To our best knowledge, this study is the first to investigate PVN PRR-mediated ANG II-independent signaling transduction on the control of sympathetic outflow and blood pressure. Our results suggest that PVN PRR-induced sympathoexcitation is possibly mediated by ROS-AP-1-iNOS signal transduction pathways.
Keywords: paraventricular nucleus, (pro)renin receptor, reactive oxygen species, sympathetic nerve activity
Abstract
Previous studies have indicated that hyperactivity of brain prorenin receptors (PRR) is implicated in neurogenic hypertension. However, the role of brain PRR in regulating arterial blood pressure (ABP) is not well understood. Here, we test the hypothesis that PRR activation in the hypothalamic paraventricular nucleus (PVN) contributes to increased sympathetic nerve activity (SNA). In anaesthetized adult Sprague-Dawley (SD) rats, bilateral PVN microinjection of human prorenin (2 pmol/side) significantly increased splanchnic SNA (SSNA; 71 ± 15%, n = 7). Preinjection of either prorenin handle region peptide, the PRR binding blocker (PRRB), or tiron (2 nmol/side), the scavenger of reactive oxygen species (ROS), significantly attenuated the increase in SSNA (PRRB: 32 ± 5% vs. control, n = 6; tiron: 8 ± 10% vs. control, n = 5; P < 0.05) evoked by prorenin injection. We further investigated the effects of PRR activation on ROS production as well as downstream gene expression using cultured hypothalamus neurons from newborn SD rats. Incubation of brain neurons with human prorenin (100 nM) dramatically enhanced ROS production and induced a time-dependent increase in mRNA levels of inducible nitric oxide synthase (iNOS), NAPDH oxidase 2 subunit cybb, and FOS-like antigen 1 (fosl1), a marker for neuronal activation and a component of transcription factor activator protein-1 (AP-1). The maximum mRNA increase in these genes occurred 6 h following incubation (iNOS: 201-fold; cybb: 2 -fold; Ffosl1: 11-fold). The increases in iNOS and cybb mRNA were not attenuated by the AT1 receptor antagonist losartan but abolished by the AP-1 blocker curcumin. Our results suggest that PVN PRR activation induces sympathoexcitation possibly through stimulation of an ANG II-independent, ROS-AP-1-iNOS signaling pathway.
NEW & NOTEWORTHY
To our best knowledge, this study is the first to investigate PVN PRR-mediated ANG II-independent signaling transduction on the control of sympathetic outflow and blood pressure. Our results suggest that PVN PRR-induced sympathoexcitation is possibly mediated by ROS-AP-1-iNOS signal transduction pathways.
accumulating evidence indicates that alteration of central (pro)renin receptor (PRR) is involved in the development of cardiovascular diseases including neurogenic hypertension (32, 46). The importance of the brain PRR in cardiovascular control is further strengthened by the observation that increased PRR expression in brain cardiovascular control areas altered body fluid homeostasis (38) and genetic knockdown of the PRR induced long-term depressor effects in hypertensive animals (21, 22, 38). However, the detailed mechanism underlying the role of brain PRR on blood pressure regulation is not well understood.
PRR is a single transmembrane protein that binds both renin and prorenin. Binding of prorenin to the PRR leads to nonproteolytic activation of prorenin and mediates local ANG II formation (28). In addition, the PRR-(pro)renin complex stimulates a variety of signal transduction pathways independent of ANG II (8, 28, 46). Our previous study has shown that PRR is highly expressed in the brain cardiovascular control areas such as the hypothalamic supraoptic nucleus (SON), and its expression is significantly increased in the spontaneous hypertensive rat (SHR) compared with normotensive Wistar-Kyoto (WKY) controls (38). Chronic overexpression of this receptor in the SON alters body fluid homeostasis in normotensive rats, while genetic knockdown of the expression of SON PRR significantly attenuated the age-dependent blood pressure increase in the SHR (38), indicating hypothalamic PRR plays a critical role in the control of blood pressure. PRR is also highly expressed in the hypothalamic paraventricular nucleus (PVN) (7, 38). The PVN is one of the most important brain areas controlling sympathetic outflow. PVN neurons receive signals from visceral and sensory nerves arising via the nucleus of the solitary tract (NTS) and integrate this with input from the limbic system, circumventricular organs, and other hypothalamus structures and control sympathetic outflow by sending projections directly to the spinal cord or the other sympathetic regulatory brain area, the rostral ventrolateral medulla (RVLM). Activation of these PVN neurons can lead to sympathetic discharge (20, 45). Multiple factors affect sympathetic outflow such as sodium, ANG II, glutamate, cytokines, oxidative stress, and nitric oxide (NO) (11, 12, 34, 41, 47). Previous studies have demonstrated that brain PRR activation can increase the production of inflammatory cytokines, oxidative stress, and ANG II (32, 37, 46). Based on these observations, we speculate that activation of PVN PRR may be involved in increased sympathetic outflow. A long-term and persistent increase in sympathetic outflow may contribute to hypertension. The objectives of this study are to investigate whether PVN PRR activation will elicit an increase in sympathetic nerve activity (SNA) and whether ANG II-independent signaling is involved in this SNA regulation. We focus on ANG II-independent signal pathways because PRR activation can trigger a variety of signal transduction cascades and their role in cardiovascular function control is not well addressed.
METHODS
Animals.
Male adult Sprague-Dawley (SD) rats (350–500 g) were purchased from Charles River Laboratories (Wilmington, MA). They were housed individually and kept on a 12:12-h light-dark cycle in a climate-controlled room. Rat chow and water were provided ad libitum. All of the animal experiments followed protocols that were approved by the Michigan Technological University Institutional Animal Care and Use Committee.
PRR immunoreactivity in the PVN.
Immunofluorescence staining of PRR was performed as described previously (38, 46). Briefly, 10 μm of brain sections containing the PVN from male adult SD rats were submerged in −20°C acetone for 5 min, followed by washing with TBS for another 5 min. Sections were then incubated with 3% horse serum in TBS for 20 min and then incubated overnight at 4°C in a cocktail consisting of 1:200 dilution of goat anti-ATP6AP2 (a PRR-specific antibody; Abcam, Cambridge, MA) and either 1:250 dilution of mouse anti-neuronal nuclear (NeuN) antibody (EMD Millipore, Billerica, MA; Chemicon, Temecula, CA) or 1:500 dilution of rabbit anti-glial fibrillary acidic protein (GFAP) antibody (Abcam, San Francisco, CA). Afterwards, sections were washed in TBS twice for 5 min and incubated for 1 h at room temperature in a mixture of secondary antibodies (Alexa Fluor 594 donkey anti-goat IgG and Alexa Fluor 488 donkey anti-mouse IgG; or Alexa Fluor 594 donkey anti-goat IgG and Alexa Fluor 488 goat anti-rabbit IgG; all diluted 500-fold). The sections were mounted in Vectashield (Vector Labs, Burlingame, CA) and images were taken with a Leica TCS SP2, a laser-disk scanning confocal microscope.
Animal surgery.
Animal surgery was performed following the previously described protocol (13). Briefly, rats were anesthetized with an intraperitoneal injection containing a mixture of α-chloralose (80 mg/kg) and urethane (800 mg/kg). Adequate depth of anesthesia was assessed before surgery by the absence of pedal and corneal reflexes and by failure to withdraw the hindlimb in response to pinching the paw. Animals were instrumented with an arterial catheter inserted into the aorta through a femoral artery. The catheter was connected to a pressure transducer to measure arterial blood pressure (ABP). Heart rate (HR) was obtained from the R-wave of the electrocardiogram (ECG) (lead I). A catheter was also placed in the left femoral vein to administer drugs. After tracheal cannulation, rats were paralyzed with gallamine triethiodide (25 mg·kg−1·h−1 iv) and artificially ventilated with oxygen-enriched room air. After paralysis, anesthesia was monitored by the stability of ABP and HR, and supplements equal to 10% of the initial dose were given when needed. End-tidal Pco2 was continuously monitored and maintained within normal limits (35–40 mmHg) by adjusting ventilation rate (80–100 breaths/min) and/or tidal volume (2.0–3.0 ml). Body temperature was held at 37°C with a water-circulating pad.
Recording of SNA.
With the use of a left flank incision, a left renal and splanchnic sympathetic nerve bundle was isolated from surrounding tissue(13, 21, 42), mounted on a stainless steel wire electrode (0.127-mm OD; A-M Systems), and covered with a silicon-based impression material (Light Body; Coltene) to insulate the recording from body fluids. The recorded signal was directed to an AC amplifier (P511; Grass Technologies) equipped with half-amplitude filters (band pass: 100-1,000 Hz) and a 60-Hz notch filter. The processed signal was rectified, integrated (10-ms time constant), and digitized at a frequency of 5,000 Hz using a 1401 Micro3 analog-to-digital converter and Spike 2 software (7.04 version; Cambridge Electronic Design, Cambridge, UK). The background noise was determined by a bolus injection of hexamethonium (30 mg/kg iv), a ganglionic blocker, at the end of the experiment and was subtracted from all the integrated values of SNA.
PVN microinjection of drugs.
Animals were placed in a stereotaxic head frame, and the skull was leveled between bregma and lambda. A small piece of skull was removed so that a single-barreled glass microinjector pipette could be lowered vertically into the PVN. The stereotaxic coordinates used were the following: 1.2–1.6 mm caudal to bregma; 0.5–0.7 mm lateral to midline; and 7.0–7.4 mm ventral to dura. Prorenin for microinjection was purchased from Molecular Innovations (Novi, MI). The prorenin handle region peptide with the sequence H2N-RILLKKMPSV-OH, a PRR and prorenin binding blocker (PRRB), was synthesized at New England Peptide (Gardner, MA). The PRRB and tiron were dissolved in saline. All compounds were microinjected into the PVN bilaterally in a volume of 100 nl per side with a pneumatic pump (WPI). The interval between two bilateral injections was ∼5 min. The volume of each injection was determined by measuring the movement of the fluid meniscus within the microinjector pipette using a dissecting microscope equipped with an eyepiece reticule.
In vivo experimental protocols.
Human prorenin (Molecular Innovations) was used to test PRR activation on sympathetic outflow and gene regulation throughout the study. Animals were allowed to stabilize for at least 2 h after surgery, at which time the response to PVN-microinjected prorenin (2 pmol/side) was determined. Changes in mean arterial pressure (MAP), splanchnic SNA (SSNA), and renal SNA (RSNA) were recorded in response to each injection. To control for possible nonspecific effects of the injected volume (100 nl), responses to PVN injection of prorenin were determined after vehicle injection of saline. To test whether the prorenin agonist elicited the sympathoexcitatory response and whether activation of the PRR was involved in these responses, the effects of PVN injection of prorenin on MAP, SSNA, and RSNA were determined after pretreatment with the PRRB (200 pmol/side) in a separate group of animals. To test whether the prorenin induced increase in SSNA was mediated by reactive oxygen species (ROS) production, the response to PVN injection of prorenin (2 pmol/side) on MAP and SSNA was determined after pretreatment of the PVN with tiron (2 nmol/side), a ROS scavenger, in a separate group of animals. At the end of each experiment, Chicago blue dye solution (2% in saline, 100 nl) was injected into the PVN to mark the site of each injection. Brains were removed and post fixed for 5 days at room temperature in 4% paraformaldehyde. Brain coronal sections containing the PVN were cut, and microinjection sites were identified under the light microscopy. Rats with injection site(s) not inside the PVN were excluded from data analysis.
Preparation of neuronal cultures.
Neuronal cultures were made from the hypothalamus of 1-day-old SD rats as described previously (37). Neuronal cultures contained >95% neurons (remaining cells were primarily astroglia). The cultures were maintained for 10–14 days before their use in experiments.
Intracellular ROS determination.
Brain neuronal cultures from the hypothalamus containing the PVN of newborn SD rats were incubated with human prorenin (100 nM) or vehicle control for 1 h. The generation of ROS was determined by the oxidant-sensitive fluorogenic probe dihydroethidium (DHE; 1 μM) staining for 30 min (43). Ethidium fluorescence within neurons was detected by a fluorescent microscope. Each treatment condition was run in triplicate within experiments, and each set of experiments was performed using three separate culture dishes.
Measurement of the prorenin stimulation effect on cultured brain neurons.
Cultured brain neurons were incubated with 100 nmol/l of human prorenin or vehicle control for differing time periods (1, 3, 6, 12, and 24 h). Cells were collected, and quantitative real-time PCR was performed to measure mRNA levels of genes of interest including inducible nitric oxide synthase (iNOS); FOS-like antigen 1 (fosl1), a neuronal activation marker; and the NAPDH oxidase 2 subunit cybb, using specific primers and probes (Applied Biosystems, Foster City, CA) in the Step One Plus Real Time PCR System (Applied Biosystems). Based on the results from the above time-course experiment, the maximum increase in most of the genes was observed 6 h postincubation with prorenin. We then coincubated neuronal cultures with 100 nmol/l of human prorenin with or without the ANG II type I receptor (AT1-R) blocker losartan (2 μmol/l) and the activator protein-1 (AP-1) inhibitor curcumin (50 μmol/l) for 6 h. The mRNA levels of iNOS, fosl1, and cybb were determined by quantitative RT-PCR as described above. Each set of experiments were performed using three separate culture dishes, and all cDNA samples were assayed in duplicate. The whole experiment was repeated at least two times. Data were normalized to GAPDH mRNA.
Data analysis of study.
Summary data are expressed as means ± SE. SSNA and RSNA were determined as an average of the rectified, integrated signal. Baseline values of all recorded variables were obtained by averaging a 10-min segment of data recorded immediately before PVN microinjection in anesthetized rats. SSNA, RSNA, MAP, and HR responses to vehicle control, prorenin, and tiron were obtained by averaging a 2-min period centered on the maximal response. Data are presented as percent (%) change from baseline after subtracting background noise determined with bolus injection of the ganglionic blocker hexamethonium (30 mg/kg). Both in vivo and in vitro data were analyzed by either one-way ANOVA with post hoc Newman-Keuls multiple comparison or unpaired student t-test. Differences were considered statistically significant at a critical value of P < 0.05.
RESULTS
PVN PRR is primarily distributed in neurons.
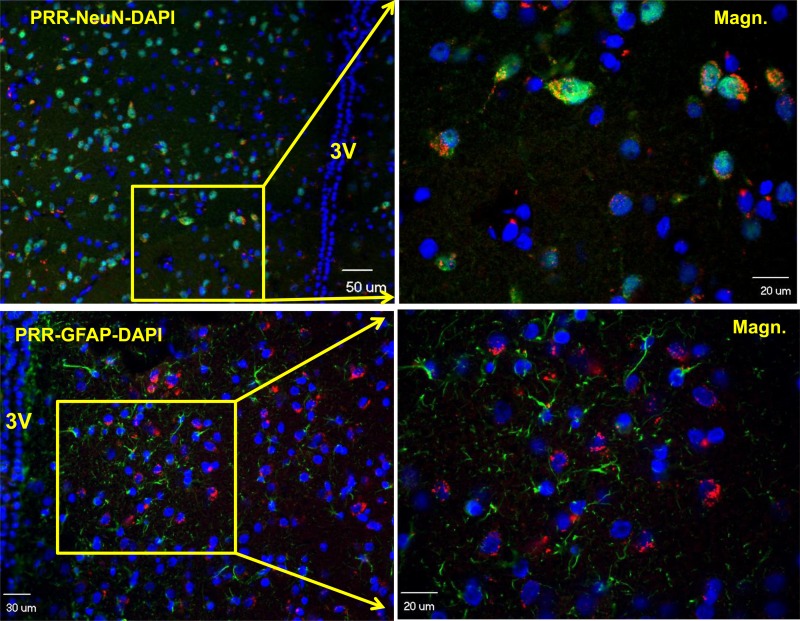
First, we performed immunohistochemistry to assay PRR distribution in the PVN using brain coronal sections containing the hypothalamus from male adult SD rats. The results showed that PRR immunoreactivity was primarily localized in NeuN-positive cells (NeuN is a marker for neuronal nuclei); no significant staining of PRR was observed with GFAP-positive cells (GFAP is an astroglial marker; Fig. 1). These results indicate that PVN PRR is predominantly localized in neurons.
Fig. 1.
The paraventricular nucleus (PVN) prorenin receptor (PRR) is primarily expressed in neurons. Representative confocal images showing coimmunofluorescence staining of PRR (red) and neuronal nuclear (NeuN; green), a marker for neuronal nuclei (top), or PRR (red) and glial fibrillary acidic protein (GFAP; green), a marker for astrocyte (bottom). Left: low-magnification images. Right: high-magnification images. Cell nuclei were stained with DAPI (blue).
Effects of PVN PRR activation on SSNA, RSNA, MAP, and HR.
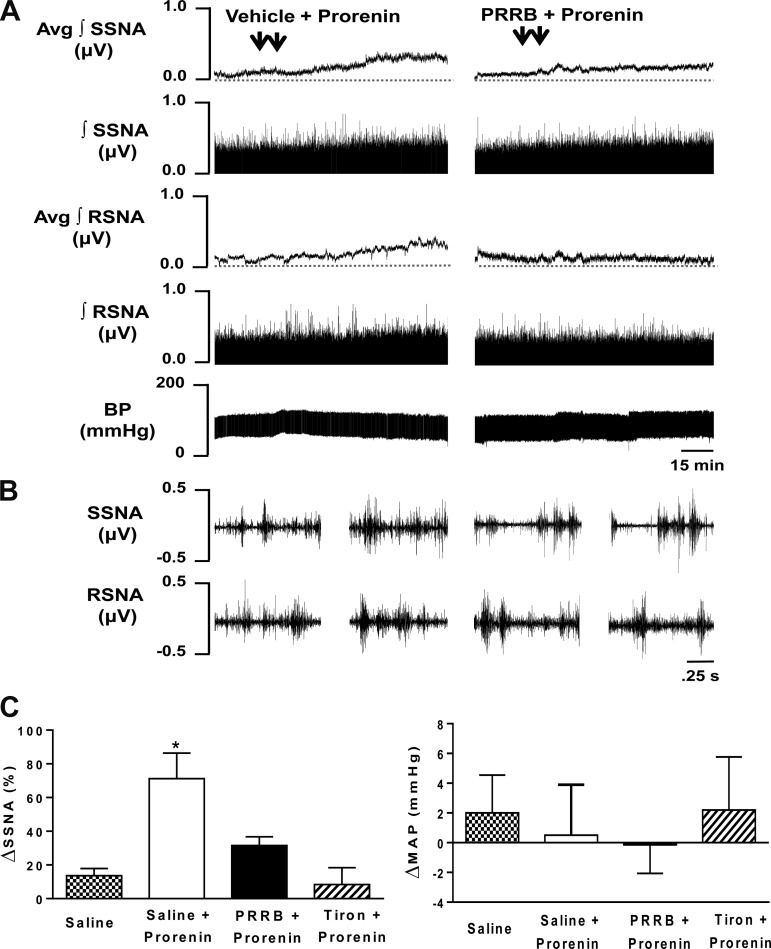
Since PVN PRR is primary distributed in neurons, and PVN neurons play an important role in regulating sympathetic outflow, we then investigated whether activation of PVN PRR would result in sympathoexcitation. Bilateral PVN microinjection of the PRR ligand prorenin increased SSNA and RSNA. Figure 2, A and B, left, shows an example of the SSNA and RSNA responses to human prorenin (2 pmol/side) bilateral injection into the PVN. Sympathoexcitatory responses began within 15–20 min after microinjection, reached a plateau within 45–60 min, and remained at an elevated steady state throughout the 110-min experimental period. Maximum increases in SSNA and RSNA were 71 ± 15% (n = 7) and 21 ± 10% (n = 7), respectively. To test whether the prorenin-induced sympathoexcitatory response involved local activation of PRR, SSNA, RSNA, and MAP, responses to bilateral PVN microinjections of the PRRB (200 pmol/side) followed by bilateral microinjections of prorenin (2 pmol/side) were determined. Figure 2, A and B, right, shows an example of the response of PRRB pretreatment followed by prorenin injected into the PVN. Note that the elevation in SSNA evoked by human prorenin was significantly blunted by preinjection of PRRB (32 ± 5%; P < 0.05 vs. vehicle control; n = 6). The elevation in RSNA was blocked by preinjection of PRRB but failed to reach the statistically significant difference (3 ± 11%; P = 0.24 vs. vehicle control; n = 7). To test whether the excitatory response in SSNA induced by PRR activation was mediated by ROS, SSNA, and MAP responses to bilateral PVN microinjections of tiron (2 nmol/side), the ROS scavenger, followed by bilateral PVN microinjections of prorenin (2 pmol/side) were determined. The elevation in SSNA evoked by prorenin was almost completely abolished by preinjection of tiron (8 ± 10%; P < 0.05 vs. vehicle control; n = 5), while there was no effect on blood pressure (Fig. 2C).
Fig. 2.
Activation of PVN PRR increases sympathetic nerve activity (SNA). A: representative traces showing splanchnic sympathetic nerve activity (SSNA), renal sympathetic nerve activity (RSNA), and mean arterial blood pressure (BP; MAP) responses to bilateral microinjections of 0.9% saline (vehicle control) followed by bilateral microinjections of prorenin (2 pmol/side) into the PVN (left) and bilateral microinjections of prorenin handle region peptide, a prorenin receptor blocker (PRRB; 200 pmol/side), followed by bilateral microinjections of prorenin (2 pmol/side) into the PVN (right). Each injection (100 nl) of saline, PRRB, and prorenin (arrowhead) was completed over a period of ∼1 min. The interval between saline or PRRB and prorenin microinjection was ∼10 min. B, left: 2.5-s specimen traces of SSNA (top) and RSNA (bottom) before injection of saline into the PVN and after microinjection of prorenin into the PVN. B, right: 2.5-s specimen traces of SSNA (top) and RSNA (bottom) before injection of PRRB into the PVN and after microinjection of prorenin into the PVN. C: summary data showing changes in SSNA and MAP in response to bilateral microinjections of saline (n = 7): saline + prorenin (n = 7), PRRB + prorenin (n = 6), and tiron + prorenin (n = 5). *P < 0.05 compared with all other 3 groups.
PRR activation increases ROS production.
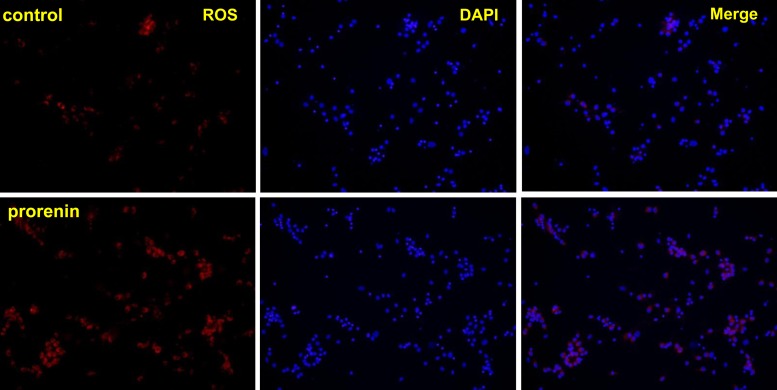
The results from the above experiment suggest that ROS may be involved in PRR-mediated sympathoexcitation. We then tested whether ROS production is increased in PVN neurons with an increased availability of the PRR ligand prorenin. Twelve-day-old cultured neurons from the hypothalamus containing the PVN of newborn SD rats were incubated with 100 nM of human prorenin for 1 h. DHE staining showed that the production of ROS was significantly increased in prorenin-treated cells compared with vehicle control (Fig. 3).
Fig. 3.
PRR activation in brain neurons increases reactive oxygen species (ROS) production. Representative micrographs of dihydroethidium (DHE) staining showing ROS production. Top: control neurons. Bottom: neurons incubated with prorenin (100 nM) for 1 h. Left: DHE staining showing ROS (red). Middle: DAPI staining showing cell nuclei (blue). Right: merged micrographs.
PRR activation increases the expression of iNOS, NAPDH oxidase, and neuronal activity.
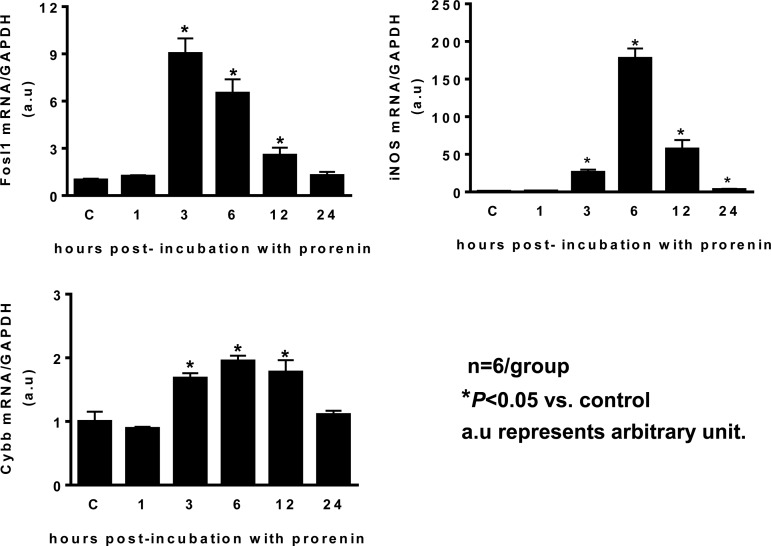
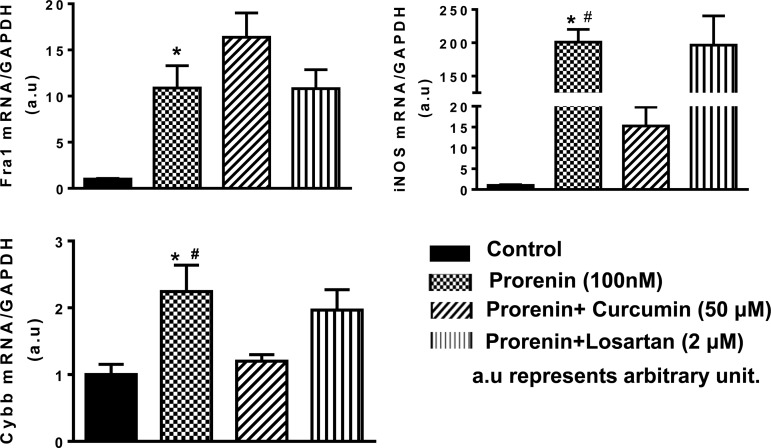
In this study, we investigated the effects of PRR activation on the regulation of neuronal activity and related genes. Cultured brain neurons from the hypothalamus of SD rats were used since PVN PRR is primarily distributed in neurons. Incubation of cultured neurons with human prorenin (100 nmol/l) resulted in a time-dependent increase in fosl1, as well as iNOS and the NAPDH oxidase 2 subunit cybb, with the maximum effect 3 h following prorenin treatment in fosl1 and 6 h following prorenin treatment in the rest of genes (Fig. 4). Fosl1 is a neuronal activation marker; it is also a component of transcription factor AP-1. Maximum increase in fosl1 occurred earlier than other genes suggesting that PRR-mediated increases in other target genes may be through activation of AP-1. PRR effect may also be through ANG II-AT1-R signaling since PRR activation can result in ANG II formation (28, 37). To test this hypothesis, we coincubated cultured neurons with human prorenin (100 nmol/l) with or without the AT1-R antagonist losartan (2 μM), and the AP-1 inhibitor, curcumin (50 μmol/l), for 6 h. Neurons were collected and real-time PCR was performed to assay the mRNA levels of the genes of interest. The results showed that prorenin incubation significantly increased the expression of fosl1 (11-fold), iNOS (201-fold), and cybb (2-fold). These stimulatory effects in iNOS and cybb were almost completely (>90%) blocked by curcumin. In contrast, the fosl1 expression is not affected by curcumin. Losartan did not show any attenuation effect for these three genes (Fig. 5).
Fig. 4.
PRR activation in brain neurons increases the mRNA levels of FOS-like antigen 1 (fosl1), inducible nitric oxide synthase (iNOS), and the NAPDH oxidase 2 subunit cybb. Brain neurons were incubated in primary cultures from the hypothalamus of new born Sprague-Dawley rats containing the PVN with prorenin (100 nM) for different time courses, the neurons were then collected, RNA was purified, and real-time PCR was performed using specific primers and probes specific for iNOS, fosl1, and cybb. The mRNA level in the control sample was assigned to be arbitrary unit (a.u) 1. *P ≤ 0.05 vs. control; n = 6 each group.
Fig. 5.
PRR-mediated increases in target genes are attenuated by the activator protein-1 (AP-1) inhibitor curcumin. Cultured brain neurons were incubated with 100 nM of human prorenin with or without curcumin (50 μmol/l), an AP-1 inhibitor, or losartan (2 μmol/l), an antagonist of ANG II type I receptor (AT1-R), for 6 h, and the neurons were then subjected to real-time PCR to assay mRNA levels of fosl1, iNOS, and cybb. The mRNA level in the control sample was assigned to be arbitrary unit 1. *P ≤ 0.05 vs. control; #P ≤ 0.05 vs. prorenin + curcumin, n = 6 each group.
DISCUSSION
The present study reports two novel findings: 1) the activation of PVN PRR contributes to sympathoexcitation, and 2) PRR-mediated ANG II-independent signaling contributes to neuronal activation and may involve neuronal ROS-AP-1-iNOS signaling. The central nervous system plays a critical role in the long-term regulation of ABP in both health and disease states through alterations in sympathetic outflow (9, 14). Increased sympathetic outflow is implicated in the etiology of hypertension. Compelling evidence indicates that brain PRR is involved in the regulation of ABP, and its hyperactivity in the brain is associated with hypertension (23, 38). Thus the objectives of this study are to investigate whether or not the PVN PRR is involved in regulating SNA. If it does, what is the potential signaling pathway mediating this regulation?
Microinjection of prorenin into the PVN elicited a significant increase in SSNA and a minor increase in RSNA (Fig. 2). The increase in SSNA was effectively blocked by PRRB, a blocker preventing PRR from binding with (pro)renin, indicating that PRR activation induced sympathoexcitation. Further study showed that the prorenin-induced increase in SSNA was completely abolished by a ROS scavenger. This result suggests that increased ROS production is involved in PRR-mediated sympathoexcitation. To be consistent with this result, DHE staining showed that prorenin treatment of PVN neurons dramatically increased ROS production (Fig. 3). However, we did not observe an obvious increase in ABP in response to PVN prorenin microinjection, although SNA is elevated. The discrepancy could be explained by the following possibilities: the present study utilized an anesthetized preparation, a potential limitation in considering the effect of prorenin on both SNA and ABP since anesthesia may inhibit the activity of PVN neurons to alter cardiovascular responses. Previous studies have shown differential responses in ABP and sympathetic outflow in response to PVN stimulation between conscious and anesthetized rats. Either electrical or chemical stimulation of the PVN in anesthetized rats evoked a depressor response accompanied with a decrease in sympathetic outflow (17, 44), while in conscious rats, electrical stimulation evoked increases in ABP and SNA (16). Therefore, anesthesia could potentially underestimate the role of PRR in the PVN in regulating both SNA and ABP. Important to this study is that activation of PRR expressed in the PVN plays a role in the regulation of sympathetic outflow. Another possibility could be that the overall increase in sympathetic outflow induced by activation of PRR in the PVN may not be high enough to elevate ABP in physiological conditions, although SSNA increased up to ∼70% (Fig. 2). This may due to either an insufficient prorenin dose used for injection or low level of endogenous PRR expression in the PVN of normotensive rat. It has been reported that the expression of PRR in the central nervous system is relatively lower in normotensive animals compared with that in animals with hypertension (21, 22, 38). Therefore, it is possible that sympathoexcitation elicited by activation of PRR in the PVN might not be sufficient to elevate ABP in normotensive animals. It would be expected that microinjection of prorenin into the PVN of hypertensive animals could induce sympathoexcitation and presser responses in a greater degree since their PRR expression is upregulated (22, 38, 46). Whether upregulation of PRR expression in hypertensive animals is able to enhance sympathoexcitation and induce a pressor response remains to be studied in the future.
It should be mentioned that there is a differential sympathoexcitatory response to PVN prorenin injection between SSNA and RSNA. The mechanisms responsible for the differences between the remarkable increase in SSNA and the negligible effect on RSNA are not clear. Differential sympathetic responses have been found in the neurogenic hypertensive model, which is characterized by increased SSNA, decreased RSNA, and no change in muscle SNA (30). A likely mechanism for this different sympathoexcitatory response to PVN PRR activation between SSNA and RSNA could be explained by the possibility that enhanced or attenuated sympathoexcitatory responses to PVN prorenin reflect the effective activation or inhibition of distal synaptic targets of the PVN, e.g., in the RVLM. This possibility was supported by the fact that RVLM neurons are organized topographically (24, 25). In addition, anatomic and electrophysiological studies have identified several groups of autonomic PVN neurons (5, 6, 36). One group of PVN neurons innervates the RVLM neurons. Another group of PVN neurons has axons innervating the NTS in the dorsal brain stem. The third group of PVN neurons has axons projecting to the spinal intermediolateral nucleus. Therefore, it is likely that different anatomic PVN neuronal pathways could innervate different sympathetic targets, supporting the orchestration of sympathoexcitatory responses. Whether or not PRR selectively expressed in the presympathetic PVN neurons contributes to the different patterns of sympathoexcitation remains to be investigated.
Next, we determined what signal pathway is potentially involved in mediating the sympathoexcitation evoked by activation of PRR in the PVN. Since ROS blockage almost completely abolished the prorenin effect (Fig. 2), we then focused on the possible downstream mechanism affected by ROS. Previous study demonstrated that excessive O2− can affect PVN neurons by modulating activity of transcription factor AP-1 (1). Studies also showed that increased ROS production (26, 31) produced by increased expression in NAPDH oxidase, a ROS-generating enzyme, and excessive NO production induced by iNOS (18, 19) are implicated in hypertensive state compared with normotensive condition. We then tested whether PRR activation activates AP-1 and increases the expression of iNOS and NAPDH oxidase. We performed this study in vitro using cultured neurons from the hypothalamus containing the PVN from newborn SD rats since brain PRR is primarily distributed in neurons (38). Incubation of cultured brain neurons with the PRR ligand prorenin resulted in a dramatic, time-dependent increase in the mRNA levels of fosl1, a marker for neuronal activation as well as a subunit of transcription factor AP-1; cybb, a subunit of NAPDH oxidase 2; and iNOS (Fig. 4). AP-1 controls transcription of multiple genes through binding with the consensus sequence in or near their promoter regions. There are AP-1 binding sites in the promoter regions of both iNOS and cybb. This evidence, coupled with the observation that the maximum increase of fosl1 occurred earlier than iNOS and cybb (3 h in fosl1 and 6 h in iNOS and cybb following prorenin incubation; Fig. 4), suggests that increased AP-1 may increase the transcriptions of iNOS and cybb. Preincubation of cultured brain neurons with curcumin, an AP-1 inhibitor, completely attenuated the increase in mRNA levels of iNOS and cybb, confirming our hypothesis that the increase in the mRNA levels of iNOS and cybb is through the activation of transcription factor AP-1. Intriguingly, curcumin also increased the mRNA transcription of fosl1, which might be a compensatory mechanism for AP-1 inhibition. Increases in iNOS, cybb, and fosl1 were not attenuated by the AT1-R antagonist losartan, suggesting the PRR effects on the increases in these genes are not mediated by ANG II formation. This is reasonable given that the human prorenin, as used in this study, can bind to rat PRR with a comparable affinity but is unable to act on the endogenous rat angiotensinogen to generate ANG II (27, 46).
Brain ROS have been implicated in cardiovascular disease and hypertension through sympathetic activation (2, 15, 33). NO signaling in the brain may be pro- or antihypertensive depending on the NOS isoform that generates this gas molecule and the site of action (4, 35). Unlike endothelial NOS (eNOS) and neuronal NOS (nNOS), which constitutively express in endothelial cells or other specific neurons (10, 29), brain iNOS is rarely expressed in normal physiological conditions, and its expression could be dramatically enhanced in pathogenesis condition or under oxidative stress (29). The NO produced by iNOS functions as a neuronal neuromodulator and can regulate the release of neurotransmitters and alter neuronal activity (34). Studies have shown that iNOS expression was higher in the RVLM of SHR compared with normotensive WKY rats (18). Specific blockage of the RVLM iNOS normalized the ABP in the SHR (18), while chronic overexpression of iNOS in the RVLM elicits hypertension by activating the sympathetic nervous system (19). Previous studies from others and ours have demonstrated that PRR expression is significantly increased in the PVN of some hypertensive animal models (22, 39). This evidence coupled with our observation that PRR activation in cultured PVN neurons dramatically increase mRNA levels of iNOS suggests that increased PVN PRR expression may result in excessive NO production through upregulating iNOS expression. Excessive NO may, in turn, enhance SNA and promote the development of hypertension. Whether or not iNOS is involved in PRR-mediated SNA regulation and contributes to development of hypertension will be our future study direction.
To summarize, the current study showed that in anesthetized SD rats, PVN prorenin injection induced an increase in sympathetic outflow and blockage of ROS attenuated the increase in SNA, indicating PRR acute activation promotes sympathetic activity by stimulating ROS production. In vitro chronic study showed that PRR activation in PVN neurons in primary culture activates AP-1-iNOS/NAPDH signaling. These data combined with the observation that PRR expression is increased in the PVN of hypertensive animal models, as well as the importance of ROS and iNOS in regulating sympathetic outflow and blood pressure (2, 3, 19), suggest that PVN PRR may contribute to hypertension development by promoting sympathetic tone through ROS-AP-1-iNOS signaling.
One of the limitations in the in vitro study is that we used cultured neurons from the hypothalamus, which contain parvocellular and magnocellular neurons. In addition, we focused on the signaling pathway of neuronal PRR. It should be mentioned that PRR is also expressed in microglia in the brain (39). Our recently published data showed that PRR activation in PVN microglia induced proinflammatory cytokines expression and secretion (39), while cytokines are also neuronal activity modulators (40). Therefore, we cannot rule out the possibility that activation of PRR expressed in PVN microglia may also contribute to sympathetic activation.
Perspectives
Compelling evidence suggests that brain PRR, the newest member of the renin-angiotensin system, plays an important role in regulating cardiovascular function. The present study indicates that PRR in the hypothalamic PVN is involved in the regulation of sympathetic outflow and this regulation may be mediated by production of ROS, which, in turn, activates the transcription factor AP-1. AP-1 results in excessive NO production and further ROS formation. Further studies will investigate 1) whether PRR expression and ROS-AP-1-iNOS/ROS signaling are enhanced in the PVN in hypertensive rats, and 2) if chronic blockage of iNOS or ROS will prevent hypertension development or reverse end organ damage in hypertension.
GRANTS
This work was funded by American Heart Association Grant 11SDG7420029 (to Z. Shan) and National Heart, Lung, and Blood Institute Grant 1-R15-HL-122952 (to Q.-H. Chen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.J.H., R.B., and A.E.C. performed experiments; M.J.H., R.B., and Z.S. analyzed data; M.J.H. and Z.S. interpreted results of experiments; M.J.H., Q.-H.C., and Z.S. prepared figures; M.J.H. and Z.S. drafted manuscript; M.J.H., C.C., Q.-H.C., and Z.S. edited and revised manuscript; M.J.H., R.B., Q.-H.C., and Z.S. approved final version of manuscript; Q.-H.C. and Z.S. conception and design of research.
REFERENCES
- 1.Burmeister MA, Young CN, Braga VA, Butler SD, Sharma RV, Davisson RL. In vivo bioluminescence imaging reveals redox-regulated activator protein-1 activation in paraventricular nucleus of mice with renovascular hypertension. Hypertension 57: 289–297, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campese VM, Ye S, Zhong H, Yanamadala V, Ye Z, Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am J Physiol Heart Circ Physiol 287: H695–H703, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Campos RR. Oxidative stress in the brain and arterial hypertension. Hypertens Res 32: 1047–1048, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Chan SH, Chan JY. Brain stem NOS and ROS in neural mechanisms of hypertension. Antioxid Redox Signal 20: 146–163, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Chen QH, Toney GM. Identification and characterization of two functionally distinct groups of spinal cord-projecting paraventricular nucleus neurons with sympathetic-related activity. Neuroscience 118: 797–807, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Chen QH, Toney GM. In vivo discharge properties of hypothalamic paraventricular nucleus neurons with axonal projections to the rostral ventrolateral medulla. J Neurophysiol 103: 4–15, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Contrepas A, Walker J, Koulakoff A, Franek KJ, Qadri F, Giaume C, Corvol P, Schwartz CE, Nguyen G. A role of the (pro)renin receptor in neuronal cell differentiation. Am J Physiol Regul Integr Comp Physiol 297: R250–R257, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 327: 459–463, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Cuadra AE, Shan Z, Sumners C, Raizada MK. A current view of brain renin-angiotensin system: is the (pro)renin receptor the missing link? Pharmacol Ther 125: 27–38, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forstermann U, Closs EI, Pollock JS, Nakane M, Schwarz P, Gath I, Kleinert H. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension 23: 1121–1131, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Fujita M, Ando K, Kawarazaki H, Kawarasaki C, Muraoka K, Ohtsu H, Shimizu H, Fujita T. Sympathoexcitation by brain oxidative stress mediates arterial pressure elevation in salt-induced chronic kidney disease. Hypertension 59: 105–112, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Fujita M, Ando K, Nagae A, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in salt-sensitive hypertension. Hypertension 50: 360–367, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Gui L, LaGrange LP, Larson RA, Gu M, Zhu J, Chen QH. Role of small conductance calcium-activated potassium channels expressed in PVN in regulating sympathetic nerve activity and arterial blood pressure in rats. Am J Physiol Regul Integr Comp Physiol 303: R301–R310, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Hirooka Y. Oxidative stress in the cardiovascular center has a pivotal role in the sympathetic activation in hypertension. Hypertens Res 34: 407–412, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Kannan H, Hayashida Y, Yamashita H. Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am J Physiol Regul Integr Comp Physiol 256: R1325–R1330, 1989. [DOI] [PubMed] [Google Scholar]
- 17.Kannan H, Niijima A, Yamashita H. Inhibition of renal sympathetic nerve activity by electrical stimulation of the hypothalamic paraventricular nucleus in anesthetized rats. J Auton Nerv Syst 21: 83–86, 1987. [DOI] [PubMed] [Google Scholar]
- 18.Kimura Y, Hirooka Y, Kishi T, Ito K, Sagara Y, Sunagawa K. Role of inducible nitric oxide synthase in rostral ventrolateral medulla in blood pressure regulation in spontaneously hypertensive rats. Clin Exp Hypertens 31: 281–286, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Kimura Y, Hirooka Y, Sagara Y, Ito K, Kishi T, Shimokawa H, Takeshita A, Sunagawa K. Overexpression of inducible nitric oxide synthase in rostral ventrolateral medulla causes hypertension and sympathoexcitation via an increase in oxidative stress. Circ Res 96: 252–260, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Li DP, Pan HL. Increased group I metabotropic glutamate receptor activity in paraventricular nucleus supports elevated sympathetic vasomotor tone in hypertension. Am J Physiol Regul Integr Comp Physiol 299: R552–R561, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Peng H, Cao T, Sato R, McDaniels SJ, Kobori H, Navar LG, Feng Y. Brain-targeted (pro)renin receptor knockdown attenuates angiotensin II-dependent hypertension. Hypertension 59: 1188–1194, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Peng H, Mehaffey EP, Kimball CD, Grobe JL, van Gool JM, Sullivan MN, Earley S, Danser AH, Ichihara A, Feng Y. Neuron-specific (pro)renin receptor knockout prevents the development of salt-sensitive hypertension. Hypertension 63: 316–323, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W, Peng H, Seth DM, Feng Y. The prorenin and (pro)renin receptor: new players in the brain renin-angiotensin system? Int J Hypertens 2012: 290635, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McAllen RM, Dampney RA. Vasomotor neurons in the rostral ventrolateral medulla are organized topographically with respect to type of vascular bed but not body region. Neurosci Lett 110: 91–96, 1990. [DOI] [PubMed] [Google Scholar]
- 25.McAllen RM, May CN. Differential drives from rostral ventrolateral medullary neurons to three identified sympathetic outflows. Am J Physiol Regul Integr Comp Physiol 267: R935–R944, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Montezano AC, Touyz RM. Oxidative stress, Noxs, and hypertension: experimental evidence and clinical controversies. Ann Med 44, Suppl 1: S2–16, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Morimoto S, Sigmund CD. Angiotensin mutant mice: a focus on the brain renin-angiotensin system. Neuropeptides 36: 194–200, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliveira-Paula GH, Lacchini R, Tanus-Santos JE. Inducible nitric oxide synthase as a possible target in hypertension. Curr Drug Targets 15: 164–174, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Osborn JW, Fink GD. Region-specific changes in sympathetic nerve activity in angiotensin II-salt hypertension in the rat. Exp Physiol 95: 61–68, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care 31, Suppl 2: S170–180, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Peng H, Li W, Seth DM, Nair AR, Francis J, Feng Y. (Pro)renin receptor mediates both angiotensin II-dependent and -independent oxidative stress in neuronal cells. PLoS One 8: e58339, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterson JR, Sharma RV, Davisson RL. Reactive oxygen species in the neuropathogenesis of hypertension. Curr Hypertens Rep 8: 232–241, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol 64: 51–68, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Sakuma I, Togashi H, Yoshioka M, Saito H, Yanagida M, Tamura M, Kobayashi T, Yasuda H, Gross SS, Levi R. NG-methyl-L-arginine, an inhibitor of L-arginine-derived nitric oxide synthesis, stimulates renal sympathetic nerve activity in vivo. A role for nitric oxide in the central regulation of sympathetic tone? Circ Res 70: 607–611, 1992. [DOI] [PubMed] [Google Scholar]
- 36.Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res 257: 275–325, 1982. [DOI] [PubMed] [Google Scholar]
- 37.Shan Z, Cuadra AE, Sumners C, Raizada MK. Characterization of a functional (pro)renin receptor in rat brain neurons. Exp Physiol 93: 701–708, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shan Z, Shi P, Cuadra AE, Dong Y, Lamont GJ, Li Q, Seth DM, Navar LG, Katovich MJ, Sumners C, Raizada MK. Involvement of the brain (pro)renin receptor in cardiovascular homeostasis. Circ Res 107: 934–938, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi P, Grobe JL, Desland FA, Zhou G, Shen XZ, Shan Z, Liu M, Raizada MK, Sumners C. Direct pro-inflammatory effects of prorenin on microglia. PLoS One 9: e92937, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi P, Raizada MK, Sumners C. Brain cytokines as neuromodulators in cardiovascular control. Clin Exp Pharmacol Physiol 37: e52–57, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi Z, Gan XB, Fan ZD, Zhang F, Zhou YB, Gao XY, De W, Zhu GQ. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf) 203: 289–297, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Stocker SD, Muntzel MS. Recording sympathetic nerve activity chronically in rats: surgery techniques, assessment of nerve activity, and quantification. Am J Physiol Heart Circ Physiol 305: H1407–H1416, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su Q, Qin DN, Wang FX, Ren J, Li HB, Zhang M, Yang Q, Miao YW, Yu XJ, Qi J, Zhu Z, Zhu GQ, Kang YM. Inhibition of reactive oxygen species in hypothalamic paraventricular nucleus attenuates the renin-angiotensin system and proinflammatory cytokines in hypertension. Toxicol Appl Pharmacol 276: 115–120, 2014. [DOI] [PubMed] [Google Scholar]
- 44.Yamashita H, Kannan H, Kasai M, Osaka T. Decrease in blood pressure by stimulation of the rat hypothalamic paraventricular nucleus with L-glutamate or weak current. J Auton Nerv Syst 19: 229–234, 1987. [DOI] [PubMed] [Google Scholar]
- 45.Ye ZY, Li DP, Pan HL. Regulation of hypothalamic presympathetic neurons and sympathetic outflow by group ii metabotropic glutamate receptors in spontaneously hypertensive rats. Hypertension 62: 255–262, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zubcevic J, Jun JY, Lamont G, Murca TM, Shi P, Yuan W, Lin F, Carvajal JM, Li Q, Sumners C, Raizada MK, Shan Z. Nucleus of the solitary tract (pro)renin receptor-mediated antihypertensive effect involves nuclear factor-kappaB-cytokine signaling in the spontaneously hypertensive rat. Hypertension 61: 622–627, 2013. [DOI] [PubMed] [Google Scholar]
- 47.Zucker IH, Gao L. The regulation of sympathetic nerve activity by angiotensin II involves reactive oxygen species and MAPK. Circ Res 97: 737–739, 2005. [DOI] [PubMed] [Google Scholar]