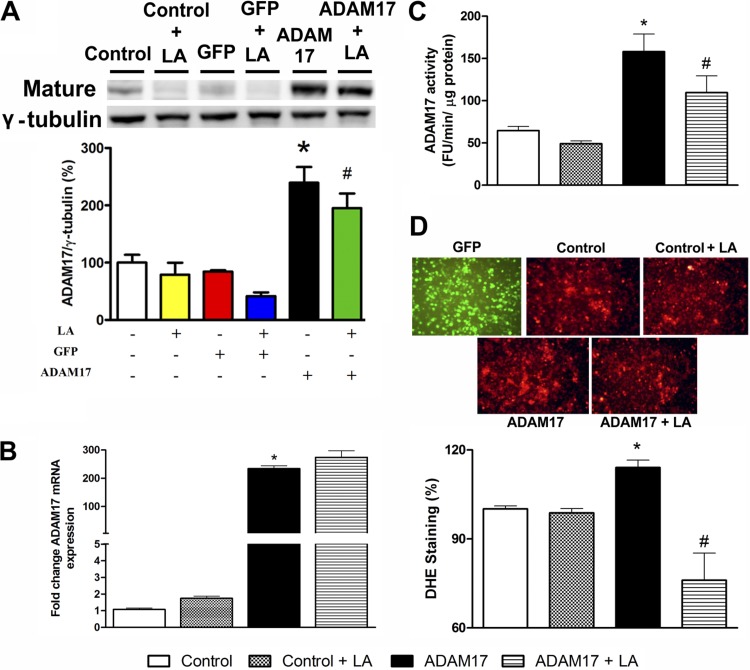
To clarify the mechanisms involved in LA-mediated reduction of ADAM17 expression, cells were transfected with a plasmid containing ADAM17. Western blotting analysis revealed that transfection with the ADAM17 plasmid led to ∼100% increase in sheddase expression in Neuro2A cells compared with control (239.3 ± 27.6 vs. 100.0 ± 16.5%, P < 0.05, Fig. 2A). ADAM17 overexpression tends to reduce after 24 h of LA treatment (239.3 ± 27.6 vs. 195.0 ± 25.6%, Fig. 2A).
Fig. 2.
Effects of LA on ADAM17 overexpression induced by ADAM17 plasmid in Neuro2A cells. A: representative Western blot and group data showing a trend to reduce the overexpression of ADAM17 induced by LA. B: QRT-PCR shows no change in ADAM17 expression after LA treatment. C: ADAM17 activity is reduced by LA treatment. D: representative dihydroethidium (DHE)-stained sections and group data showing that ADAM17 overexpression significantly increases reactive oxygen species (ROS) production in Neuro2A cells and that LA reduces ROS accumulation. GFP, green fluorescent protein. *P < 0.05 vs. control and #P < 0.05 vs. ADAM17. All data are means ± SE.
To investigate a possible effect of LA on ADAM17 transcriptional regulation, we first measured mRNA levels for ADAM17. As expected, ADAM17 overexpression significantly increased mRNA expression for ADAM17 compared with control (234.3 ± 10.1 vs. 1.7 ± 0.1-fold, P < 0.05, Fig. 2B). However, LA was unable to affect the increase in ADAM17 mRNA expression following transfection with the ADAM17 plasmid in Neuro2A cells, as shown in Fig. 2B. These data rule out a potential effect of LA on ADAM17 transcriptional regulation, suggesting that LA directly acts on ADAM17 protein expression and/or activation.
To determine whether these changes are restricted to ADAM17 protein expression, ADAM17 activity was assessed. Neuro2A cells transfected with the ADAM17 plasmid showed an approximately twofold increase in ADAM17 activity (158.0 ± 20.0 vs. 77.3 ± 3.3 FU·min−1·μg protein−1, P < 0.05, Fig. 2C). LA reduced ADAM17 activity in Neuro2A (109.5 ± 19.8 vs. 158.0 ± 20.0 FU·min−1·μg protein−1, P < 0.05, Fig. 2C).
LA prevents ADAM17-mediated oxidative stress.
To further investigate whether ADAM17 overexpression could be involved in oxidative stress and whether LA decreases ADAM17 expression and activity by its antioxidant effect, cells were transfected with ADAM17 plasmid and treated with LA. Oxidative stress was assessed by DHE staining. ADAM17 overexpression significantly increased superoxide levels in Neuro2A cells (114.1 ± 2.5 vs. 101.0 ± 1.0%, P < 0.05, Fig. 2D), whereas LA treatment attenuated the ADAM17-induced oxidative stress (76.0 ± 9.1 vs. 114.1 ± 2.5%, P < 0.05, Fig. 2D). Taken together, these data support the beneficial effect of LA in preventing ADAM17-mediated ROS formation in neurons.
LA prevents DOCA-salt-induced hypertension by improving baroreflex sensitivity and autonomic function.
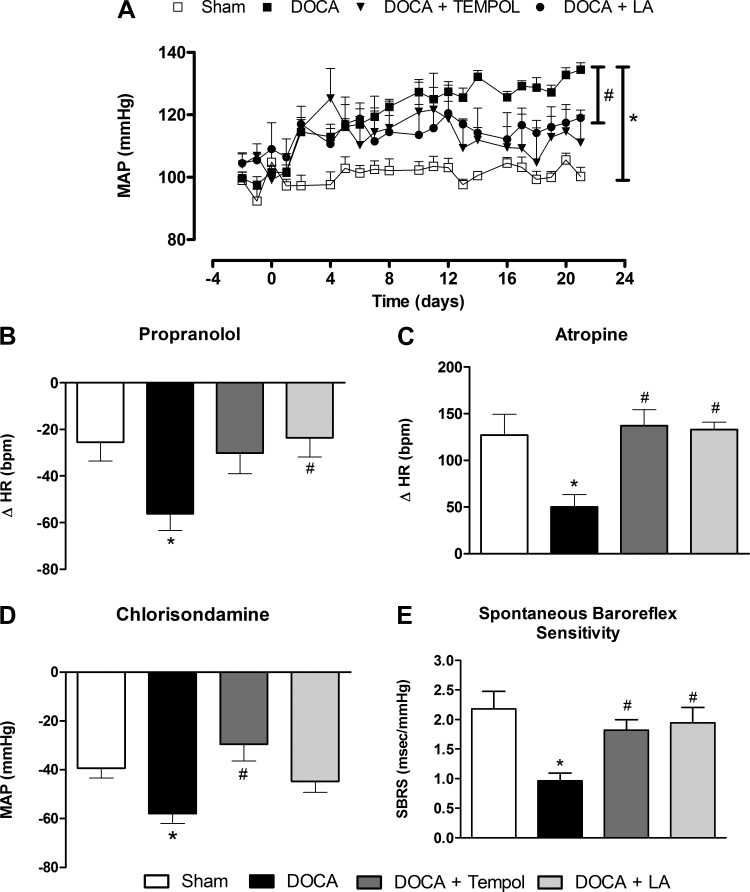
DOCA-salt treatment elicited a rapid rise in MAP within 48 h (119.4 ± 2.7 vs. 102.5 ± 2.4 mmHg, P < 0.05, n = 7, Fig. 3A) that reached a plateau in the 2nd wk compared with the sham group (131.4 ± 2.2 vs. 100.2 ± 2.9 mmHg, P < 0.05, n = 7, Fig. 3A). Importantly, LA and tempol produced a similar abatement of hypertension (Fig. 3A), as observed in DOCA + LA (119.0 ± 2.4 vs. 131.4 ± 2.2 mmHg, P < 0.05, n = 7) and DOCA + tempol (111.0 ± 8.7 vs. 131.4 ± 2.2 mmHg, P < 0.05, n = 7) groups, confirming that oxidative stress is a critical contributor in this model.
Fig. 3.
LA prevents DOCA-salt-induced hypertension. A: average mean arterial pressure changes in the various groups before and after DOCA-salt treatment. LA improves impaired cardiac sympathetic (B) and vagal (C) tone in DOCA-salt hypertension without affecting vascular sympathetic drive (D). LA improves baroreflex sensitivity in DOCA-salt-induced hypertensive mice (E). *P < 0.05 vs. sham and #P < 0.05 vs. DOCA. All data are means ± SE.
Cardiac and vascular sympathetic drive were increased after DOCA-salt treatment compared with sham (P < 0.05, Fig. 3, B and D), whereas the vagal tone was blunted in DOCA-salt hypertensive mice (P < 0.05, Fig. 3C). Tempol and LA similarly reduced dysautonomia (i.e., reduced sympathetic drive and enhanced vagal tone) although LA exhibited a more modest effect on vascular sympathetic drive, with only a trend to reduce vagal tone. To determine the role of antioxidant therapy in DOCA-salt hypertension, we assessed SBRS using the sequence method (14, 36). As expected, SBRS was significantly impaired in DOCA-salt hypertensive mice compared with sham (0.96 ± 0.1 vs. 2.2 ± 0.3 ms/mmHg, P < 0.05, Fig. 3E). However, both tempol and LA prevented the reduction of SBRS in DOCA-salt hypertensive mice (1.8 ± 0.2 and 1.94 ± 0.2, respectively, vs. 0.96 ± 0.1 ms/mmHg, P < 0.05, Fig. 3E). Altogether, these data suggest that LA and tempol reduce DOCA-salt hypertension by preventing autonomic dysfunction and normalizing baroreflex sensitivity.
LA reduces ACE2 shedding in DOCA-salt hypertension.
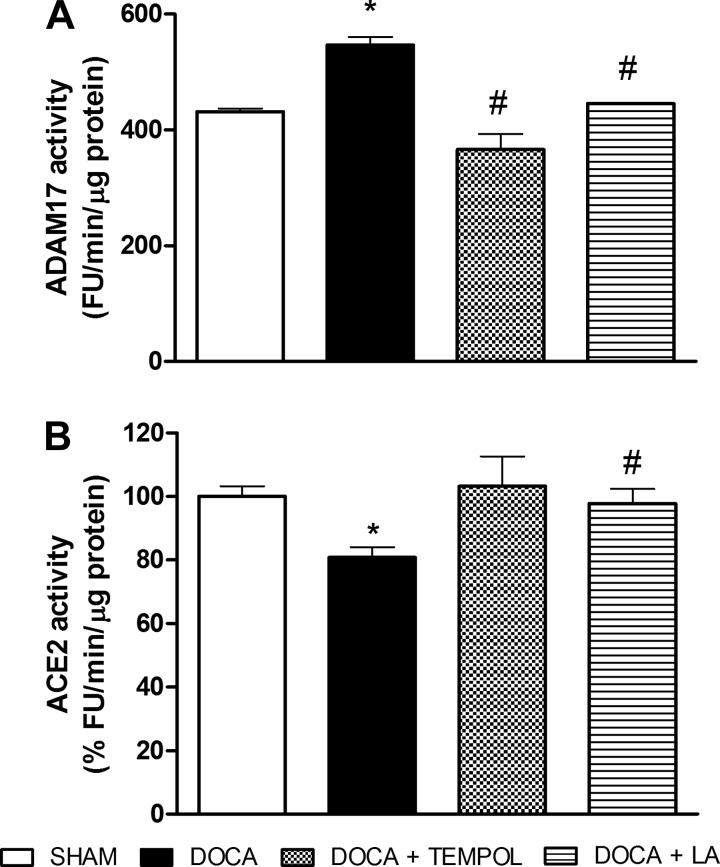
We previously reported that ADAM17 promotes ACE2 shedding in DOCA-salt hypertension (37). Accordingly, ADAM17 activity was increased by 25%, and ACE2 activity was reduced by 20% in the hypothalamus after 3 wk of DOCA-salt treatment (P < 0.05 vs. sham, Fig. 4). Interestingly, ADAM17 activity was blunted by tempol (366.1 ± 27.0 vs. 546.8 ± 14.0 FU·min−1·μg protein−1, P < 0.05, Fig. 4A) and LA (445.0 ± 0.9 vs. 546.8 ± 14.0 FU·min−1·μg protein−1, P < 0.05, Fig. 4A). In addition, LA prevented the reduction in ACE2 activity (97.8 ± 4.5 vs. 80.8 ± 3.3%FU·min−1·μg protein−1, P < 0.05, Fig. 4B). Our data suggest that the reduction of oxidative stress by LA restores ACE2 activity by preventing ADAM17-mediated shedding.
Fig. 4.
ADAM17 and type 2 angiotensin-converting enzyme (ACE2) activities in the hypothalamus of DOCA-salt hypertensive mice. The increase of hypothalamic ADAM17 activity (A) and the decrease of ACE2 activity (B) were reduced by LA treatment in DOCA-salt hypertensive mice. *P < 0.05 vs. sham and #P < 0.05 vs. DOCA. All data are means ± SE.
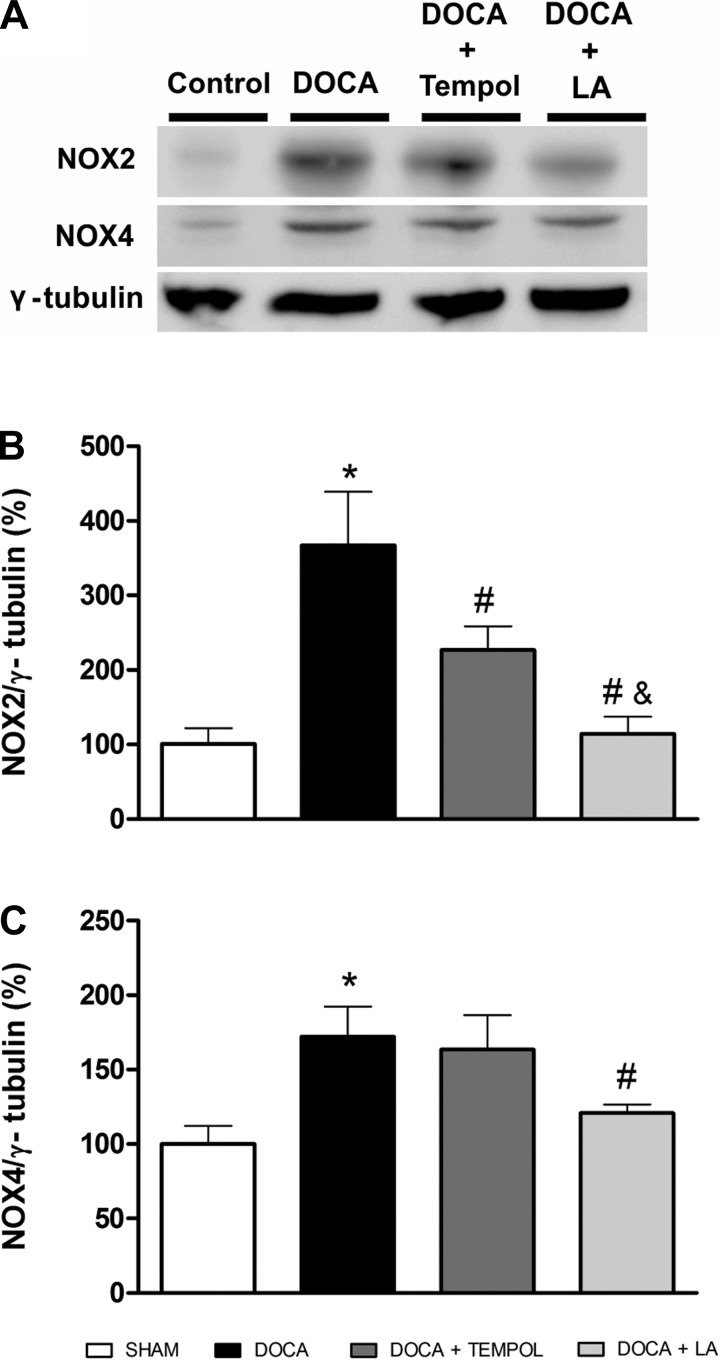
LA reduces the enhanced brain expression of Nox2 and Nox4 in DOCA-salt hypertension.
Because Nox2 and Nox4 are members of the NADPH oxidase family of enzymes involved in ROS production in hypertension, we tested whether these enzymes were altered in DOCA-salt hypertension and whether their protein expression is affected by LA treatment. As shown in Fig. 5, oxidative stress was increased in DOCA-salt hypertension with an increase in protein expression for Nox2 (367.1 ± 71.0 vs. 100.0 ± 21.0%, P < 0.05, Fig. 5, A and B) and Nox4 (172.3 ± 20.1 vs. 100.0 ± 12.1%, P < 0.05, Fig. 5, A and C). LA was more effective than tempol in preventing the increase in both members of the Nox family (P < 0.05, Fig. 5, A–C).
Fig. 5.
Hypothalamic NADPH oxidase expression in DOCA-salt hypertensive mice. Representative Western blot (A) and group data showing that LA reduces the increase in NAD(P)H oxidase (NOX2, B) and Nox4 (C) protein expression in the hypothalamus of DOCA-salt hypertensive mice. *P < 0.05 vs. sham, #P < 0.05 vs. DOCA, and &P < 0.05 vs. DOCA + tempol. All data are means ± SE.
DISCUSSION
Using a combination of in vitro and in vivo approaches, we tested the hypothesis that oxidative stress-mediated activation of ADAM17 and the resulting ACE2 shedding are blunted by LA in neurogenic hypertension. The major findings of this study are that LA attenuates the DOCA-salt hypertension-mediated dysautonomia and impaired baroreflex sensitivity, leading to a reduction in high BP. In neuronal cells, LA reduced the increase in ADAM17 expression and activity. Finally, the beneficial effects of LA were associated with its antioxidant activity.
Our data show that ANG II treatment in Neuro2A cells induces an increase in ADAM17 protein expression. Increase in ADAM17 expression has been reported in several models of cardiovascular diseases, including hypertension (25). Several mechanisms have been proposed by which ANG II induces ADAM17 activation (24, 26, 46). An important factor involved in ADAM17 activation is through ROS production. Strong evidence suggests that ROS stimulates the removal of the inhibitory prodomain of ADAMs (47), and this leads to activation of ectodomain shedding (30). ROS also could mediate the phosphorylation of p38 mitogen-activated protein kinase, which in turn phosphorylates and activates the threonine-735 residue on ADAM17 protein (33, 42). However, N-acetyl-l-cysteine was shown to impair metalloprotease-dependent ectodomain shedding by preventing ROS-mediated ADAM17 activation (30). Our study extends previous findings since LA not only reduced ADAM17 overexpression but also blunted ADAM17 activity, suggesting an inhibitory response in ADAM17 expression/activity due to an antioxidant effect.
ANG II induces ROS production, including superoxide, in neurons of cardiovascular regulatory brain areas such as the paraventricular nucleus (PVN) of the hypothalamus, the subfornical organ, and the rostral ventrolateral medulla (5, 7, 48) and decreases ACE2 expression in Neuro2A cells (38). These alterations could be both the cause and consequence of an increase in ADAM17 expression and/or activity. Therefore, we assessed whether ADAM17 overexpression could trigger ROS production and whether LA decreases the sheddase by its antioxidant effects. DHE staining showed strong evidence that LA induces ADAM17 impairment by reducing oxidative stress. Previous data have shown that adenovirus-mediated ACE2 overexpression reduces ROS formation (15). Furthermore, ACE2 depletion could lead to an increase in ROS production (38); therefore, we identified ACE2 activation as a new “antioxidant” effect. From our knowledge, the current study provides the first demonstration that elevated ROS production induces ADAM17 activation in neurons.
DOCA-salt hypertension is a well-established model of hypertension, depending on high-salt and water intake leading to hypervolemia (17). In this model, excess of mineralocorticoids leads to an imbalance in renal sodium handling by increasing sodium and water reabsorption (45). Notably, after chronic DOCA-salt treatment, the peripheral RAS, a major regulator of BP (18), is suppressed, whereas in the brain, there is overactivity of this system (20, 21). Previous studies have supported the hypothesis that DOCA-salt hypertension is a result of ACE upregulation in the brain (21) or increase in AT1R density in some regions of the brain such as subfornical organ, nucleus tractus solitarius, area prostrema, and PVN (19). RAS overactivity associated with AT1R increase in the brain led us to suggest oxidative stress as a key mechanism in ANG II-mediated neurogenic hypertension (7). Increased oxidative stress has been found in DOCA-salt hypertension (2, 32). Oxidative stress mediates increased transcription of genes, including activation of nuclear factor-kB (NF-kB), responsible for the early inflammatory response in mineralocorticoid hypertensive animals contributing to end-organ damage (32). In the current study, we demonstrated that DOCA-salt hypertensive mice presented a greater increase in MAP and that either LA or the classic antioxidant tempol attenuated the hypertension. Antioxidant therapies, including ROS scavengers and vitamins, superoxide dismutase mimetics, or NADPH oxidase inhibitors have been shown to attenuate or prevent the development of hypertension (4, 8, 29). Our previous study showed that LA reduced hypertension in 2K1C rats (29). Besides, LA was able to decrease systolic BP of DOCA-salt hypertensive rats at a dose considerably higher compared with the one used in our study (35). We previously demonstrated that spontaneous baroreflex sensitivity and sympathetic activity are impaired in DOCA-salt hypertension (37). In our study, LA treatment improved impaired cardiac sympathetic and vagal tone with only a trend to affect the vascular sympathetic drive. Previous studies have shown that tempol treatment induced a reduction in BP by inhibition of sympathetic nerve activity (SNA) in DOCA-salt hypertension (41). In fact, tempol administration produced a greater depressor effect in DOCA-salt hypertensive rats compared with control animals, indicating that SNA acts as an important mediator of high BP in DOCA-salt hypertension (40). Curiously, the depressor effect induced by tempol in DOCA-salt hypertension was oxidative stress-independent (40), suggesting a direct effect of tempol on SNA without affecting ROS-induced mechanisms in this model. On the other hand the effect observed for LA seems to be different from that induced by tempol. In prior studies and in the present work, we showed that LA reduces the SNA and BP by an oxidative stress-dependent mechanism (4, 29). In addition, LA increased SBRS in DOCA-salt hypertensive animals. Moreover, our group previously showed that improvement of autonomic function is associated with ACE2-mediated reduction of oxidative stress in the central nervous system (38). Other work demonstrated that ACE2 gene therapy can restore baroreflex and autonomic functions and prevent the development of hypertension (14). Additionally, we documented that knockdown of ADAM17 prevents the reduction of brain ACE2 levels and inhibits DOCA-salt hypertension, suggesting that ACE2 shedding contributes to the development of neurogenic hypertension (37).
We next assessed the activity of ADAM17 and ACE2 in hypothalamus of DOCA-salt hypertensive mice. Activity of ADAM17 was augmented while ACE2 activity was decreased in DOCA-salt hypertensive animals. These responses were previously observed by us (37); however, both activities were restored by antioxidant treatment with LA. A previous study showed that ACE2 overexpression in the PVN increased ACE2 activity and downregulated AT1R expression contributing to the reduction of ANG II-mediated hypertension (34). ANG II mediates ADAM17 activation and ACE2 shedding through AT1R, acting as a feedforward mechanism in the RAS. The ACE2-ANG-(1–7)-MasR pathway is the main negative regulator of ACE/ANG II/AT1R effects (43). Therefore, oxidative stress from ANG II-mediated Nox activation can induce an upregulation of ADAM17, which in turn induces ACE2 shedding and drives the feedforward mechanism of hypertension. These responses corroborate the idea that both antioxidant therapy and an increase in ACE2 expression counteract ROS formation in the brain and are pivotal to prevent neurogenic hypertension. The impaired ACE2 activity and ADAM17 activation found in pathological conditions, such as in DOCA-salt hypertension, allow for the uncoupling of the ACE2-ANG-(1–7)-MasR pathway and promote the increase of soluble ACE2 in cardiovascular diseases (9, 13).
A recent study reported that ADAM17 activation induces oxidative stress via upregulation of Nox4 protein expression and increased Nox activity leading to extracellular matrix accumulation in kidney (16). In contrast, another study demonstrated that Nox4 and ROS promote increase in ADAM17 expression by ANG II-mediated EGFR activation to induce cardiac hypertrophy (46). Another study suggested that Nox2 seems to be a more prominent mediator of the injurious effects of ANG II in the central nervous system during hypertension (11) while injections of adenoviral vectors encoding small-interfering RNA targeting Nox2 or Nox4, to knock down expression in the PVN, showed that both attenuated the development of aldosterone/NaCl-mediated hypertension (44). Of note, our study shows that both Nox2 and Nox4 are upregulated in hypothalamus of DOCA-salt hypertension mice. In addition, LA treatment to avoid ROS formation blunted Nox subunit overexpression. This suggests that Nox has an important role in DOCA-salt hypertension development. Therefore, ROS formation from Nox could induce ADAM17 overexpression and subsequently ACE2 shedding in the brain, thus promoting neurogenic hypertension.
Clearly, the mechanisms by which LA acts to promote the effects presented in this study need to be further investigated. However, we already know that LA regenerates endogenous antioxidants (vitamins C and E) and has the ability to scavenge free radicals (3). LA is widely known to reduce the expression of metalloproteinase-9 through the inhibition of NF-kB (22), which is important to regulate an inflammatory response induced by ROS. In addition, one must keep in mind that ACE2 is only one of multiple targets for ADAM17, and therefore, by reducing ADAM17 levels, LA could exert its beneficial effects via numerous other players, including EGFR, TNF-α, and various cytokines (12), ultimately inhibiting ROS formation and reducing hypertension.
In summary, our data suggest that LA might preserve ACE2 compensatory activity by breaking the feedforward cycle between oxidative stress and ADAM17 due to its antioxidant properties. Therefore, ADAM17 could be a new target to prevent neurogenic hypertension.
GRANTS
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico/Brazil, National Institutes of Health Grants HL-093178 and GM-106392, and American Heart Association Established Investigator Award 12EIA8030004.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: T.M.d.Q., C.M.F., V.A.B., and E.L. conception and design of research; T.M.d.Q., H.X., and E.L. performed experiments; T.M.d.Q., H.X., C.M.F., V.A.B., and E.L. analyzed data; T.M.d.Q., C.M.F., V.A.B., and E.L. interpreted results of experiments; T.M.d.Q. and E.L. prepared figures; T.M.d.Q. and E.L. drafted manuscript; T.M.d.Q., H.X., C.M.F., V.A.B., and E.L. edited and revised manuscript; T.M.d.Q., H.X., C.M.F., V.A.B., and E.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Kim B. Pedersen and Srinivas Sriramula for technical expertise and discussions.
Present address for C. M. Filipeanu: Dept. of Pharmacology, Howard University College of Medicine, Washington, DC 20059.
REFERENCES
- 1.Arribas J, Esselens C. ADAM17 as a therapeutic target in multiple diseases. Curr Pharm Des 15: 2319–2335, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Beswick RA, Zhang H, Marable D, Catravas JD, Hill WD, Webb RC. Long-term antioxidant administration attenuates mineralocorticoid hypertension and renal inflammatory response. Hypertension 37: 781–786, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Biewenga GP, Haenen GRMM, Bast A. The pharmacology of the antioxidant lipoic acid. Gen Pharmacol 29: 315–331, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Botelho-Ono MS, Pina HV, Sousa KHF, Nunes FC, Medeiros IA, Braga VA. Acute superoxide scavenging restores depressed baroreflex sensitivity in renovascular hypertensive rats. Auton Neurosci 159: 38–44, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Braga VA. Dietary salt enhances angiotensin-II-induced superoxide formation in the rostral ventrolateral medulla. Auton Neurosci 155: 14–18, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Brill A, Chauhan AK, Canault M, Walsh MT, Bergmeier W, Wagner DD. Oxidative stress activates ADAM17/TACE and induces its target receptor shedding in platelets in a p38-dependent fashion. Cardiovasc Res 84: 137–144, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burmeister MA, Young CN, Braga VA, Butler SD, Sharma RV, Davisson RL. In vivo bioluminescence imaging reveals redox-regulated activator protein-1 activation in paraventricular nucleus of mice with renovascular hypertension. Hypertension 57: 289–297, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Touyz RM, Park JB, Schiffrin EL. Antioxidant effects of vitamins C and E are associated with altered activation of vascular nadph oxidase and superoxide dismutase in stroke-prone SHR. Hypertension 38: 606–611, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Chodavarapu H, Grobe N, Somineni HK, Salem ESB, Madhu M, Elased KM. Rosiglitazone treatment of type 2 diabetic db/db mice attenuates urinary albumin and angiotensin converting enzyme 2 excretion. PLOS ONE 8: e62833, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow FL, Fernandez-Patron C. Many membrane proteins undergo ectodomain shedding by proteolytic cleavage. Does one sheddase do the job on all of these proteins? IUBMB Life 59: 44–47, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Chrissobolis S, Banfi B, Sobey CG, Faraci FM. Role of Nox isoforms in angiotensin II-induced oxidative stress and endothelial dysfunction in brain. J Appl Physiol 113: 184–191, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dreymueller D, Pruessmeyer J, Groth E, Ludwig A. The role of ADAM-mediated shedding in vascular biology. Eur J Cell Biol 91: 472–485, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Epelman S, Tang WHW, Chen SY, Van Lente F, Francis GS, Sen S. Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin-angiotensin-aldosterone system. J Am Coll Cardiol 52: 750–754, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RAS, Speth RC, Sigmund CD, Lazartigues E. Brain-selective overexpression of human angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ Res 106: 373–382, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Y, Yue X, Xia H, Bindom SM, Hickman PJ, Filipeanu CM, Wu G, Lazartigues E. Angiotensin-converting enzyme 2 overexpression in the subfornical organ prevents the angiotensin ii-mediated pressor and drinking responses and is associated with angiotensin ii type 1 receptor downregulation. Circ Res 102: 729–736, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford BM, Eid AA, Gooz M, Barnes JL, Gorin YC, Abboud HE. ADAM17 mediates Nox4 expression and NADPH oxidase activity in the kidney cortex of OVE26 mice. Am J Physiol Renal Physiol 305: F323–F332, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garwitz ET, Jones AW. Aldosterone infusion into the rat and dose-dependent changes in blood pressure and arterial ionic transport. Hypertension 4: 374–381, 1982. [DOI] [PubMed] [Google Scholar]
- 18.Gavras H, Brunner HR, Laragh JH, Vaughan ED, Koss M, Cote LJ, Gavras I. Malignant hypertension resulting from deoxycorticosterone acetate and salt excess: role of renin and sodium in vascular changes. Circ Res 36: 300–309, 1975. [DOI] [PubMed] [Google Scholar]
- 19.Grobe JL, Buehrer BA, Hilzendeger AM, Liu X, Davis DR, Xu D, Sigmund CD. Angiotensinergic signaling in the brain mediates metabolic effects of deoxycorticosterone (DOCA)-salt in C57 mice. Hypertension 57: 600–607, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilzendeger AM, Cassell MD, Davis DR, Stauss HM, Mark AL, Grobe JL, Sigmund CD. Angiotensin type 1a receptors in the subfornical organ are required for deoxycorticosterone acetate-salt hypertension. Hypertension 61: 716–722, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itaya Y, Suzuki H, Matsukawa S, Kondo K, Saruta T. Central renin-angiotensin system and the pathogenesis of DOCA-salt hypertension in rats. Am J Physiol Heart Circ Physiol 251: H261–H268, 1986. [DOI] [PubMed] [Google Scholar]
- 22.Kim HS, Kim HJ, Park KG, Kim YN, Kwon TK, Park JY, Lee KU, Kim JG, Lee IK. α-Lipoic acid inhibits matrix metalloproteinase-9 expression by inhibiting NF-[kappa]B transcriptional activity. Exp Mol Med 39: 106–113, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Lai ZW, Hanchapola I, Steer DL, Smith AI. Angiotensin-converting enzyme 2 ectodomain shedding cleavage-site identification: determinants and constraints. Biochemistry 50: 5182–5194, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Mifune M, Ohtsu H, Suzuki H, Nakashima H, Brailoiu E, Dun NJ, Frank GD, Inagami T, Higashiyama S, Thomas WG, Eckhart AD, Dempsey PJ, Eguchi S. G protein coupling and second messenger generation are indispensable for metalloprotease-dependent, heparin-binding epidermal growth factor shedding through angiotensin ii type-1 receptor. J Biol Chem 280: 26592–26599, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Odenbach J, Wang X, Cooper S, Chow FL, Oka T, Lopaschuk G, Kassiri Z, Fernandez-Patron C. MMP-2 mediates angiotensin ii-induced hypertension under the transcriptional control of MMP-7 and TACE. Hypertension 57: 123–130, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Ohtsu H, Dempsey PJ, Frank GD, Brailoiu E, Higuchi S, Suzuki H, Nakashima H, Eguchi K, Eguchi S. ADAM17 mediates epidermal growth factor receptor transactivation and vascular smooth muscle cell hypertrophy induced by angiotensin II. Arterioscler Thromb Vasc Biol 26: e133–e137, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 86: 747–803, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen KB, Sriramula S, Chhabra K, Xia H, Lazartigues E. Species-specific inhibitor sensitivity of angiotensin-converting enzyme 2 (ACE2) and its implication for ACE2 activity assays. Am J Physiol Regul Integr Comp Physiol 301: R1293–R1299, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Queiroz TM, Guimaraes DD, Mendes LG Jr, Braga VA. α-Lipoic acid reduces hypertension and increases baroreflex sensitivity in renovascular hypertensive rats. Molecules 17: 13357–13367, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanderson MP, Abbott CA, Tada H, Seno M, Dempsey PJ, Dunbar AJ. Hydrogen peroxide and endothelin-1 are novel activators of betacellulin ectodomain shedding. J Cell Biochem 99: 609–623, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta 1790: 1149–1160, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Somers MJ, Mavromatis K, Galis ZS, Harrison DG. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation 101: 1722–1728, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Soond SM, Everson B, Riches DWH, Murphy G. ERK-mediated phosphorylation of Thr735 in TNFα-converting enzyme and its potential role in TACE protein trafficking. J Cell Sci 118: 2371–2380, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Sriramula S, Cardinale JP, Lazartigues E, Francis J. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc Res 92: 401–408, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takaoka M, Kobayashi Y, Yuba M, Ohkita M, Matsumura Y. Effects of α-lipoic acid on deoxycorticosterone acetate-salt-induced hypertension in rats. Eur J Pharmacol 424: 121–129, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Xia H, Feng Y, Obr TD, Hickman PJ, Lazartigues E. Angiotensin II type 1 receptor-mediated reduction of angiotensin-converting enzyme 2 activity in the brain impairs baroreflex function in hypertensive mice. Hypertension 53: 210–216, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia H, Sriramula S, Chhabra K, Lazartigues E. Brain ACE2 shedding contributes to the development of neurogenic hypertension. Circ Res 113: 1087–1096, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia H, Suda S, Bindom S, Feng Y, Gurley SB, Seth D, Navar LG, Lazartigues E. ACE2-mediated reduction of oxidative stress in the central nervous system is associated with improvement of autonomic function. PLoS One 6: e22682, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao L, Gao L, Lazartigues E, Zucker IH. Brain-selective overexpression of angiotensin-converting enzyme 2 attenuates sympathetic nerve activity and enhances baroreflex function in chronic heart failure. Hypertension 58: 1057–1065, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu H, Fink GD, Galligan JJ. Nitric oxide-independent effects of tempol on sympathetic nerve activity and blood pressure in DOCA-salt rats. Am J Physiol Heart Circ Physiol 283: H885–H892, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Xu H, Fink GD, Galligan JJ. Tempol lowers blood pressure and sympathetic nerve activity but not vascular O2- in DOCA-salt rats. Hypertension 43: 329–334, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Xu P, Derynck R. Direct activation of TACE-mediated ectodomain shedding by p38 MAP kinase regulates EGF receptor-dependent cell proliferation. Mol Cell 37: 551–566, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu P, Sriramula S, Lazartigues E. ACE2/Ang-(1–7)/Mas pathway in the brain: the Axis of Good. Am J Physiol Regul Integr Comp Physiol 300: R804–R817, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xue B, Beltz TG, Johnson RF, Guo F, Hay M, Johnson AK. PVN adenovirus-siRNA injections silencing either NOX2 or NOX4 attenuate aldosterone/NaCl-induced hypertension in mice. Am J Physiol Heart Circ Physiol 302: H733–H741, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yemane H, Busauskas M, Burris SK, Knuepfer MM. Neurohumoral mechanisms in deoxycorticosterone acetate (DOCA)-salt hypertension in rats. Exp Physiol 95: 51–55, 2010. [DOI] [PubMed] [Google Scholar]
- 46.Zeng SY, Chen X, Chen SR, Li Q, Wang YH, Zou J, Cao WW, Luo JN, Gao H, Liu PQ. Upregulation of Nox4 promotes angiotensin ii-induced epidermal growth factor receptor activation and subsequent cardiac hypertrophy by increasing ADAM17 expression. Can J Cardiol 29: 1310–1319, 2013. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z, Oliver P, Lancaster JJR, Schwarzenberger PO, Joshi MS, Cork J, Kolls JK. Reactive oxygen species mediate tumor necrosis factor alpha-converting, enzyme-dependent ectodomain shedding induced by phorbol myristate acetate. FASEB J 15: 303–305, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res 95: 210–216, 2004. [DOI] [PubMed] [Google Scholar]