Abstract
Objective To estimate the association between guideline recommended drugs and death in older adults with multiple chronic conditions.
Design Population based cohort study.
Setting Medicare Current Beneficiary Survey cohort, a nationally representative sample of Americans aged 65 years or more.
Participants 8578 older adults with two or more study chronic conditions (atrial fibrillation, coronary artery disease, chronic kidney disease, depression, diabetes, heart failure, hyperlipidemia, hypertension, and thromboembolic disease), followed through 2011.
Exposures Drugs included β blockers, calcium channel blockers, clopidogrel, metformin, renin-angiotensin system (RAS) blockers; selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs); statins; thiazides; and warfarin.
Main outcome measure Adjusted hazard ratios for death among participants with a condition and taking a guideline recommended drug relative to participants with the condition not taking the drug and among participants with the most common combinations of four conditions.
Results Over 50% of participants with each condition received the recommended drugs regardless of coexisting conditions; 1287/8578 (15%) participants died during the three years of follow-up. Among cardiovascular drugs, β blockers, calcium channel blockers, RAS blockers, and statins were associated with reduced mortality for indicated conditions. For example, the adjusted hazard ratio for β blockers was 0.59 (95% confidence interval 0.48 to 0.72) for people with atrial fibrillation and 0.68 (0.57 to 0.81) for those with heart failure. The adjusted hazard ratios for cardiovascular drugs were similar to those with common combinations of four coexisting conditions, with trends toward variable effects for β blockers. None of clopidogrel, metformin, or SSRIs/SNRIs was associated with reduced mortality. Warfarin was associated with a reduced risk of death among those with atrial fibrillation (adjusted hazard ratio 0.69, 95% confidence interval 0.56 to 0.85) and thromboembolic disease (0.44, 0.30 to 0.62). Attenuation in the association with reduced risk of death was found with warfarin in participants with some combinations of coexisting conditions.
Conclusions Average effects on survival, particularly for cardiovascular study drugs, were comparable to those reported in randomized controlled trials but varied for some drugs according to coexisting conditions. Determining treatment effects in combinations of conditions may guide prescribing in people with multiple chronic conditions.
Introduction
Most deaths in developed countries occur in people aged more than 65 years who have multiple chronic conditions that cause, or contribute to, death.1 2 3 4 5 Guidelines for chronic conditions recommend drugs based on evidence that they reduce mortality or benefit condition specific outcomes.6 7 8 9 10 11 Prescribing decisions based on guidelines for each condition result in people with multiple conditions taking large numbers of drugs.12 Almost 40% of those aged 65 years and older take at least five prescription drugs; the number increases with number of chronic conditions.13 The benefits of drugs prescribed for a single condition, however, are difficult to determine in the presence of multiple conditions and drugs.
Not only are the benefits of drugs uncertain, greater numbers of drugs reduce adherence, compound the burden of treatment, and increase the likelihood of adverse drug effects.13 14 15 16 Strategies to determine drug effects in those with multiple conditions are needed to minimize these potential harms and burdens and to guide prescribing decisions that maximize benefits.17 Evaluating the effect of drugs on universal health outcomes such as survival, function, and symptom burden that are affected by most conditions and are important to people could lay the foundation for an evidence based approach to drug decision making for people with multiple coexisting conditions.
Though randomized clinical trials remain the ideal, they are not feasible for studying all possible combinations of conditions and drugs of potential benefit for people with multiple chronic conditions. A recent Cochrane review showed that observational study results often are similar to those found in randomized controlled trials, suggesting this design may be suitable for studying drug effects.18 Average effect in either randomized controlled trials or observational studies, however, is not a sufficient measure of drug effects among older adults with multiple chronic conditions. Results in people with key combinations of chronic conditions are also needed to guide clinical decision making. We estimated the association between nine guideline recommended and commonly prescribed drugs and death in a nationally representative sample of older adults with multiple chronic conditions, including common combinations of coexisting conditions.
Methods
Study population
The study sample included Medicare Current Beneficiary Survey participants enrolled from 2005-09, with follow-up through 2011.19 The Medicare Current Beneficiary Survey is a representative sample of Medicare—the federal health insurance for older adults and people with disabilities—beneficiaries in the United States obtained using stratified multistage sampling from the Centers for Medicare and Medicaid Services enrollment file.19 We included all participants aged 65 years or more with at least two of nine chronic conditions, identified by at least one inpatient or two other kinds of claims (outpatient, physician, skilled nursing, home health) during the first two years of participation. Of the 20 026 participants aged 65 years or more, 2682 were Medicare Advantage participants who lacked claims data. Of the 17 344 remaining, 6984 did not have multiple chronic conditions as defined by having at least two study chronic conditions. Of the 10 360 participants (60%) with at least two study conditions, 1505 were non-respondents and 277 lacked data on drugs; the remaining 8578 participants (83% of those with multiple chronic conditions) constituted the study population.
Patient involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in the design or analysis of the study. There are no plans to involve patients in dissemination.
Data sources and data
Participants completed a baseline and three yearly interviews from 2006 to 2010. Sociodemographic, behavioral, health, and functional data were obtained from annual in person interviews.
Chronic conditions
We identified the study conditions in steps with the aim of selecting conditions present in at least 10% of the overall cohort that are associated with an increased risk of mortality and for which there is at least one oral prescription drug recommended by disease guidelines that was used by at least 10% of the final study cohort. These criteria were selected to ensure a sufficient number of participants with the conditions receiving the drugs to evaluate the association between drug use and death. In the first step we identified 23 chronic conditions that were present in at least 10% of the overall cohort based on ICD-9 codes (international classification of diseases, ninth revision) in claims data that were categorized using Clinical Classification Software.20 Of these 23 conditions, 12 were associated with an increased risk of mortality. Of these 12 conditions, we removed cerebrovascular disease and heart valve disease because no guideline recommended prescription drug exists for everyone with the condition. We also removed chronic obstructive pulmonary disease and dementia because there was no oral prescription drug used by at least 10% of the study population for these two conditions. These four excluded conditions were all included as covariates.
The study chronic conditions included atrial fibrillation,8 coronary artery disease,6 depression10 (defined by at least two claims for depression or by self reported depression), diabetes mellitus,7 heart failure,9 hyperlipidemia,21 and hypertension.11 Though thromboembolic disease (pulmonary embolism and venous thrombosis) was present in less than 10% of the population, it was included because of its high mortality risk.22 Though no drug class met the criteria for inclusion for chronic kidney disease, we included this common chronic condition because of its effect on use of several of the study drugs and its association with mortality.
Drugs
The study drugs consisted of all oral prescription drugs (non-prescription ones such as aspirin were not available in the dataset), used by at least 10% of the study population and recommended in US national disease guidelines for any study condition.6 7 8 9 10 11 21 22 We accepted any evidence level in identifying the study drugs.23 The nine drugs included β blockers (cardioselective or αβ blockers); calcium channel blockers; clopidogrel; metformin; renin-angiotensin system (RAS) blockers, including angiotensin converting enzyme inhibitors and angiotensin II receptor blockers; selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs); statins; thiazide diuretics; and warfarin. Some drugs, such as β blockers and statins, were indicated for more than one condition. We ascertained prescription drugs by direct observation of the drug containers of currently used drugs during in-home interviews. To account for initiation and discontinuation of treatment, we updated the use of prescription drugs on a yearly basis. We considered drugs to be discontinued if they were included in the group of drugs shown to the interviewer during an earlier visit but no subsequent visit. The drugs recommended in the national guidelines that were not included in the current study because they are not used orally, are not prescription drugs in the United States, or were used by less than 10% of the population is listed in the footnote in table 1. During follow-up, the amount of missing data for study drugs ranged from 1.0% (86/8578) to 1.3% (112/8578).
Outcome
We identified all cause mortality through the Medicare vital status file, with follow-up to three years, death, or the end of study participation.
Covariates
Covariates were identified because they are associated with risk of death or with the likelihood of receiving any of the study drugs. These included age (≤80 years or >80 years), sex, race (white or non-white), ethnicity (Hispanic or non-Hispanic), yearly income (<$25 000 or ≥$25 000), percentage of days alive spent in the hospital, living situation (community or facility), body mass index >30, smoking status (current or non-current), hearing and vision impairments, number of drugs other than the study drugs, use of assistive device, urinary incontinence, insurance coverage for prescription drugs, cognitive impairment, physical function level,24 and individual Elixhauser chronic conditions.25 26 We considered cognitive impairment to be present if there was self reported memory loss, plus either trouble concentrating or difficulty making decisions that interfered with activities of daily living, or a claim for dementia. Physical function was measured by amount of difficulty stooping or kneeling; lifting heavy items; reaching or extending arms; writing; grasping; and walking 0.25 miles (0.40 km), with each activity rated from no difficulty (score 0) to unable to do it (score 4).24 The scores ranged from 0 to 20, with higher scores indicating more difficulty performing the activities. These covariate data were obtained during the face to face interviews, with missingness ranging from 0.1% (9/8578) to 1.6% (137/8578).
Statistical analysis
We summarized baseline characteristics and prevalence of drugs by indicating chronic condition, overall and by vital status, as frequencies and percentages or means and standard deviations. All individual conditions were fit with a set of multivariable Cox regression models for time until death, with censoring of survivors at the end of their follow-up. To explore whether the association between study drugs and survival varied by presence of different coexisting conditions, we repeated the analyses for participants with four designated coexisting conditions. To assess the effects of β blockers, calcium channel blockers, RAS blockers, statins, and thiazides, we identified patients who had coronary artery disease, hypertension, and hyperlipidemia—the three most common study cardiovascular conditions—plus at least one of the remaining study conditions. To assess the effects of clopidogrel, metformin, SSRIs or SNRIs, and warfarin, we identified participants with the study conditions for which one of these drugs is recommended (that is, atrial fibrillation, depression, coronary artery disease, diabetes, or thromboembolic disease) plus the three most common study conditions that coexisted with that condition.
A participant entered the risk set for a condition at the first annual interview in which the condition was present. The model for each condition—or coexisting conditions—included the main effects of each drug indicated for the conditions, as well as time varying main effects for the remaining study conditions and time varying interactions between the remaining conditions and their indicated drugs.27 We adjusted all models for the other study conditions and covariates. Multiple imputation with 10 replicates was used for missing drug and covariate data, which were all <1.6% (137/8578), using SAS/STAT PROC MI and MIANALYZE (SAS Institute). No data were missing for chronic conditions or the outcome of death. We calculated hazard ratios for participants with a condition or combination of conditions and who were taking a guideline recommended drug relative to those with the condition not taking the drug. These hazard ratios reflect the risk of death with the guideline recommended drug relative to not taking the drug for a given condition after accounting for the other conditions, drugs, and covariates. Analytical approaches employing causal inference techniques, such as propensity scores, were not feasible because we studied multiple drugs and conditions simultaneously. Recent studies report similar results between conventional regression adjustment and propensity score techniques.28 We assessed model assumptions and goodness of fit. No drugs violated the proportional hazards assumption.
We used SAS version 9.4 (SAS Institute, Cary, NC). A P value of 0.05 (two tailed) was used to denote statistical significance.
Results
The mean age of the 8578 participants was 77.4 (standard deviation 7.7) years; 36% (n=3073) were aged more than 80 years, 59% (n=5026) were women, and 87% (n=7471) were white (table 1). The most common chronic conditions were hypertension (92%; n=7911), hyperlipidemia (77%; n=6603), diabetes (40%; n=3408), and coronary artery disease (39%; n=3383). The drugs used most often included RAS blockers (54%; n=4592) and statins (53%; n=4553); thiazide diuretics (n=4007) and β blockers (n=3987) were both used by 47% of participants (table 1). Over half of participants took at least three of the nine study drugs. The mean number of total drugs was 10.0 (standard deviation 5.9). The median duration of follow-up was 24 (interquartile range 12-36) months. A total of 1287 (15%) participants died during follow-up.
Table 1.
Baseline characteristics by vital status. Values are numbers (percentages) unless stated otherwise
| Characteristics | All participants (n=8578) | Alive (n=7291) | Deceased (n=1287) |
|---|---|---|---|
| Age >80 years | 3073 (35.8) | 2278 (31.2) | 795 (61.8) |
| Women | 5026 (58.6) | 4284 (58.8) | 742 (57.7) |
| White | 7471 (87.1) | 6367 (87.3) | 1104 (85.8) |
| Hispanic ethnicity | 497 (5.8) | 431 (5.9) | 66 (5.1) |
| Income <$25 000 | 4542 (52.9) | 3670 (50.3) | 872 (67.8) |
| Current smoker | 655 (7.6) | 566 (7.8) | 89 (6.9) |
| Body mass index >30 | 2242 (26.1) | 1980 (27.2) | 262 (20.4) |
| Prescription drug insurance | 5465 (63.7) | 4825 (66.2) | 640 (49.7) |
| Urinary incontinence | 1763 (20.6) | 1308 (17.9) | 455 (35.4) |
| Assistive device required | 2293 (26.7) | 1598 (21.9) | 695 (54.0) |
| Hearing impairment | 733 (8.5) | 540 (7.4) | 193 (15.0) |
| Vision impairment | 708 (8.3) | 523 (7.2) | 185 (14.4) |
| Cognitive impairment | 2156 (25.1) | 1460 (20.0) | 696 (54.1) |
| Community dwelling | 7777 (90.7) | 6867 (94.2) | 910 (70.7) |
| Mean (SD) percentage days alive spent in hospital | 1.3 (4.8) | 0.6 (1.8) | 5.0 (10.9) |
| Mean (SD) physical function* | 6.6 (5.6) | 5.8 (5.2) | 11.0 (5.8) |
| Study chronic conditions: | |||
| Atrial fibrillation | 1649 (19.2) | 1207 (16.6) | 442 (34.3) |
| Coronary artery disease | 3383 (39.4) | 2742 (37.6) | 641 (49.8) |
| Depression | 2221 (25.9) | 1725 (23.7) | 496 (38.5) |
| Diabetes | 3408 (39.7) | 2827 (38.8) | 581 (45.1) |
| Heart failure | 1743 (20.3) | 1144 (15.7) | 599 (46.5) |
| Hyperlipidemia | 6603 (77.0) | 5885 (80.7) | 718 (55.8) |
| Hypertension | 7911 (92.2) | 6718 (92.1) | 1193 (92.7) |
| Kidney disease | 1004 (11.7) | 678 (9.3) | 326 (25.3) |
| Thromboembolic disease | 476 (5.5) | 328 (4.5) | 148 (11.5) |
| Other conditions:† | |||
| Alcohol misuse | 90 (1.0) | 65 (0.9) | 25 (1.9) |
| Blood loss anemia | 207 (2.4) | 141 (1.9) | 66 (5.1) |
| Chronic pulmonary disease | 2178 (25.4) | 1677 (23.0) | 501 (38.9) |
| Coagulopathy | 435 (5.1) | 310 (4.3) | 125 (9.7) |
| Deficiency anemia | 808 (9.4) | 605 (8.3) | 203 (15.8) |
| Fluid and electrolyte disorders | 1688 (19.7) | 1135 (15.6) | 553 (43.0) |
| Hypothyroidism | 1894 (22.1) | 1576 (21.6) | 318 (24.7) |
| Liver disease | 252 (2.9) | 183 (2.5) | 69 (5.4) |
| Lymphoma | 91 (1.1) | 69 (0.9) | 22 (1.7) |
| Metastatic cancer | 174 (2.0) | 79 (1.1) | 95 (7.4) |
| Neurological disorders | 642 (7.5) | 414 (5.7) | 228 (17.7) |
| Paralysis | 143 (1.7) | 85 (1.2) | 58 (4.5) |
| Peptic ulcer disease | 148 (1.7) | 114 (1.6) | 34 (2.6) |
| Psychoses | 244 (2.8) | 148 (2.0) | 96 (7.5) |
| Peripheral vascular disorders | 1738 (20.3) | 1304 (17.9) | 434 (33.7) |
| Pulmonary circulation disorders | 353 (4.1) | 240 (3.3) | 113 (8.8) |
| Rheumatoid arthritis or collagen disease | 467 (5.4) | 391 (5.4) | 76 (5.9) |
| Solid tumor without metastasis | 1108 (12.9) | 835 (11.5) | 273 (21.2) |
| Valvular disease | 1223 (14.3) | 959 (13.2) | 264 (20.5) |
| Weight loss | 423 (4.9) | 248 (3.4) | 175 (13.6) |
| Study drugs:‡ | |||
| β blocker§ | 3987 (46.5) | 3373 (46.3) | 614 (47.7) |
| Calcium channel blocker | 2810 (32.8) | 2406 (33.0) | 404 (31.4) |
| Clopidogrel | 1148 (13.4) | 951 (13.0) | 197 (15.3) |
| Metformin | 1198 (14.0) | 1058 (14.5) | 140 (10.9) |
| Renin-angiotensin system blocker | 4592 (53.5) | 3977 (54.5) | 615 (47.8) |
| SSRI/SNRI | 1774 (20.7) | 1398 (19.2) | 376 (29.2) |
| Statin | 4553 (53.1) | 4053 (55.6) | 500 (38.9) |
| Thiazide | 4007 (46.7) | 3282 (45.0) | 725 (56.3) |
| Warfarin | 1227 (14.3) | 978 (13.4) | 249 (19.3) |
| No of study drugs: | |||
| 0 | 389 (4.5) | 305 (4.2) | 84 (6.5) |
| 1-2 | 3078 (35.9) | 2657 (36.4) | 421 (32.7) |
| 3-5 | 4637 (54.1) | 3943 (54.1) | 694 (53.9) |
| 6-9 | 474 (5.5) | 386 (5.3) | 88 (6.8) |
| Mean (SD) no of non-study drugs | 7.1 (5.3) | 6.8 (5.1) | 8.8 (6.1) |
| Mean (SD) total drugs¶ | 10.0 (5.9) | 9.7 (5.7) | 11.7 (6.7) |
SSRI=selective serotonin reuptake inhibitor; SNRI=serotonin norepinephrine reuptake inhibitor.
*Measured by amount of difficulty stooping or kneeling; lifting heavy items; reaching or extending arms; writing; grasping; and walking 0.25 miles, with each activity rated from no difficulty (0) to unable to do it (4). Scores ranged from 0-20, with higher scores indicating more difficulty performing activities.
†Conditions include Elixhauser comorbidities excluding study conditions and HIV (absent in participants).
‡Recommended drugs in national disease guidelines6-11 21 22 but not included in current study as not used orally, not prescription drugs in the US (and thus not available in dataset), or used by less than 10% of the population, including amiodarone, apixaban, aspirin, dabigatran, digoxin, dofetilide, dronedarone, flecainide, propafenone, rivaroxaban, and sotalol for atrial fibrillation; aspirin for coronary artery disease; bupropion, mirtazapine, monoamine oxidase inhibitors, serotonin modulators, and tricyclic antidepressants for depression; α glucosidase inhibitors, amylin agonists, dipeptidyl peptidases-4, glinides, insulin, sulfonylureas, and thiazolidinediones for diabetes; hydral nitrates, loop diuretics, and spironolactone for heart failure; dabigatran, low molecular weight heparin, and rivaroxaban for thromboembolic disease.
§3400 participants received a cardioselective β blocker and 687 received an αβ blocker.
¶Includes all prescription drugs, including study and other drugs, taken over first year of follow-up.
Table 2 lists the proportion of participants with a study condition who received each guideline recommended study drug. For example, β blockers (n=1258) and warfarin (n=1095) were the two most commonly used guideline recommended drugs among the 1946 participants with atrial fibrillation. The frequency of use for most drugs was similar for all participants with a condition, including those with combinations of coexisting conditions (table 2).
Table 2.
Prevalence of guideline recommended drugs for study chronic conditions*
| Conditions | β blocker | Calcium channel blocker | Clopidogrel | Metformin | RAS blocker | SSRI/SNRI | Statin | Thiazide | Warfarin |
|---|---|---|---|---|---|---|---|---|---|
| Atrial fibrillation (n=1946) | 1258 (64.6) | 795 (40.9) | 320 (16.4) | 1095 (56.3) | |||||
| Coronary artery disease (n=3780) | 2535 (67.1) | 1084 (28.7) | 2386 (63.1) | 2492 (65.9) | |||||
| Depression (n=2630) | 1471 (55.9) | ||||||||
| Diabetes (n=3715) | 1335 (35.9) | 2440 (65.7) | 2353 (63.3) | ||||||
| Heart failure (n=2169) | 1454 (67.0) | 1408 (64.9) | |||||||
| Hyperlipidemia (n=6853) | 4761 (69.5) | ||||||||
| Hypertension (n=8074) | 4301 (53.3) | 3180 (39.4) | 4986 (61.8) | 4405 (54.6) | |||||
| Thromboembolic disease (n=619) | 372 (60.1) | ||||||||
| Coexisting conditions:† | |||||||||
| AF-CAD-HL-HTN (n=910) | 662 (72.7) | 389 (42.7) | 232 (25.5) | 608 (66.8) | 664 (73.0) | 636 (69.9) | 539 (59.2) | ||
| AF-DM-HL-HTN (n=633) | 457 (72.2) | 287 (45.3) | 137 (21.6) | 197 (31.1) | 433 (68.4) | 462 (73.0) | 457 (72.2) | 366 (57.8) | |
| AF-DEP-HL-HTN (n=407) | 278 (68.3) | 184 (45.2) | 75 (18.4) | 247 (60.7) | 236 (58.0) | 277 (68.1) | 273 (67.1) | 213 (52.3) | |
| AF-HF-HL-HTN (n=689) | 497 (72.1) | 294 (42.7) | 151 (21.9) | 472 (68.5) | 473 (68.7) | 547 (79.4) | 402 (58.3) | ||
| DEP-CAD-HL-HTN (n=877) | 587 (66.9) | 372 (42.4) | 263 (30.0) | 568 (64.8) | 494 (56.3) | 634 (72.3) | 524 (59.7) | ||
| DEP-DM-HL-HTN (n=797) | 458 (57.5) | 319 (40.0) | 273 (34.3) | 545 (68.4) | 473 (59.3) | 565 (70.9) | 475 (59.6) | ||
| DEP-HF-HL-HTN (n=548) | 388 (70.8) | 220 (40.1) | 368 (67.2) | 308 (56.2) | 377 (68.8) | 415 (75.7) | |||
| DM-CAD-HL-HTN (n=1471) | 1084 (73.7) | 595 (40.4) | 482 (32.8) | 482 (32.8) | 1048 (71.2) | 1121 (76.2) | 936 (63.6) | ||
| DM-HF-HL-HTN (n=863) | 642 (74.4) | 345 (40.0) | 265 (30.7) | 621 (72.0) | 630 (73.0) | 685 (79.4) | |||
| HF-CAD-HL-HTN (n=1129) | 870 (77.1) | 454 (40.2) | 361 (32.0) | 799 (70.8) | 816 (72.3) | 865 (76.6) |
RAS=renin-angiotensin system; AF=atrial fibrillation; CAD=coronary artery disease; DEP=depression; DM=diabetes; HF=heart failure; HL=hyperlipidemia; HTN=hypertension; metform=metformin; SSRI=selective serotonin reuptake inhibitor; SNRI=serotonin norepinephrine reuptake inhibitor.
*Percentages based on number of participants in row with indicated condition who took the drug at any time during the study period. Participants with a specific condition may have another condition for which the drug is indicated.
†See statistical analysis section for criteria for selecting groups of coexisting conditions.
We ascertained changes in drug use over the study period. The percentage of participants starting a study drug after baseline ranged from 2% (180/7380) for metformin to 13% (517/3986) for RAS blockers, while the proportion discontinuing a study drug present at baseline ranged from 8% for statins (386/4553) and β blockers (316/3987) to 18% (208/1148) for clopidogrel.
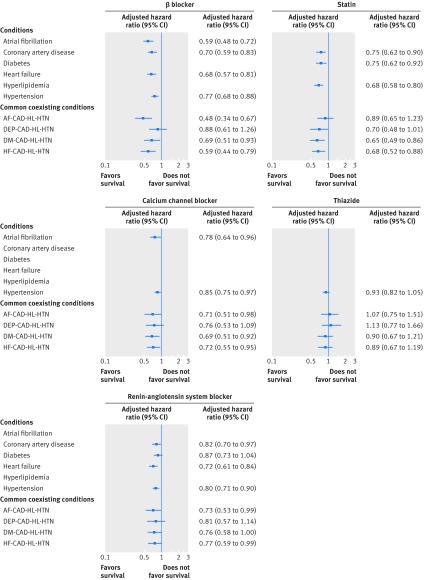
Mortality was 26.6% (517/1946) among those with atrial fibrillation, 18.7% (707/3780) among those with coronary artery disease, 16.6% (617/3715) among those with diabetes, 32.8% (711/2169) among those with heart failure, 10.9% (747/6853) among those with hyperlipidemia, and 15.0% (1214/8074) among those with hypertension (fig 1). Figure 1 shows the associations between the guideline recommended cardiovascular drugs and the risk of death associated with each of β blockers, calcium channel blockers, RAS blockers, statins, and thiazides among participants with the cardiovascular condition for which the drugs are indicated. To explore whether the effects of these drugs differed according to the presence of different coexisting conditions, we identified participants who had common combinations of four conditions, as described in the statistical analysis section.
Fig 1 Adjusted hazard ratios of death associated with guideline recommended cardiovascular drugs for older adults with chronic conditions. AF=atrial fibrillation; CAD=coronary artery disease; DEP=depression; DM=diabetes; HF=heart failure; HL=hyperlipidemia; HTN=hypertension. Hazard ratios are adjusted for the covariates described in the Methods section,as well as number of drugs other than the study drugs, and all coexisting study conditions and drugs
Most adjusted hazard ratios for cardiovascular drugs were similar across combinations of coexisting conditions, though there were trends toward variable effects for β blockers—adjusted hazard ratios ranged from 0.48 (95% confidence interval 0.34 to 0.67) to 0.88 (0.61 to 1.26).
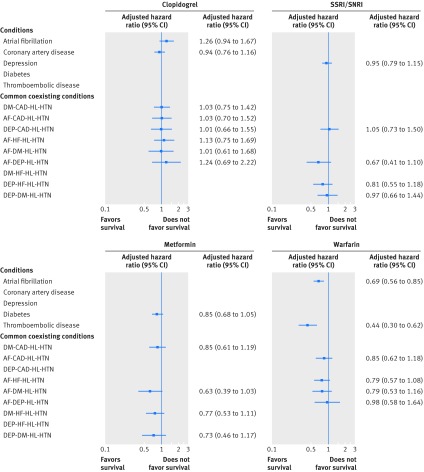
Figure 2 displays the association between each of clopidogrel, metformin, SSRIs or SNRIs, and warfarin and risk of death for participants with the conditions for which these drugs are recommended. Clopidogrel was not associated with a reduced risk of death in participants with atrial fibrillation or coronary artery disease nor was metformin in those with diabetes or SSRIs or SNRIs among participants with depression. Warfarin was associated with a reduction in death among those with atrial fibrillation (adjusted hazard ratio 0.69, 95% confidence interval 0.56 to 0.85) and thromboembolic disease (0.44, 0.30 to 0.62). The association between warfarin and risk of death was attenuated in participants with atrial fibrillation who had coexisting conditions, particularly depression, hypertension, and hyperlipidemia. The number of people with thromboembolic disease was insufficient to look at combinations of coexisting conditions.
Fig 2 Adjusted hazard ratios of death associated with commonly used guideline recommended drugs for older adults with chronic conditions. AF=atrial fibrillation; CAD=coronary artery disease; DEP=depression; DM=diabetes; HF=heart failure; HL=hyperlipidemia; HTN=hypertension; SSRIs= selective serotonin reuptake inhibitors; SNRIs=serotonin norepinephrine reuptake inhibitors. Hazard ratios are adjusted for the covariates described in the Methods section as well asnumber of drugs other than the study drug and all coexisting study conditions and drugs
Discussion
We found an association of improved survival with several study drugs, particularly cardiovascular drugs, in this representative sample of older adults with multiple chronic conditions. This is a population at high risk of dying. The three year mortality rate was 15%, escalating to nearly a third of those with heart failure. Most results were similar for participants with common combinations of coexisting conditions, although there were drugs with stronger or weaker associations with risk of death in those with some combinations of conditions. The high number of prescription drugs in the study population highlights the importance of determining the safest and most effective regimens for older adults with multiple chronic conditions.
With the exception of metformin, calcium channel blockers, and clopidogrel—which is not a first line drug for atrial fibrillation and is not indicated for everyone with coronary artery disease—over 50% of participants received the recommended drugs. The prevalence of use remained high in those with multiple coexisting conditions, suggesting that clinicians continue to follow disease guidelines in the presence of multiple conditions. The result was a high accumulated number of drugs for many participants.
The association between drug use and mortality risk usually remained similar across patterns of coexisting conditions, suggesting that benefits often remain despite comorbidity. While overlapping confidence intervals precluded definitive conclusions, however, the adjusted hazard ratios for β blockers and warfarin differed in at least one combination of coexisting conditions compared with the overall adjusted hazard ratios for participants with the indicated condition. This finding suggests that the effect of some drugs may vary in the presence of some coexisting conditions. We only studied a few of the hundreds of possible combinations of study conditions; we do not know if the association with death varies for study drugs in other combinations of coexisting conditions. We cannot exclude the possibility that these were chance findings. Further exploration of the effect of coexisting conditions on drug effects is important to ensure optimal prescribing for those with multiple chronic conditions, particularly for drugs such as warfarin with potential for both benefit and harm.8 29
Comparison with other studies
Our results corroborate those of a recent Cochrane review, which showed that drug effects were usually similar between randomized controlled trials and observational studies.18 Similar to randomized controlled trials, we found survival benefit with calcium channel blockers, RAS blockers, statins, and warfarin.8 11 21 26 30 31 32 33 34 35 The lack of survival benefit of SSRIs or SNRIs with depression mirrors the results of randomized controlled trials, which have been mixed.36 The beneficial association of β blockers in people with hypertension, not seen in randomized controlled trials,11 may reflect its beneficial effect on conditions coexisting with hypertension in this multimorbid population. As we lacked echocardiographic data to determine the type of heart failure, we cannot confirm or refute previous reports of improved survival with RAS blockers in people with heart failure and low, but not preserved, left ventricular ejection fraction.32 33 34 35
We found no survival benefit with clopidogrel in those with atrial fibrillation or coronary artery disease. The results of randomized controlled trials for survival effect for clopidogrel among those with coronary artery disease are conflicting.6 37 The marginally decreased survival with atrial fibrillation may reflect the use of clopidogrel in those at high risk for whom warfarin or newer antithrombotics, known to be effective,38 were deemed unsafe. We lacked data on aspirin use so we cannot comment on the effect of aspirin and clopidogrel combination therapy. Unlike previous studies,39 40 metformin showed no association with survival among older people with diabetes in the current study.
Strengths and limitations of this study
The observational design of this study conferred strengths as well as limitations. The large, nationally representative cohort enhances the generalizability of the results and better resembles the heterogeneous older adult population cared for in clinical practice than do participants in the randomized controlled trials that inform disease guidelines.41 42 43 The rich set of sociodemographic, health, medical, behavioral, psychological, and functional characteristics, not available in administrative data, allowed us to account for the wide range of factors that affect the likelihood both of receiving the drugs and of dying during the follow-up period. Furthermore, we accounted for functional level, amount of time in hospital, and living in a nursing facility, all of which are measures of disease severity. Along with coexisting conditions and cognitive impairment, accounted for in our analyses, these characteristics also are predictive of overall prognosis, which affects the likelihood of both receiving a drug and dying during follow-up.44
The limitations of Medicare claims data for ascertaining chronic conditions have been well chronicled, with conditions that provide more lucrative reimbursement and require more frequent medical attention being more thoroughly reported.45 46 47 We used two years of inpatient and outpatient claims to improve ascertainment of chronic conditions. Hypertension and hyperlipidemia, for example, were both present in over three quarters of the sample. We combined information from interviews with claims data to increase ascertainment of depression and cognitive impairment, two conditions underreported in claims.47 We cannot determine whether results generalize to the 24% of older Americans in Medicare Advantage plans excluded from the study because they do not have healthcare claims.48
We did not have information on time of onset of the chronic conditions or duration of drug treatment. Inception cohorts are one means recommended to limit bias in observational studies and assure that confounders are measured before the initiation of drugs.49 An inception cohort was not feasible or appropriate for the current study. Older adults have often had their conditions and been receiving treatment for many years; the clinical question is usually the benefit versus harm of continuing rather than starting drugs. Prevalent users are the appropriate participants to address this problem. Our time varying analyses accounted for incident conditions, as well as initiation or discontinuation of drugs during follow-up.
Not all guideline recommended drugs were investigated. We limited the study to oral drugs, included only drugs used by at least 10% of participants, and studied drug classes rather than individual drugs. For example, because of small numbers we did not separate dihydropyridine and non-dihydropyridine calcium channel blockers despite the fact that the latter are recommended for atrial fibrillation.8 Only 21 of 1946 people with atrial fibrillation received a calcium channel blocker and did not have concomitant hypertension for which they may have received either a dihydropyridine or a non-dihydropyridine calcium channel blocker. We did not include drug doses, which likely varied during the study period. Because lower doses and less aggressive treatment targets (for example, higher blood pressure or glycated hemoglobin) are often recommended for older adults or frailer patients, there may be dose-response relations that affected the association between drugs and mortality that we could not detect. Adherence is another factor that may affect results. Though we did not have direct information on adherence, drug use was ascertained by direct observation of the drugs so we at least know whether the participants filled the prescriptions and had the drugs available.
Despite adjustment for a wide array of confounding factors, we used observational data and cannot exclude the possibility of unmeasured confounders. Although there is biologic plausibility for potential survival benefit for the drugs studied, and most of our findings are similar to those of randomized controlled trials, we cannot establish a causal relation in this observational study. The observed beneficial effects of the drugs could reflect healthy user bias in which healthier patients are more likely to be prescribed, and to take, a drug than those who are frail or more chronically ill.50 Investigators recommend the use of analytical approaches employing causal inference techniques such as instrumental variables, marginal structural models, or propensity scores. However, causal methods have not been developed when multiple factors are intended for inference and not every participant is at risk of receiving every drug. Of note, recent investigations, including a recent Cochrane review, found similar results between conventional adjusted regression and propensity score techniques.18 28 Observational studies may help to determine drug effects in older adults with multiple chronic conditions.
Conclusions and policy implications
Notwithstanding these limitations and the fact that results from this single study are not definitive, findings suggest that the average survival benefit with many cardiovascular drugs in older adults with major complications and comorbidities is similar to that reported for participants in randomized controlled trials. Survival effects for some drugs, however, varied by coexisting conditions. Eventually, more individualized estimates, perhaps ascertained through large well characterized databases of people with major complications and comorbidities may guide treatment decisions with the aim of maintaining the beneficial effects of drugs while reducing the burden and risk of adverse effects of polypharmacy. Survival was the outcome in the current study but older adults with multiple chronic conditions vary in the health outcome of highest priority.51 Many questions remain to be addressed. It will be important to look at other important cross disease, universal health outcomes such as function or symptom burden.5 Future work should also address other combinations of conditions and drugs and the effect of key characteristics such as age, frailty, functional status, and adherence on the association between drugs and health outcomes in people with multiple chronic conditions. Eventually it should be possible to estimate and compare benefits of several guideline recommended drugs for any given combination of conditions. The ultimate goal would be to select the drug combination that maximizes benefit and minimizes drug burden within the context of each individual’s priorities for health outcome and constellation of conditions.
What is already known on this topic
Most older adults have multiple chronic conditions and take many drugs for these in accordance with disease guidelines
Drug effects, including effect on mortality risk, may differ in older adults with multiple chronic conditions from those reported for participants of randomized clinical trials that inform disease guidelines
What this study adds
The beneficial effects on survival of β blockers, calcium channel blockers, renin-angiotensin system blockers, statins, and warfarin are comparable to those reported in randomized controlled trials but vary for β blockers and warfarin according to coexisting conditions
Clopidogrel, metformin, and serotonin reuptake inhibitors or serotonin norepinephrine reuptake inhibitors were not associated with survival benefit
Determining effects in combinations of coexisting conditions may guide prescribing in people with multiple chronic conditions
Contributors: MET obtained funding, provided supervision, conceived and designed the study, interpreted the data, drafting, and critically revised the manuscript. GMcA conceived and designed the study, analyzed and interpreted the data, drafted and critically revised the manuscript, and did the statistical analysis. ABC interpreted the data and critically revised the manuscript. MET interpreted the data, did the statistical analysis, and critically revised the manuscript. HGA obtained funding, provided supervision, conceived and designed the study, analyzed and interpreted the data, drafted and critically revised the manuscript, and did the statistical analysis. MET and HGA are the guarantors of the study.
Funding: This study was supported by grant R21 AG045148 and by the Yale Pepper Center (P30 AG021342), both from the National Institute on Aging. The researchers conducted this study independently from the funders.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This study was exempt from review by the Yale University Human Investigation Committee because it involved existing, publicly available, deidentified data.
Data sharing: The Medicare Current Beneficiary Study is in a publicly available dataset (www.cms.gov/Research-Statistics-Data-and-Systems/Research/MCBS/index.html?redirect=/mcbs).
Transparency: The lead authors (MET and HGA) affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Cite this as: BMJ 2015;351:h4984
References
- 1.Gorina Y, Lentzer H. Multiple causes of death in old age. Aging Trends 2008;(9):1-9. [PubMed]
- 2.National Center for Health Statistics. Mortality data, multiple-cause-of-death public-use data files. 2015. www.cdc.gov/nchs/data_access/VitalStatsOnline.htm#Mortality_Multiple.
- 3.Hoyert DL, Xu JQ. Deaths: preliminary data for 2011. National Vital Statistics Reports; Vol 61 No 6. National Center for Health Statistics; 2012. [PubMed]
- 4.Tinetti ME, McAvay G, Murphy T, Allore H, Gross, C, Lin H. Contribution of individual diseases to death in older adults with multiple diseases. J Am Geriatr Soc 2012;60:1448-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tinetti ME, McAvay G, Chang SS, et al. Contribution of multiple chronic conditions to universal health outcomes. J Am Geriatr Soc 2011;59:1686-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 2011;124:2458-73. [DOI] [PubMed] [Google Scholar]
- 7.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.January CT, Wann LS, Alpert JS. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2014;130:2071-104. [DOI] [PubMed] [Google Scholar]
- 9.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147-239. [DOI] [PubMed] [Google Scholar]
- 10.Work Group on Major Depressive Disorder. Practice guideline for the treatment of patients with major depressive disorder. 3 ed. American Psychiatric Publishing; 2010. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.
- 11.James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report From the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507-20. [DOI] [PubMed] [Google Scholar]
- 12.Bajcar JM, Li Wang L, Moineddin R, Nie JX, Tracy CS, Upshur REG. From pharmaco-therapy to pharmaco-prevention: trends in prescribing to older adults in Ontario, Canada, 1997-2006. BMC Fam Pract 2010;11:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Center for Health Statistics. Health, United States, 2013: with special feature on prescription drugs. CDC, 2014. www.cdc.gov/nchs/data/hus/hus13.pdf. [PubMed]
- 14.Tran VT, Harrington M, Montori VM, Barnes C, Wicks P, Ravaud P. Adaptation and validation of the Treatment Burden Questionnaire (TBQ) in English using an internet platform. BMC Med 2014;12:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taché SV, Sönnichsen A, Ashcroft DM. Prevalence of adverse drug events in ambulatory care: a systematic review. Ann Pharmacother 2011;45:977-89. [DOI] [PubMed] [Google Scholar]
- 16.Hakkarainen KM, Hedna K, Petzold M, Hägg S. Percentage of patients with preventable adverse drug reactions and preventability of adverse drug reactions: a meta-analysis. PLoS One 2012;7:e33236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uhlig K, Leff B, Kent D, et al. A framework for crafting clinical practice guidelines that are relevant to the care and management of people with multimorbidity. J Gen Intern Med 2014;29:670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anglemyer A, Horvath HT, Bero L. Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. Cochrane Database Syst Rev 2014;4:MR000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medicare Current Beneficiary Survey (MCBS). 2015.www.cms.gov/ Research-Statistics-Data-and-Systems/Research/MCBS/index.html.
- 20.Healthcare Cost and Utilization Project (HCUP). Clinical classification system. Agency for Healthcare Research and Quality. 2012. www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. [PubMed]
- 21.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(25 Suppl 2):S1-45. [DOI] [PubMed] [Google Scholar]
- 22.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ, for the American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive Summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2_suppl):7S-47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.GRADE Working Group. Grading the quality of evidence and the strength of recommendations (2010). The GRADE Working Group. 2015. www.gradeworkinggroup.org/intro.htm.
- 24.Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc 1976;54:439-67. [PubMed] [Google Scholar]
- 25.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8-27. [DOI] [PubMed] [Google Scholar]
- 26.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130-9. [DOI] [PubMed] [Google Scholar]
- 27.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. Springer-Verlag; 2000.
- 28.Sturmer T, Joshia M, Glynn RJ, et al. A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol 2006;59:437-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Brien EC, Kim S, Hess PL. Effect of the 2014 atrial fibrillation guideline revisions on the proportion of patients recommended for oral anticoagulation. JAMA Intern Med 2015;175:848-50. [DOI] [PubMed] [Google Scholar]
- 30.The AFFIRM Investigators. Relationships Between Sinus Rhythm, Treatment, and Survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation 2004;109:1509-13. [DOI] [PubMed] [Google Scholar]
- 31.Afilalo DJ, Duque G, Russell Steele R, et al. Statins for secondary prevention in elderly patients; a hierarchical bayesian meta-analysis. Am Coll Cardiol 2008;51:437-51. [DOI] [PubMed] [Google Scholar]
- 32.Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet 2003;362:759-66. [DOI] [PubMed] [Google Scholar]
- 33.Massi BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456-67. [DOI] [PubMed] [Google Scholar]
- 34.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The Perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 2006;27:2338-2345. [DOI] [PubMed] [Google Scholar]
- 35.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003;362:777-81. [DOI] [PubMed] [Google Scholar]
- 36.Bogner HR, Morales KH, Post EP, Bruce ML. Diabetes, depression, and death. A randomized controlled trial of a depression treatment program for older adults based in primary care (PROSPECT). Diabetes Care 2007;30:3005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatt DL, Fox KA, Hacke W, et al Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1706-17. [DOI] [PubMed] [Google Scholar]
- 38.Dogliotti A, Paolasso E, Giuliano RP. Current and new oral antithrombotics in non-valvular atrial fibrillation: a network meta-analysis of 79808 patients. Heart 2014;100:396-405. [DOI] [PubMed] [Google Scholar]
- 39.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577-89. [DOI] [PubMed] [Google Scholar]
- 40.Roussel R, Tavert F, Pasquet B. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch Intern Med 2010;170:1892-9. [DOI] [PubMed] [Google Scholar]
- 41.Dhruva SS, Redberg RF. Variations between clinical trial participants and Medicare beneficiaries in evidence used for Medicare national coverage decisions. Arch Intern Med 2008;168:136-40. [DOI] [PubMed] [Google Scholar]
- 42.American Geriatrics Society expert panel on the care of older adults with multimorbidity. Patient-centered care for older adults with multiple chronic conditions: a stepwise approach from the American Geriatrics Society. J Am Geriatr Soc 2012;60:1957-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lugtenberg M, Burgers JS, Clancy C, Westert GP, Schneider EC. Current guidelines have limited applicability to patients with comorbid conditions: a systematic analysis of evidence-based guidelines. PLoS One 2011;6:e25987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pilotto A, Addante F, Ferrucci L, et al. The multidimensional prognostic index predicts short- and long-term mortality in hospitalized geriatric patients with pneumonia. J Gerontol A Biol Sci Med Sci 2009;64:880-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klabunde CN, Harlan LC, Warren JL. Data sources for measuring comorbidity. A comparison of hospital records and Medicare claims data for cancer patients. Med Care 2006;44:921-8. [DOI] [PubMed] [Google Scholar]
- 46.Lentine KL, Schnitzler MA, Abbott KC, Bramesfeld K, Buchanan PM, Brennan DC. Sensitivity of billing claims for cardiovascular disease events among kidney transplant recipients. Clin J Am Soc Nephrol 2009;4:1213-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noyes K, Liu H, Lyness JM, Friedman B. Medicare beneficiaries with depression: Comparing diagnoses in claims data with the results of screening. Psychiatr Serv 2011;62:1159-66. [DOI] [PubMed] [Google Scholar]
- 48.Total Medicare health plan enrollment, 1999-2014. MPR/Kaiser Family Foundation Analysis of CMS Medicare Advantage enrollment files 2008-2014. http://kff.org/medicare/fact-sheet/medicare-advantage-fact-sheet.
- 49.Johnson ES, Bartman BA, Briesacher BA, et al. The incident user design in comparative effectiveness research. Pharmacoepidemiol Drug Saf 2012;22:1-6. [DOI] [PubMed] [Google Scholar]
- 50.Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med 2011;26:546-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fried TR, McGraw S, Agostini JV, Tinetti ME. Views of older persons with multiple conditions on competing outcomes and clinical decision-making. J Am Geriatr Soc 2008;56:1839-44. [DOI] [PMC free article] [PubMed] [Google Scholar]