ABSTRACT
In regenerative medicine using transplantation of mesenchymal stem cells (MSCs), the importance of regulating the quality of MSCs has been well recognized; however, there is little information concerning the relationship between the population doubling level (PDL) and the stemness of MSCs in equine medicine. In this study, we showed that the amount of glycosaminoglycan (GAG) secreted by bone marrow-derived MSCs (BMSCs) decreases with increase of PDL. Enzymatic digestion and two-dimensional electrophoresis revealed that a main component of GAG produced by BMSCs was hyaluronan with a small amount of chondroitin sulfate. Increase of PDL downregulated the expression of MSC CD markers, including CD44, CD73, CD90, CD105, and CD146, along with loss of differentiation capacity. Thus, the effect of hyaluronan supplement to the growth medium on both expression of CD markers and the tri-lineage potential of BMSCs was evaluated. Expression of CD73 and CD90 was preserved by continuous addition of hyaluronan to the growth medium, whereas mRNA levels corresponding to CD44, CD105 and CD146 were not preserved by supplementation of hyaluronan. BMSCs subcultured with hyaluronan-supplemented growth medium to PDL-12 showed osteogenic capacity, however adipogenic and chondrogenic activities at PDL-12 were not preserved by exogenous hyaluronan. These results suggest that downregulation of CD44, CD105 and CD146 might not affect the osteogenic capacity. Taken together, the results suggested that supplementation of hyaluronan to the growth medium might be effective at maintaining the osteogenic capacity of equine BMSCs.
Keywords: CD, hyaluronan, osteogenesis, stemness
Mesenchymal stem cells (MSCs) are fibroblastic multipotent stem cells with a high capacity for self-renewal and have been isolated from bone marrow, adipose tissue, umbilical cord, tendon, lung, and the periosteum [5]. These cells can differentiate into several cell lineages including adipocytes, osteocytes and chondrocytes [6, 8, 14, 24]. Many cell surface markers of MSCs including STRO-1, CD29 (integrin β1), CD44 (HCAM), CD71 (transferrin receptor protein 1), CD73 (5′-nucleotidase), CD90 (Thy-1), CD105 (endoglin), CD106 (VCAM-1), CD146 (MCAM), CD166 (ALCAM) and Sca-1 (stem cell antigen-1) have been identified [6, 13, 15, 19, 21]. In human medicine, the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) proposed three criteria to define MSC as follows: 1) adherence to plastic dish with fibroblastic morphology under standard culture conditions; 2) expression of CD73, CD90 and CD105 with lack of several leukocyte/macrophage-related antigens including CD11b, CD14, CD19, CD34, CD45, and CD79a, and an MHC class II cell surface receptor; 3) differentiation into osteoblastic, adipogenic and chondrogenic lineages [3, 7]. Accordingly, these CD molecules have been used as an index for identification of human MSCs. Among these CD molecules, CD73 is known as a 5′-nucleotidase that preferably catalyzes AMP, and CD105 functions as a receptor for the TGFβ superfamily. On the other hand, the function of CD90 has not yet been fully elucidated, but roles in nerve regeneration, apoptosis and fibrosis are speculated. Unlike human MSCs, it was reported that a significant level of CD73 is not present in equine umbilical cord-derived MSCs, whereas expression of CD90, CD105 and CD146 has been detected [13]. These results suggest that the minimum requirement for cell surface antigen expression varies among animal species and the origin of tissues. In equine medicine, the technical methods for autologous implantation of bone marrow-derived MSCs (BMSCs) into injured SDF tendon have been developed [9, 14, 26]. As BMSCs exist at very low levels (0.01–0.001%) in the bone marrow, extensive passage will be needed to achieve the numbers necessary for clinical use [4, 16, 25]. It has been reported that adipogenic capacity decreases significantly with increasing passage in human BMSCs [16], whereas osteogenic activity is maintained until population doubling level (PDL)-16 with no significant change of expression of CD73, CD90 and CD105 [25] in human umbilical cord-derived MSCs. These results suggest that increasing passage of MSCs results in loss of differentiation capacity; however the cause of this loss remains unclear.
Elucidation of the relationship between the stemness of MSCs and in vitro senescence has been recognized as being important for clinical applications and cell-based therapy. Lo Surdo et al. recently reported that the decreases in proliferation and adipogenic capacity in human BMSCs with passage correlate with the decrease in percentage of colony forming units [16]. Other reports indicate that the effects of long-term passage are negligible except for the proliferation capacity in tonsil-derived MSCs [3], and that umbilical cord-derived MSCs maintain stable expression of MSC-related CD markers, including CD13, CD29, CD44, CD49e, CD73 and CD90, as well as stable osteogenic activity [13]. Many cell surface markers of MSCs have been found, and the ISCT proposed that human MSCs must express CD73, CD90 and CD105 [5, 7], but there is no report in the literature concerning the effect of long-term passage of equine BMSCs on expression of CD markers and differentiation activities.
It has been recognized that the maintenance of the stemness in MSCs is quite important for the quality control of MSCs. Several biomaterials have been proposed as artificial niches for engineering stem cells, and hyaluronan is considered to be an important constituent of the stem cell niche [18, 20]. In hyaluronan-based hydrogel, rat BMSCs preserved the properties of stem cells over 28 days of culture [18]. Additionally, exogenous hyaluronan reduced cellular senescence and differentiation potentials of murine adipose-derived MSCs [2]. These results suggest that hyaluronan may contribute to the maintenance of the stemness of MSCs. On the other hand, it has been reported that human BMSCs secrete hyaluronan into the medium, suggesting autocrine maintenance of the hyaluronan niche [20]. However, the PDL-related change in secreted hyaluronan has not been evaluated. Thus, in this study, we examined the PDL-dependent change of hyaluronan secreted into the medium, and the effects of hyaluronan supplementation to the growth medium on both expression of cell surface antigens and the tri-lineage potential (osteogenic/adipogenic/chondrogenic).
Materials and Methods
Cell culture
BMSCs were established from four Thoroughbred racehorses (3–5 years old) as previously described [17] and their pluripotency of them was confirmed by their ability to differentiate into the three cell lineages, including osteocytes, adipocytes and chondrocytes as previously reported [14]. All animal procedures were approved by the Ethics Committee for Laboratory Animals of the Japan Racing Association Equine Research Institute. These BMSCs were propagated in a growth medium consisting of Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich Inc., St. Louis, MO, U.S.A.) supplemented with 20% fetal bovine serum (FBS, Invitrogen Corp., Carlsbad, CA, U.S.A.) and a penicillin-streptomycin-neomycin solution (PSN, Sigma-Aldrich) in an atmosphere of humidified air, 5% CO2 at 37°C. These BMSCs were then mixed and subcultured to population doubling level (PDL)-12 by repeated division into two. At every PDL point, the medium was changed to serum-free DMEM and cultured for 3 days. Then the medium was collected to analyze the glycosaminoglycan (GAG) content and the molecular species of GAG. To examine the effect of hyaluronan on the preservation of the stemness, BMSCs were subcultured to PDL-12 with 50 µg/ml of hyaluronan (from human umbilical cord, Sigma-Aldrich) -supplemented growth medium throughout.
To induce osteogenesis and adipogenesis, cells of PDL-2 and PDL-12 were plated on ϕ6 cm dishes at 3 × 105 cells and cultured for 2 days. For osteogenic induction, the medium was then changed to Iscove’s Modified Dulbecco’s Media (IMDM, Sigma-Aldrich) supplemented with 10% FBS, PSN, 1 nM dexamethasone (Sigma-Aldrich), 200 mM β-glycerol phosphate (Sigma-Aldrich), and 2 mM ascorbate-2-phosphate (Wako Pure Chemical Industries, Osaka, Japan) for an additional 14 days. The resulting cultures were fixed with 4% paraformaldehyde (PFA) and stained with 0.5% alizarin red solution [22]. For adipogenesis, the medium was then changed to adipogenesis induction medium (IMEM) supplemented with 10% FBS, PSN, 100 nM dexamethasone, 500 µM 3-isobutyl-1-methyl xanthine (Sigma-Aldrich), 100 µM indomethacin (Sigma-Aldrich) and 1% ITS-A (1 mg/ml insulin, 550 µg/ml transferrin, 670 ng/ml sodium selenite) (Invitrogen) for 3 days. The induction medium was then replaced with the adipogenic maintenance medium (IMEM supplemented with 10% FBS, PSN and 1% ITS-A) for one day. This cycle was repeated 4 times. The adipogenic cultures were then fixed with 4% PFA and stained with Oil Red O solution [1]. Chondrogenesis was induced in micromass pellet cultures (2 × 106 cells) placed in a 15 ml polypropylene conical tube. The pellets derived from BMSCs of PDL-2 and PDL-12 were cultured at 37°C with 5% CO2 in 2 ml of chondrogenic medium (IMEM supplemented with 10% FBS, PSN, 10 ng/ml of TGFβ1, 50 µM ascorbic acid-2-phosphate and 1% ITS-A). The medium was replaced every 2–3 days. After a 4 week incubation period, the pellet was fixed in 4% PFA, and then dehydrated with graded ethanol and embedded in paraffin. Four-micrometer sections were prepared and stained with alcian blue and nuclear fast red [19]. These differentiated cells were also used to analyze differentiation-specific phenotypes in qRT-PCR.
Analysis of GAG secreted by MSCs
The media were harvested from BMSCs culture at the indicated PDL. GAG in serum-free medium was precipitated by the addition of cetylpyridinium chloride (Wako) to a final concentration of 0.2% and stirred overnight at 4°C. The GAG fraction was collected by centrifugation at 8,000 × g for 20 min at 4°C, and then the pellet was washed with 10% potassium acetate/ethanol for three times. After dissolving the pellet with 5 mM CaCl2/50 mM Tris-HCl at pH 7.6, the remaining protein in the solution was completely digested with Pronase E (Wakenyaku Co., Ltd., Kyoto, Japan) for 48 hr at 50°C. After elimination of the proteinous components by precipitation in a final concentration of 10% trichloroacetic acid, the supernatant was completely dialyzed against ultrapure water, and then lyophilized as the MSC-derived GAG fraction.
After the GAG fraction has been dissolved in ultrapure water, identification of the MSC-derived GAG was determined by its sensitivity to enzymes and two-dimensional electrophoresis. The total amount of uronic acid was determined by the carbazole-sulfuric acid reaction. Briefly, 200 µl of GAG sample was mixed with 1 ml of 0.95% borate/sulfuric acid, and then boiled for 10 min. After cooling to room temperature, 40 µl of 1.25% carbazole/methanol was added to the resulting mixture, and then boiled for 15 min. After cooling with running water, the optical absorbance was measured at 530 nm. The standard curve was prepared using graded concentrations of glucuronic acid. Next, the sensitivity to several enzymes of the MSC-derived GAG was examined. Appropriate amounts of the MSC-derived GAG fraction were digested with testicular hyaluronidase (Sigma-Aldrich), streptomyces hyaluronidase (Sigma-Aldrich), or chondroitinase ABC (Sigma-Aldrich) according to the manufacturer’s instruction. One microliter of these digests were spotted on a cellulose acetate membrane and stained with 0.1% alcian blue/0.1% acetic acid and destained with 0.1% acetic acid. Two-dimensional electrophoresis on a cellulose acetate membrane was performed following a previously described method [11]. After apply the GAG sample onto the cellulose acetate membrane, electrophoresis was performed in 0.1 M pyridine/0.47 M formic acid in the first dimension, and in 0.1 M barium acetate in the second dimension, respectively. The resulting membrane was developed by 0.1% alcian blue/0.1% acetic acid.
Quantitative RT-PCR (qRT-PCR)
Total RNA in undifferentiated and differentiated BMSCs were isolated by RNAiso Plus (Takara, Shiga, Japan) according to the manufacturer’s protocol. Complementary DNA was prepared by reverse transcription of DNase I (Roche)-treated total RNA with One Step SYBR PrimeScript RT-PCR Kit (TAKARA), then qRT-PCR was performed in a Thermal Cycler Dice Real Time System (Takara) using SYBR Premix ExTaq II (Takara). Several cell surface antigens corresponding to CD44, CD73, CD90, CD105, and CD146 and differentiation markers, including osteocalcin, peroxisome proliferator-activated receptor-γ (PPARγ), and type II collagen α1 chain (Col2a1), were amplified using the specific primer sets shown in Table 1. Nucleotide sequences of the amplified DNA fragments were confirmed using an autosequencer (ABI, Life Technologies Japan, Tokyo, Japan).
Table 1. Sequences of primer sets used for qRT-PCR.
| Gene | Upper primer | Lower primer | Annealing °C | Size (bp) |
|---|---|---|---|---|
| GAPDH | 5′- GTGTCCCCACCCCTAACG -3′ | 5′- AGTGTAGCCCAGGATGCC -3′ | 55.1 | 131 |
| CD44 | 5′- TTTATGACAGCCGACGAG -3′ | 5′- AAAAGACCCGAATGAAGC -3′ | 52.4 | 169 |
| CD73 | 5′- CATTCTTCTCAACAGCAG -3′ | 5′- ATAGCATCACAAATCAGG -3′ | 48.0 | 183 |
| CD90 | 5′- TTCACCACCAAGGACGAG -3′ | 5′- CAGCAGAGGCAGGGACAG -3′ | 56.3 | 180 |
| CD105 | 5′- CTGCCTTTGTGCGGTTGG -3′ | 5′- CTTTCGGGGTCCTTCAGC -3′ | 57.1 | 193 |
| CD146 | 5′- AAAGGAGAGGAAGGTGTG -3′ | 5′- ATTCAGGATGCTCAGGAC -3′ | 51.4 | 151 |
| OCN | 5′- AACGGTGCTGGAGACTGG -3′ | 5′- ACCTCACTGCCCTCTTGC -3′ | 58.1 | 196 |
| PPARg | 5′- TTAGATGACAGCGACTTG -3′ | 5′- GTAGGAGTGGGTGAAGAC -3′ | 50.8 | 274 |
| Col2a1 | 5′- TCAAGTCCCTCAACAACC -3′ | 5′- ATCCAGTAGTCTCCGCTC -3′ | 53.5 | 124 |
Statistical analysis
The results are shown as the mean ± SD of a representative experiment performed in triplicate. Statistical significances of the differences between the values of the respective experimental groups and PDL-2 were determined using Mann-Whitney’s U test and values of P<0.05 were considered significant.
Results
Feature of GAG secretion by BMSCs
The standard curve shows the reliability of measurements between 10 to 100 mg/ml in graded concentrations of glucuronic acid and it has a correlation coefficient of 0.996 (Fig. 1a). Measurements of GAG content that secreted into the medium demonstrated that an increase in PDL elicited decrease of secreted GAG in the medium (Fig. 1b). Secretion of GAG began to diminish at PDL-3 and the amount decreased to a one-fifth or less of the PDL-2 value after PDL-4. To identify molecular species of GAG secreted by BMSCs, the enzymatic sensitivity was examined. Testicular hyaluronidase completely digested BMSC-derived GAG, whereas small amount of undigested material were seen in the digest with streptomyces hyaluronidase or chondroitinase ABC (Fig. 1c), suggesting that BMSC-derived GAG is not pure hyaluronan. Two-dimensional electrophoresis revealed that the main component of BMSC-derived GAG was hyaluronan and that a small amount of chondroitin sulfate was also present (Fig. 1d).
Fig. 1.
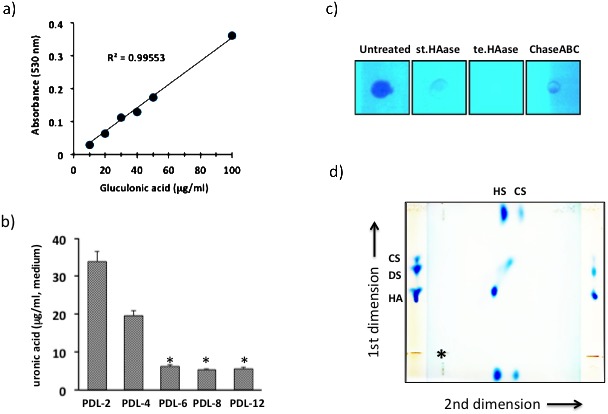
The standard curve of graded concentrations of glucuronic acid has a correlation coefficient of 0.996 (a) and the changes in secreted GAG secreted into the medium were estimated (b). The values are expressed as the mean ± SD of three independent experiments, and significant differences between the values of the respective experimental groups and PDL-2 are indicated (*P<0.05). MSC-derived GAG was digested with streptomyces hyaluronidase (st.HAase), testicular hyaluronidase (te.HAase), or chondroitinase ABC (ChaseABC), and then stained with alcian blue (c). Two-dimensional electrophoresis of MSC-derived GAG was performed on cellulose acetate membrane. Purified hyaluronan (HA), dermatan sulfated (DS) and chondroitin sulfate (CS) were used as molecular markers, and the starting point for the first dimension is indicated by an asterisk (d).
Effect of long-term passage and addition of hyaluronan on the characteristics of BMSC
Change of mRNA levels corresponding to CD44, CD73, CD90, CD105 and CD146 in accompanying the increased of PDL were examined using qRT-PCR analysis. Increase of PDL elicited decrease of mRNA expression of the CD markers examined approximately 1/3 to 1/10 of their levels at PDL-2 (Fig. 2). In subculture with hyaluronan-supplemented growth medium, decreased expression of CD73 and CD90 reversed, and approximate 2-fold increase was observed in their expression. On the other hand, addition of hyaluronan did not prevent the downregulation of CD44, CD105 or CD146 expression.
Fig. 2.
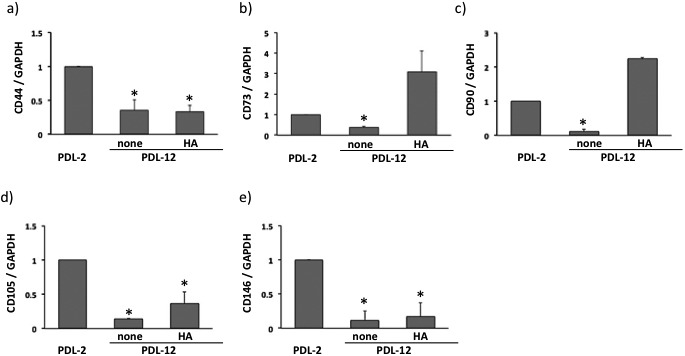
Expression of CD44 (a), CD73 (b), CD90 (c), CD105 (d) and CD146 (e) in BMSCs at PDL-2 or PDL-12 cultured with the growth medium alone (none) or hyaluronan (HA)-supplemented medium. The values are expressed relative to the value of BMSCs at PDL-2 as the mean ± SD of three independent experiments and significant differences between the values of the respective experimental groups and PDL-2 are indicated (*P<0.05).
In osteogenic induction, BMSCs of PDL-12 showed decreased osteogenic ability (Fig. 3b), while BMSCs of PDL-2 successfully formed alizarin red-positive osteoid (Fig. 3a). When BMSCs were subcultured to PDL-12 with hyaluronan-supplemented growth medium, they maintained their capacity for osteogenesis (Fig. 3c). Additionally, the induced osteoid seemed to be distributed in more widely than the osteoid induced at BMSCs in PDL-2. On the other hand, the adipogenic (Fig. 3d–3f) and chondrogenic abilities of BMSCs (Fig. 3g–3i) were not observed at PDL-12 when they were subcultured with either growth medium alone (Fig. 3e and 3h) or with hyaluronan-supplemented medium (Fig. 3f and 3i), while BMSCs of PDL-2 showed the adipogenic ability with lipid droplets (Fig. 3d) as well as chondrogenicity with extracellular accumulation of alcian blue-positive matrix (Fig. 3g). These results were confirmed by qRT-PCR for differentiation-specific markers. Increased PDL elicited decrease in all phenotype specific expressions including osteocalcin, PPARγ and Col2a1. Subculture with hyaluronan-supplemented growth medium maintained the expression of osteocalcin as an osteogenic marker and its mRNA level rather increased about 5-fold compared to PDL-2 BMSC. On the other hand, expression of PPARγ and Col2a1 tended to weakly increase with supplementation of hyaluronan, but these increase were not statistically significant.
Fig. 3.
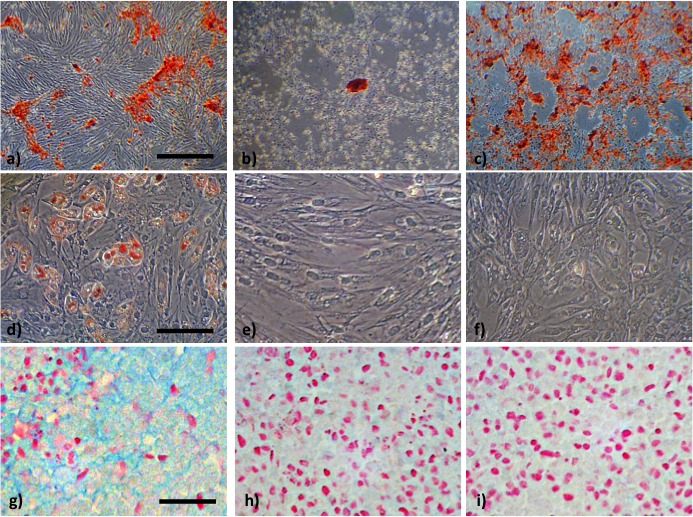
Osteogenic capacity was demonstrated by stainings with alizarin red (a, b, c) and adipogenesis was demonstrated by the presence of intracellular lipid droplets after staining with Oil Red O (d, e, f). Chondrogenic capacity was evaluated with alcian blue and counter stainings was performed with nuclear fast red (g, h, i). Bars indicated 100 µm (a, b, c), 25 µm (d, e, f) and 50 µm (g, h, i), respectively.
Discussion
In the present study, we examined how increased of PDL in equine BMSCs affected the expression of CD markers and their differentiation potency. During the experiments, we first found GAG content in the culture medium decreased with increase of PDL, and the major GAG component secreted by MSCs was hyaluronan, with a small quantity of chondroitin sulfate (Fig. 1). Hyaluronan secreted by BMSCs has been proposed to be function as an autocrine niche to maintain the stemness of BMSCs [20]. According to that hypothesis, the decrease of hyaluronan secretion by BMSCs, shown in this study, would be explained as the result of the PDL-related decline of the autocrine niche, and the breakdown of the niche might bring loss of the stemness including downregulation of CD expression. Our results also indicated that BMSCs secreted a small amount of chondroitin sulfate in addition to hyaluronan. There is no reports in the literature concerning the molecular species of GAG secreted by MSCs, but it has been reported that sulfated hyaluronan promoted osteogenic and chondrogenic differentiation of human MSCs [12, 15, 23], suggesting the importance of sulfated GAG in the differentiation capacity of MSCs.
To examine the correlation between secreted hyaluronan and the stemness of equine BMSCs, BMSCs were cultured until PDL-12 in a medium supplemented with hyaluronan, and then changes in the mRNA levels corresponding to positive CD markers were assessed. Expressions of all CD markers in BMSCs at PDL-12 had decreased compared to PDL-2. Exogenous hyaluronan stimulated expression of CD73 and CD90, but the expression of the other CD markers, such as CD44, CD105 and CD146, was not recovered by the addition of hyaluronan (Fig. 2). Next, the abilities of BMSCs to differentiate into three lineages of these cells were examined. With increasing PDL, osteogenic activity decreased but weakly remained, whereas the adipogenic and chondrogenic activities were completely lost (Fig. 3). These results were confirmed by analysis of phenotype-specific expression. Expression of osteocalcin was also preserved by exogenous hyaluronan, but the expression of adipogenic and chondrogenic markers were not fully preserved (Fig. 4). Although the mechanism of hyaluronan on upregulation of CD73 and CD90 is not yet known, it has been reported that hyaluronan may select the osteogenic population in human embryonic stem cells [10], and that addition of hyaluronan stimulated hyaluronan synthesis in porcine BMSCs, which together stimulated osteogenic differentiation [27]. These results suggested that CD73 and CD90 may play important roles in osteogenic induction, while CD44, CD105 and CD146 might not affect the osteogenic capacity of equine BMSCs. Murine adipose tissue-derived MSCs showed subculture-dependent decrease of differentiation capacity for osteogenesis and adipogenesis, but the change in CD markers’ expression was not evaluated [2]. On the other hand, long-term passage of human umbilical cord-derived MSCs maintained the reactivity of antibodies to CD29, CD44, CD49e, CD73, CD90, CD105, and CD166 along with the osteogenic activity was maintained [24], whereas adipogenic capacity decreased significantly with increasing passage in human BMSCs [16]. Our results suggested that exogenous hyaluronan preserves a part of the stemness of equine BMSCs. Until now, there has been no report concerning the relationship between the expression of MSC-related CD markers and the tri-lineage potential. As CD73 is known to produce adenosine from AMP, adenosine might affect the osteogenesis of BMSCs. Also, the function of CD90 has not been fully elucidated, and this membrane-anchored protein might have some roles in cell-cell interaction. To confirm the effect of hyaluronan on osteogenic capacity shown in this study, CD73 and/or CD90-knockdown BMSCs should be established and used in long-term culture experiments with exogenous hyaluronan.
Fig. 4.
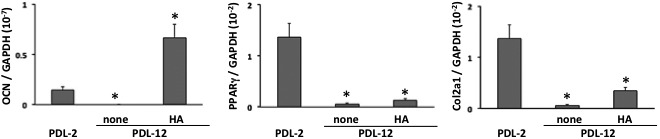
Expression of osteocalcin (a), peroxisome proliferator-activated receptor-γ (PPARγ, b) and type II collage a1 chain (Col2a1) in BMSCs of PDL-2 or PDL-12 cultured with the growth medium alone (none) or hyaluronan (HA)-supplemented medium. The values are expressed as the mean ± SD of three independent experiments and significant differences between the values of the respective experimental groups and PDL-2 are indicated (*P<0.05).
In this study, we showed that increase of PDL elicited decreases in the amounts of secreted hyaluronan along with suppression of CD markers and loss of a tri-lineage potential. These results suggested that supplementation of hyaluronan to the growth medium might be effective at maintaining the osteogenic capacity of equine BMSCs. Although the minimum requirement of cell surface antigen expression in equine BMSCs has not been clarified, our data suggested that expression of CD73 and CD90 might be needed for the osteogenesis of BMSCs.
References
- 1.Barbero A., Ploegert S., Heberer M., Martin I. 2003. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 48: 1315–1325. [DOI] [PubMed] [Google Scholar]
- 2.Chen P.Y., Huang L.L., Hsieh H.J. 2007. Hyaluronan preserves the proliferation and differentiation potentials of long-term cultured murine adipose-derived stromal cells. Biochem. Biophys. Res. Commun. 360: 1–6. [DOI] [PubMed] [Google Scholar]
- 3.Choi J.S., Lee B.J., Park H.Y., Song J.S., Shin S.C., Lee J.C., Wang S.G., Jung J.S. 2015. Effects of donor age, long-term passage culture, and cryopreservation on tonsil-derived mesenchymal stem cells. Cell. Physiol. Biochem. 36: 85–99. [DOI] [PubMed] [Google Scholar]
- 4.Corselli M., Crisan M., Murray I.R., West C.C., Scholes J., Codrea F., Khan N., Péault B. 2013. Identification of perivascular mesenchymal stromal/stem cells by flow cytometry. Cytometry A 83: 714–720. [DOI] [PubMed] [Google Scholar]
- 5.Crisan M., Yap S., Casteilla L., Chen C.W., Corselli M., Park T.S., Andriolo G., Sun B., Zheng B., Zhang L., Norotte C., Teng P.N., Traas J., Schugar R., Deasy B.M., Badylak S., Buhring H.J., Giacobino J.P., Lazzari L., Huard J., Péault B. 2008. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301–313. [DOI] [PubMed] [Google Scholar]
- 6.da Silva Meirelles L., Caplan A.I., Nardi N.B. 2008. In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26: 2287–2299. [DOI] [PubMed] [Google Scholar]
- 7.De Schauwer C., Meyer E., Van de Walle G.R., Van Soom A. 2011. Markers of stemness in equine mesenchymal stem cells: a plea for uniformity. Theriogenology 75: 1431–1443. [DOI] [PubMed] [Google Scholar]
- 8.De Ugarte D.A., Alfonso Z., Zuk P.A., Elbarbary A., Zhu M., Ashjian P., Benhaim P., Hedrick M.H., Fraser J.K. 2003. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol. Lett. 89: 267–270. [DOI] [PubMed] [Google Scholar]
- 9.Fortier L.A., Smith R.K. 2008. Regenerative medicine for tendinous and ligamentous injuries of sport horses. Vet. Clin. North Am. Equine Pract. 24: 191–201. [DOI] [PubMed] [Google Scholar]
- 10.Harkness L., Mahmood A., Ditzel N., Abdallah B.M., Nygaard J.V., Kassem M. 2011. Selective isolation and differentiation of a stromal population of human embryonic stem cells with osteogenic potential. Bone 48: 231–241. [DOI] [PubMed] [Google Scholar]
- 11.Hata R., Senoo H. 1989. L-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissuelike substance by skin fibroblasts. J. Cell. Physiol. 138: 8–16. [DOI] [PubMed] [Google Scholar]
- 12.Hintze V., Miron A., Möller S., Schnabelrauch M., Heinemann S., Worch H., Scharnweber D. 2014. Artificial extracellular matrices of collagen and sulphated hyaluronan enhance the differentiation of human mesenchymal stem cells in the presence of dexamethasone. J. Tissue Eng. Regen. Med. 8: 314–324. [DOI] [PubMed] [Google Scholar]
- 13.Hoynowski S.M., Fry M.M., Gardner B.M., Leming M.T., Tucker J.R., Black L., Sand T., Mitchell K.E. 2007. Characterization and differentiation of equine umbilical cord-derived matrix cells. Biochem. Biophys. Res. Commun. 362: 347–353. [DOI] [PubMed] [Google Scholar]
- 14.Kasashima Y., Ueno T., Tomita A., Goodship A.E., Smith R.K. 2011. Optimisation of bone marrow aspiration from the equine sternum for the safe recovery of mesenchymal stem cells. Equine Vet. J. 43: 288–294. [DOI] [PubMed] [Google Scholar]
- 15.Kliemt S., Lange C., Otto W., Hintze V., Möller S., von Bergen M., Hempel U., Kalkhof S. 2013. Sulfated hyaluronan containing collagen matrices enhance cell-matrix-interaction, endocytosis, and osteogenic differentiation of human mesenchymal stromal cells. J. Proteome Res. 12: 378–389. [DOI] [PubMed] [Google Scholar]
- 16.Lo Surdo J.L., Millis B.A., Bauer S.R. 2013. Automated microscopy as a quantitative method to measure differences in adipogenic differentiation in preparations of human mesenchymal stromal cells. Cytotherapy 15: 1527–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyabara S., Yuda Y., Kasashima Y., Kuwano A., Arai K. 2014. Regulation of tenomodulin expression via Wnt/β-catenin signaling in equine bone marrow-derived mesenchymal stem cells. J. Equine Sci. 25: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohand-Kaci F., Assoul N., Martelly I., Allaire E., Zidi M. 2013. Optimized hyaluronic acid-hydrogel design and culture conditions for preservation of mesenchymal stem cell properties. Tissue Eng. Part C Methods 19: 288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pascucci L., Curina G., Mercati F., Marini C., Dall’Aglio C., Paternesi B., Ceccarelli P. 2011. Flow cytometric characterization of culture expanded multipotent mesenchymal stromal cells (MSCs) from horse adipose tissue: towards the definition of minimal stemness criteria. Vet. Immunol. Immunopathol. 144: 499–506. [DOI] [PubMed] [Google Scholar]
- 20.Qu C., Rilla K., Tammi R., Tammi M., Kröger H., Lammi M.J. 2014. Extensive CD44-dependent hyaluronan coats on human bone marrow-derived mesenchymal stem cells produced by hyaluronan synthases HAS1, HAS2 and HAS3. Int. J. Biochem. Cell Biol. 48: 45–54. [DOI] [PubMed] [Google Scholar]
- 21.Ranera B., Lyahyai J., Romero A., Vázquez F.J., Remacha A.R., Bernal M.L., Zaragoza P., Rodellar C., Martín-Burriel I. 2011. Immunophenotype and gene expression profiles of cell surface markers of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Vet. Immunol. Immunopathol. 144: 147–154. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi Y., Sekiya I., Yagishita K., Ichinose S., Shinomiya K., Muneta T. 2004. Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirates. Blood 104: 2728–2735. [DOI] [PubMed] [Google Scholar]
- 23.Sawatjui N., Damrongrungruang T., Leeanansaksiri W., Jearanaikoon P., Hongeng S., Limpaiboon T. 2015. Silk fibroin/gelatin-chondroitin sulfate-hyaluronic acid effectively enhances in vitro chondrogenesis of bone marrow mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 52: 90–96. [DOI] [PubMed] [Google Scholar]
- 24.Sekiya I., Colter D.C., Prockop D.J. 2001. BMP-6 enhances chondrogenesis in a subpopulation of human marrow stromal cells. Biochem. Biophys. Res. Commun. 284: 411–418. [DOI] [PubMed] [Google Scholar]
- 25.Shi Z., Zhao L., Qiu G., He R., Detamore M.S. 2015. The effect of extended passaging on the phenotype and osteogenic potential of human umbilical cord mesenchymal stem cells. Mol. Cell. Biochem. 401: 155–164. [DOI] [PubMed] [Google Scholar]
- 26.Smith R.K., Korda M., Blunn G.W., Goodship A.E. 2003. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet. J. 35: 99–102. [DOI] [PubMed] [Google Scholar]
- 27.Zou L., Zou X., Chen L., Li H., Mygind T., Kassem M., Bünger C. 2008. Effect of hyaluronan on osteogenic differentiation of porcine bone marrow stromal cells in vitro. J. Orthop. Res. 26: 713–720. [DOI] [PubMed] [Google Scholar]


