Abstract
Flublok is the first recombinant hemagglutinin (HA) vaccine licensed by the US Food and Drugs Administration for the prevention of influenza in adults aged 18 and older. The HA proteins produced in insect cell culture using the baculovirus expression system technology are exact analogues of wild type circulating influenza virus HAs. The universal HA manufacturing process that has been successfully scaled to the 21,000L contributes to rapid delivery of a substantial number of doses. This review discusses the immunogenicity, efficacy and safety data from five pivotal clinical studies used to support licensure of trivalent Flublok for adults 18 years of age and older in the United States. The trial data demonstrate that the higher antigen content in Flublok results in improved immunogenicity. Data further suggest improved efficacy and a slightly lower local reactogenicity compared with standard inactivated influenza vaccine, despite the presence of more antigen (statistically significant). Flublok influenza vaccine can include HAs designed to mimic ‘drift’ in influenza viruses as the process of predicting antigenic drift advances and, at a minimum, could address late appearing influenza viruses. The implementation of the latter will require support from regulatory authorities.
Keywords: cell culture, hemagglutinin, immunogenicity, influenza, safety, vaccine
Introduction
Flublok (Protein Sciences Corporation, Meriden, CT, USA) is the only recombinant influenza vaccine licensed by the US Food and Drug Administration (FDA) for the prevention of influenza in adults 18 years of age and older. The mechanism of action of Flublok is the same as that of the licensed egg-grown inactivated influenza vaccines (IIVs). IIVs contain a standardized amount of hemagglutinin (HA) and immune correlates are well established. Regulators around the world use standardized criteria based on HA inhibition (HAI) titers to support the licensure of influenza vaccines.
The FDA initially licensed Flublok in 2013 for the prevention of influenza in adults 18–49 years of age on the basis of two placebo-controlled clinical studies, PSC01 and PSC04. The first study PSC01 demonstrated that including three times more HA antigen (45 versus 15 μg) resulted in improved antibody responses, confirming the results of an earlier clinical study [Treanor et al. 2007]. In addition, studies PSC03 and PSC06 demonstrated improved immunogenicity of Flublok for the influenza A viruses [Keitel et al. 2010; Baxter et al. 2011] as reported for increased antigen concentration of other IIVs [Keitel et al. 1994, 1996]. Study PSC04 confirmed the efficacy of the vaccine in adults 18–49 years old despite the circulation of drift viruses during the 2007/08 season when the study was conducted [Treanor et al. 2011]. In this review we include a Kaplan–Meijer plot that provides some preliminary evidence for waning vaccine efficacy (VE) later in the season.
Additional safety data for Flublok in older adults were collected in another study of adults older than 50 [ClinicalTrials.gov identifier: NCT01825200].
This review discusses five pivotal clinical studies (PSC01; PSC03; PSC04; PSC06; PSC11) supporting full approval of Flublok for adults aged 18–49 and approval under the ‘accelerated approval’ mechanism for adults over 50 (Table 1). The latter means that confirmatory efficacy studies still need to be conducted. A large comparative efficacy study in adults over 50 years old with a quadrivalent formulation is in progress during the 2014/15 season. Results expected from this double-blinded efficacy study will provide important insights as to whether variations in the HA protein caused by the egg-based manufacturing process were indeed responsible for the reported low effectiveness of the 2014/15 influenza vaccine [D’Mello et al. 2015; Skowronski et al. 2015].
Table 1.
Clinical studies discussed in this review.
| ID season | Test product | Comparator | Study population | Endpoints | N | Reference |
|---|---|---|---|---|---|---|
| PSC01 2004–2005 (phase IIb) | Flublok 135 μg A/New Caledonia A/Wyoming B/Jiangsu Flublok 75 μg | Placebo | Healthy adults 18–49 years of age | Safety, immunogenicity clinical efficacy | 458 | Treanor et al. [2007] |
| PSC03 2006–2007 (phase III) | Flublok 135 μg A/New Caledonia A/Wisconsin B/Ohio | Fluzone* A/New Caledonia A/Wisconsin B/Malaysia | Healthy adults 65–92 years of age | Safety, immunogenicity | 869 | Keitel et al. [2010] |
| PSC04 2007–2008 (phase III) | Flublok 135 μg A/Solomon Islands A/Wisconsin B/Malaysia | Placebo | Healthy adults 19–49 years of age | Safety, immunogenicity clinical efficacy | 4648 | Treanor et al. [2011] |
| PSC06 2007–2008 (phase III) | Flublok 135 μg A/Solomon Islands A/Wisconsin B/Malaysia | Fluzone* | Healthy adults 50–64 years of age | Safety, immunogenicity relative clinical efficacy | 602 | Baxter et al. [2011] |
| PSC11 2012–2013 (Phase IV) | Flublok 135 μg A/California A/Victoria B/Wisconsin | Afluria$ | Ambulatory and medically stable adults ⩾50 years of age | Safety, solicited and unsolicited hypersensitive adverse events | 2640 | Izikson et al. [2015] |
Fluzone manufactured by Sanofi-Pasteur, Swiftwater, PA, USA.
Afluria manufactured by bioCSL Pty Ltd. CSL Limited, Parkville, Vicotria 3052, Australia.
Traditional influenza vaccines are produced by growing the influenza virus in embryonated hen’s eggs. Gradually this production process may be transferred to cell culture with one manufacturer (formerly Novartis; recently acquired by CSL-bio CSL Limited, Parkville, Vicotria 3052, Australia) pioneering the field by growing the influenza viruses in Madin-Darby Canine Kidney (MDCK) cells. Virions are harvested, chemically inactivated and treated with detergent, the HA and neuraminidase (NA) proteins are partially purified to produce split-product, subvirion, or subunit vaccines [Wood, 1998]. The 60-year-old egg-based influenza manufacturing process served well; however, newer technology enables vaccine production to overcome the well recognized egg substrate disadvantages. Limitations include the following: selection or adaptation of influenza virus strains for production at high levels in eggs or cells may result in suboptimal genetic match between the vaccine strains and the disease causing influenza virus strains [Katz et al. 1987; Wang et al. 1989; Rajakumar et al. 1990; Skowronski et al. 2014]; of note the MDCK production process uses egg-derived virus seeds in its manufacturing process as well and the mutations associated with adaptation to growth in eggs remain an issue; the time required to produce a high growth reassortant; the manufacture process requires high-level biocontainment facilities or workers with appropriate immune responses against the viruses they produce; the influenza virus inactivation process requires the use of undesirable chemicals; the endotoxin content cannot be carefully controlled; and the presence of ovalbumin or residual chemicals or antibiotics required to maintain a low bioburden during processing.
IIVs are standardized to contain 15 μg of each of three or four HAs, derived from influenza A subtype H1N1, H3N2 and more recently two B lineages [Bridges et al. 2002]. HA is the dominant surface glycoprotein on the influenza virus and key antigen in both natural infection and vaccination host response to influenza virus, and the logical candidate for recombinant vaccine technology [Huber and Cullers, 2008]. NA plays an important role in initiation of infection [Matrosovich et al. 2004] and antibody to the NA induces an ‘infection permissive’ immunity most likely by a combination of mechanisms: direct inhibition of the enzyme; cross linking individual virions and thereby reducing the number of infectious viruses available to infect host cells; cementing virions to cell associated NA; and inhibiting the detachment of nascent virions from the host cell [Air et al. 1989; Huang et al. 1980a, 1980b; Kilbourne et al. 1968a, 1968b].
Flublok contains HA protein antigens derived from influenza virus strains, selected for inclusion in the annual influenza vaccine by the World Health Organization and updated on an annual basis. The proteins are produced in a proprietary nontransformed, nontumorigenic continuous cell line (expresSF+ insect cells Protein Sciences Corporation, Meriden, CT USA) grown in serum-free medium, derived from Sf9 cells of the fall armyworm, Spodoptera frugiperda. The HAs are expressed in this insect cell line using the baculovirus Autographa californica nuclear polyhedrosis virus. The individual HAs are extracted from the cells with buffer and detergent and further purified by column chromatography. Further details on the production and characterization of recombinant HA (rHA) are described elsewhere [Holtz et al. 2003; Wang et al. 2006; Cox and Hashimoto, 2011]. The mechanism of action of this vaccine candidate is expected to be similar to IIV; namely, the induction of HAI antibodies to prevent influenza infection [Kida et al. 1983; Yoden et al. 1986].
Earlier development of Flublok was reviewed elsewhere in detail [Cox et al. 2008]. Here, we review data derived from five clinical studies and focus on three aspects: safety, immunogenicity and efficacy of Flublok, comparing its performance with other commercially available influenza vaccines.
Flublok safety
Flublok contains 45 μg of each HA and is well tolerated, whereas the standard IIV contains 15 μg of each HA. The higher HA content offers the potential to provide cross protection for which preliminary evidence has been presented, but also the possibility for longer lasting and improved immunogenicity [Treanor et al. 2006; Cox and Anderson, 2007]. Data obtained with Flublok are consistent with studies that demonstrated increased doses of purified HA and subvirion vaccines produce an enhanced antibody response in both the elderly and healthy adult populations [Keitel et al. 1994, 1996].
Flublok has been administered to and safety data collected from 2497 adults, 18 through 49 years of age, 972 adults 50 through 64 years of age, and 1078 adults aged 65 years and older enrolled in five randomized, placebo- or active-controlled clinical trials.
Description of trivalent Flublok vaccine clinical studies PSC01, PSC03, PSC04, PSC06 and PSC11
Study PSC01 included 458 subjects, 18 through 49 years of age, for safety analysis, randomized to receive Flublok low dose (n = 151; vaccine contained 45 μg of H3 rHA and 15 μg of B and H1 rHA), Flublok (n = 153), or placebo (n = 2304) [Treanor et al. 2007].
Study PSC03 included 869 subjects aged 65 years and older for safety analysis, randomized to receive Flublok (n = 436) or another US-licensed trivalent influenza vaccine (Fluzone, Sanofi-Pasteur, Swiftwater, PA, USA) as an active control (n = 433) [Keitel et al. 2010].
Study PSC04 included 4648 subjects, 18 through 49 years of age, for safety analysis, randomized to receive Flublok (n = 2344) or placebo (n = 2304) [Treanor et al. 2011].
Study PSC06 included 602 subjects, 50 through 64 years of age, for safety analysis, randomized to receive Flublok (n = 300) or another US-licensed trivalent influenza vaccine (Fluzone, as an active control) (n = 302) [Baxter et al. 2011].
Study PSC11 included 2627 subjects aged 50 years and older for safety analysis, randomized to receive Flublok (n = 1314) or another US-licensed trivalent influenza vaccine (Afluria, manufactured by bioCSL Pty Ltd) as an active control (n = 1313) [ClinicalTrials.gov identifier: NCT01825200]. Among subjects 50 through 64 years of age, 672 received Flublok and 665 received Afluria. Among subjects aged 65 years and older, 642 received Flublok and 648 received Afluria.
In all studies, a series of symptoms and findings were specifically solicited by a memory aid used by subjects for the 7-day period following vaccination. All studies collected spontaneous reports of adverse events for 28 days following vaccination (see below) and subjects were actively queried about changes in their health status 6 months after vaccination for studies PSC01 and PSC03. The frequency of solicited local injection site reactions and systemic adverse reactions within 7 days of administration of Flublok or comparator vaccine are shown in Tables 2 and 3 for adults aged 50–64 and adults aged 65 and older, respectively.
Table 2.
Frequency of solicited local injection site reactions and systemic adverse reactions within 7 days of administration of Flublok or comparator in adults 50–64 years of age, studies PSC06 and PSC11, total vaccinated cohort*.
| Flublok N = 972 |
IIV3*
N = 967 |
|||||
|---|---|---|---|---|---|---|
| Any | Mod$ | Sev$ | Any | Mod$ | Sev$ | |
| Local | % | |||||
| Pain | 32 | 2 | <1 | 37 | <1 | 0 |
| Firmness/swelling | 7 | 2 | <1 | 6 | 1 | <1 |
| Redness | 6 | 2 | <1 | 5 | 1 | <1 |
| Systemic | % | |||||
| Headache | 17 | 4 | <1 | 16 | 3 | <1 |
| Fatigue | 13 | 3 | <1 | 17 | 3 | <1 |
| Muscle pain | 11 | 2 | <1 | 11 | 2 | <1 |
| Joint pain | 8 | 2 | <1 | 8 | 2 | <1 |
| Nausea | 6 | 1 | 0 | 5 | <1 | <1 |
| Shivers/chills | 5 | 1 | 0 | 4 | <1 | <1 |
| Fever‡ | <1 | <1 | <1 | <1 | 0 | 0 |
Data based on the most severe response reported by subjects. Results of at least 1% are reported to nearest whole percent; results greater than 0 but less than 1% reported as less than 1%.
Data from studies PSC06 and PSC11 were pooled. For studies PSC06 and PSC11, the US-licensed IIV3 comparators were Fluzone and Afluria, respectively.
Moderate (Mod) = had it, and it was bad enough to prevent a significant part of usual activities; severe (Sev) = had it, and it prevented most or all of normal activities, or had to see a doctor for prescription medicine.
Fever defined as at least 100.4°F (38°C): mild (⩾100.4° to <101.1°F); moderate (⩾101.2°F to <102.2°F); severe (⩾102.2°F). For fever, data for 12 Flublok recipients and 5 IIV3 recipients were missing data, making these denominators 964 and 962, respectively.
Table 3.
Frequency of solicited local injection site reactions and systemic adverse reactions within 7 days of administration of Flublok or comparator in adults at least 65 years of age, studies PSC03 and PSC11, total vaccinated cohort*.
| Flublok N = 1078 |
IIV31
N = 1081 |
|||||
|---|---|---|---|---|---|---|
| Any | Mod$ | Sev$ | Any | Mod$ | Sev$ | |
| Local | % | |||||
| Pain | 19 | <1 | <1 | 20 | <1 | <1 |
| Redness | 7 | 1 | <1 | 7 | 1 | 1 |
| Firmness/swelling | 7 | 2 | <1 | 7 | <1 | <1 |
| Systemic | % | |||||
| Fatigue | 13 | 3 | <1 | 15 | 2 | <1 |
| Headache | 10 | <1 | <1 | 9 | 1 | <1 |
| Muscle pain | 8 | 2 | <1 | 8 | 1 | <1 |
| Joint pain | 6 | 1 | <1 | 6 | 1 | <1 |
| Shivers/chills | 5 | <1 | <1 | 5 | <1 | <1 |
| Nausea | 4 | <1 | <1 | 3 | <1 | <1 |
| Fever‡ | 3 | <1 | <1 | 2 | 0 | 0 |
Data based on the most severe response reported by subjects. Results of at least 1% are reported to nearest whole percent; results greater than 0 but less than 1% reported as less than 1%.
Data were pooled from studies PSC03 and PSC11. For studies PSC03 and PSC011, the US-licensed IIV3 comparators were Fluzone and Afluria, respectively.
Moderate (Mod) = had it, and it was bad enough to prevent a significant part of usual activities; severe (Sev) = had it, and it prevented most or all of normal activities, or had to see a doctor for prescription medicine.
Fever defined as at least 100.4°F (38°C): mild (⩾100.4° to <101.1°F); moderate (⩾101.2°F to <102.2°F); severe (⩾102.2°F).
Among adults 18–49 years of age (studies PSC01 and PSC04 pooled), through 6 months post vaccination, two deaths were reported, one in a Flublok recipient and one in a placebo recipient. Both deaths occurred more than 28 days following vaccination and neither were considered vaccine related. Serious adverse events (SAEs) were reported by 32 Flublok recipients and 35 placebo recipients. One SAE in a Flublok recipient was assessed as possibly related to the vaccine: pleuropericarditis with effusions requiring hospitalization and drainage. No specific cause was identified. The patient recovered without sequelae.
Among adults 50–64 years of age (studies PSC06 and PSC11 pooled), through up to 6 months post vaccination, there were no deaths; SAEs were reported by 10 subjects, six Flublok recipients and four IIV3 recipients. Vasovagal syncope following injection of Flublok was an SAE considered related to study vaccine, although likely due to the injection procedure rather than the vaccine material. Among adults 65 years of age and older (studies PSC03 and PSC11 pooled), through up to 6 months post vaccination, there were four deaths, two in Flublok recipients and two in IIV3 recipients. None were considered related to the study vaccines. SAEs were reported from 80 subjects, 37 Flublok recipients and 43 IIV3 recipients. None were considered related to the study vaccines.
In study PSC04 (adults 18–49 years of age), the most frequent unsolicited adverse events, occurring in 1–2% of subjects, were nasopharyngitis, upper respiratory infection, headache, cough, nasal congestion, pharyngolaryngeal pain, and rhinorrhea.
Among adults 50–64 years of age (studies PSC06 and PSC11 pooled), the most frequent unsolicited adverse events, occurring in 1% of subjects, were diarrhea and cough. Among adults at least 65 years of age (studies PSC03 and PSC11 pooled), the most frequent unsolicited adverse events, occurring in 1% of subjects, were nasopharyngitis and cough.
Among adults 50 years of age and older (study PSC11) for whom the incidence of rash, urticaria, swelling, nonpitting edema, or other potential hypersensitivity reactions were actively solicited for 30 days following vaccination, a total of 2.4% of Flublok recipients and 1.6% of IIV3 recipients reported such events over the 30-day follow-up period. A total of 1.9% and 0.9% of Flublok and IIV3 recipients, respectively, reported these events in the 7 days following vaccination. Of these solicited events, rash was most frequently reported (Flublok 1.3%, IIV3 0.8%) over the 30-day follow-up period. The events adjudicated by independent experts to be likely hypersensitivity reactions were reported from 0.5% and 0.3% of Flublok and IIV3 recipients, respectively.
We compared the incidence of solicited adverse events following Flublok, Fluzone and Fluzone HD from Flublok clinical trials and from the Fluzone package insert [Sanofi-Pasteur, 2014] to evaluate the possible impact of the threefold higher content of HA in Flublok on reactogenicity (Table 4). Solicited adverse events in Flublok were generally similar to Fluzone with the exception of headache, with is slightly more common in Flublok recipients, whereas all solicited adverse events were more common in Fluzone HD recipients.
Table 4.
Solicited adverse events in the first 7 days after administration of Flublok, Fluzone or Fluzone HD.
|
Study PSC03 |
Sanofi PI high dose* |
|||
|---|---|---|---|---|
| Adults aged ⩾ 65 years |
Adults aged ⩾ 65 years |
|||
| Flublok | Fluzone | Fluzone | Fluzone HD | |
| Number of subjects | 436 | 433 | 2569–2572 | 1258–1260 |
| Local adverse events | ||||
| Pain | 22% | 23% | 24% | 36% |
| RR (95% CI) | 0.95 (0.75–1.23) | 1.50 (1.36–1.66) | ||
| Redness | 10% | 12% | 11% | 15% |
| RR (95% CI) | 0.83 (0.58–1.23) | 1.36 (1.15–1.62) | ||
| Swelling | 11% | 13% | 6% | 9% |
| RR (95% CI) | 0.85 (0.59–1.22) | 1.50 (1.19–1.89) | ||
| Systemic adverse events | ||||
| Headache | 11% | 9% | 14% | 18% |
| RR (95% CI) | 1.22 (0.82–1.83) | 1.28 (1.10–1.49) | ||
| Fatigue versus malaise | 9% | 10% | 14% | 18% |
| RR (95% CI) | 0.90 (0.60–1.36) | 1.28 (1.10–1.49) | ||
| Muscle + joint pain versus myalgia | 12% | 15% | 18% | 21% |
| RR (95% CI) | 0.80 (0.57–1.12) | 1.16 (1.02–1.34) | ||
Data derived from clinical study PSC03 [Keitel et al. 2010] and Fluzone high-dose package insert [Sanofi-Pasteur, 2014].
Relative risk (RR) was calculated using https://www.medcalc.net/tests/relative_risk.php
CI, confidence interval.
Pain at the injection site is statistically significantly lower for Flublok in comparison to Fluzone HD.
Flublok efficacy against culture-confirmed influenza
The efficacy of Flublok was evaluated in study PSC04, a randomized, observer-blind, placebo-controlled multicenter trial conducted in the US during the 2007–2008 influenza season. In this study 4648 healthy adults (mean age 32.5 years) were randomized in a 1:1 ratio to receive a single dose of Flublok (n = 2344) or saline placebo (n = 2304). The two groups were similar in demographics. Culture-confirmed influenza was assessed by active and passive surveillance for influenza-like illness (ILI) beginning 2 weeks post vaccination until the end of the influenza season, approximately 7 months post vaccination. ILI was defined as having at least two of three symptoms (no specified duration) in the following categories: fever at least 100°F; respiratory symptoms (cough, sore throat, runny nose/stuffy nose); or systemic symptoms (myalgias, arthralgias, headache, chills/sweats, tiredness/malaise). For subjects with an episode of ILI, nasal and throat swab samples were collected for viral culture. Most of the influenza isolates obtained from subjects in this study were not antigenically matched to the strains represented in the vaccine. The VE of Flublok against all strains isolated from any subject with an ILI regardless of antigenic match, not necessarily CDC-defined ILI, demonstrated an efficacy estimate of 44.8% (95% CI 24.4–60.0). Thus, Flublok is effective in the prevention of influenza illness and provides protection against drift variants [Treanor et al. 2011; Cox and Anderson 2007]. The results of this study are summarized in Table 5 for a presentation of VE by case definition and antigenic similarity.
Table 5.
Vaccine efficacy against culture-confirmed influenza in healthy adults 18–49 years of age.
| Case definition | Flublok (N = 2344) |
Saline Placebo (N = 2304) |
Flublok vaccine efficacy*, % | 95% confidence interval | ||
|---|---|---|---|---|---|---|
| Cases, n | Rate, % | Cases, n | Rate, % | |||
| Positive culture with a strain represented in the vaccine | ||||||
| CDC-ILI, all matched strains$ | 1 | 0.04 | 4 | 0.2 | 75.4 | (−148.0, 99.5) |
| Any ILI, all matched strains‡ | 2 | 0.1 | 6 | 0.3 | 67.2 | (−83.2, 96.8) |
| Positive culture with any strain, regardless of match to the vaccine | ||||||
| CDC-ILI, all strains$ | 44 | 1.9 | 78 | 3.4 | 44.6 | (18.8–62.6) |
| Subtype A | 26 | 1.1 | 56 | 2.4 | 54.4 | (26.1–72.5) |
| Type B | 18 | 0.8 | 23 | 1.0 | 23.1 | (−49.0, 60.9) |
| Any ILI, all strains‡ | 64 | 2.7 | 114 | 4.9 | 44.8 | (24.4–60.0) |
| Subtype A | 41 | 1.7 | 79 | 3.4 | 49.0 | (24.7–65.9) |
| Type B | 23 | 1.0 | 36 | 1.6 | 37.2 | (−8.9, 64.5) |
Determined under the assumption of Poisson event rates, according to Breslow and Day [1987].
Meets Centers for Disease Control (CDC) influenza-like illness (ILI) defined as fever of at least 100°F oral accompanied by cough or sore throat, on the same day or on consecutive days.
All culture-confirmed cases are considered, regardless of whether they qualified as CDC-ILI.
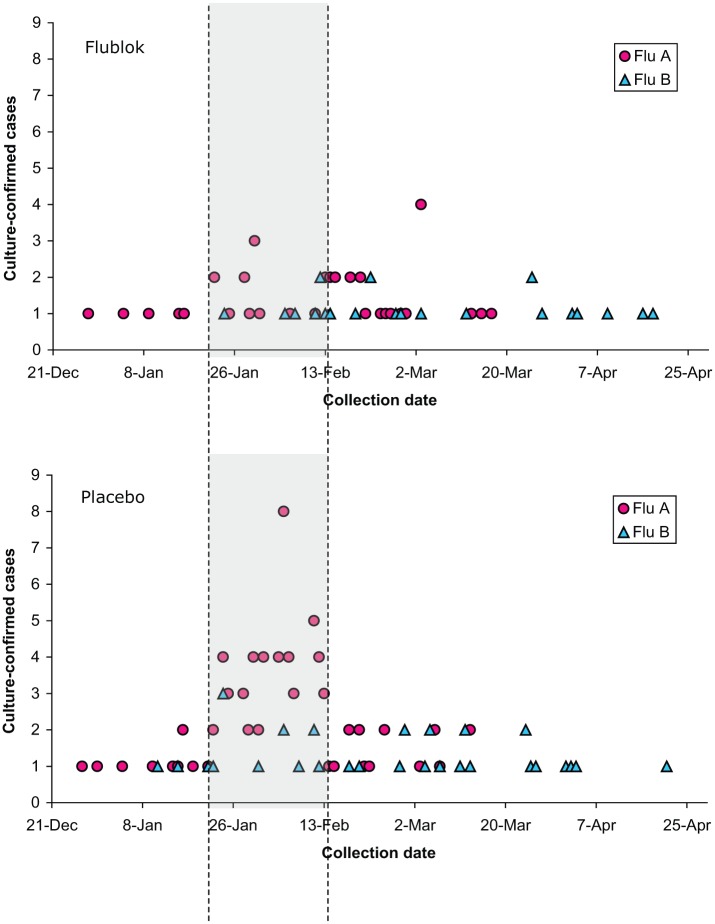
As shown in Figure 1, the majority of influenza isolates were obtained in the period mid January through early February. In the period before and including 8 February, a total of 67 influenza A isolates were obtained, of which 51 were isolated from placebo recipients and 16 from Flublok recipients, resulting in a VE of 69.2% [95% confidence interval (CI) 45.0–83.6]. In the period after 8 February 2008, another 52 influenza A isolates were obtained, 27 in placebo and 25 in Flublok, resulting in an efficacy of only 9% (95% CI −62.9, 49.3). A similar analysis for B isolates showed that in the period before and including 8 February, a total of 14 influenza B isolates were obtained, of which 11 were isolated from placebo recipients and 3 from Flublok recipients, resulting in an efficacy of 73.2% (95% CI −1.5, 95.2). In the period after 8 February 2008, 45 influenza B isolates were obtained, 25 in placebo and 20 in Flublok, resulting in an efficacy of 21.4% (95% CI −47.5, 58.6). The decrease in VE over time does not appear to coincide with an increase in drift viruses.
Figure 1.
Influenza isolates shown by collection date.
Additional limited efficacy data available from the other studies suggest that the higher antigen content in Flublok may contribute to improved protection. In study PSC01, culture-confirmed influenza infection was documented in four subjects receiving the lower dose (3%), one subject in the Flublok group (1%), and eight subjects in the placebo group (5%). The protective efficacy against all cases of culture-confirmed, symptomatic infection (regardless of whether the subject met the case definition of CDC-ILI) was 49.0% (95% CI −90.4, 88.8) for the low dose and 87.3% (95% CI 5.5–99.7) for Flublok. Two culture-positive subjects (1%) who received the low-dose formulation and seven subjects (5%) who received placebo met the case definition for CDC-ILI. There were no cases of culture-confirmed CDC-ILI among subjects vaccinated with Flublok. The protective efficacy against culture-confirmed CDC-ILI was 70.9% (95% CI −53.1, 97.0) for the low dose and 100% (95% CI 29.7–100) for Flublok 135 μg. Fisher’s exact test showed a statistically significant reduction in culture-confirmed CDC-ILI between subjects who received Flublok (versus placebo; p = 0.0146). In PSC03, only three cases of culture-confirmed CDC-ILI symptoms occurred: one in the Flublok group and two in the Fluzone group. In PSC06, 34 subjects reported ILI: 14 (5%) in the Flublok group and 20 (7%) in the Fluzone group. Unfortunately, viral cultures were not obtained uniformly from symptomatic subjects, so the incidence of culture-confirmed CDC-ILI cases could not be assessed.
Flublok immunogenicity results
In studies PSC01, PSC03, PSC04 (subset) and PSC06, HAI antibody titers to each virus strain represented in the vaccine were measured in sera obtained ~28 days after vaccination. Analysis of endpoints was performed for each HA contained in the vaccine, active control, or placebo according to the criteria specified in the FDA Guidance for Industry [FDA, 2007].
Across all studies, serum HAI antibody responses to Flublok usually met the prespecified seroconversion criteria for all three virus strains, and also the prespecified criterion for the proportion of subjects with HAI titers at least 1:40 (seroprotection). This data were reviewed and reported in detail elsewhere [Cox et al., 2008]. Here we compare the data obtained for Flublok in study PSC03 with the Sanofi high-dose (HD) influenza vaccine. As shown in Table 6, the immunogenicity of Flublok exceeds the immunogenicity of Fluzone for the influenza A viruses, but not for the influenza B viruses. Fluzone HD is always more immunogenic than Fluzone. The immunogenicity for the H3 influenza virus is similar between Flublok and Fluzone HD.
Table 6.
Immunogenicity after administration of Flublok, Fluzone or Fluzone HD.
|
Study PSC03 |
Sanofi PI high dose* |
|||
|---|---|---|---|---|
| Adults age ⩾ 65 years |
Adults age ⩾ 65 years |
|||
| Flublok | Fluzone | Fluzone | Fluzone HD | |
| Number of subjects | 436 | 433 | 1248–1249 | 2529–2531 |
| A (H1N1) | ||||
| GMT | 177 | 148 | 67 | 116 |
| GMT ratio | 1.2 | 1.7 | ||
| Seroconversion | 43 | 33 | 23 | 49 |
| Difference in seroconversion | 10 | 25 | ||
| A (H3 N2) | ||||
| GMT | 339 | 199 | 333 | 609 |
| GMT ratio | 1.7 | 1.8 | ||
| Seroconversion | 78 | 58 | 51 | 69 |
| Difference in seroconversion | 20 | 18 | ||
| B (Note: different antigen in Flublok versus Fluzone) | ||||
| GMT | 150 | 195 | 52 | 69 |
| GMT ratio | 0.8 | 1.3 | ||
| Seroconversion | 29 | 39 | 30 | 42 |
| Difference in seroconversion | −10 | 12 | ||
Data derived from clinical study PSC03 [Keitel et al. 2010] and Fluzone high-dose package insert [Sanofi-Pasteur, 2014].
The Geometric Mean Titer (GMT) ratio presented in bold shows comparable immunogenicity between Flublok and Fluzone HD.
Discussion and conclusion
Flublok is a trivalent rHA vaccine with a mechanism of action similar to that of the licensed trivalent IIV, namely the induction of HAI antibodies to prevent influenza infection [Kida et al. 1983; Yoden et al. 1986]. The five pivotal studies support the vaccine to be safe, immunogenic and effective in the prevention of influenza. The technology used to produce Flublok offers multiple advantages including the following: the vaccine will be an exact genetic match with the disease causing influenza virus; the manufacturing time is shortened; the manufacture process does not require biocontainment as no influenza virus is used in the process; no undesirable chemicals like formaldehyde are used in the process; the endotoxin content is carefully controlled; and no ovalbumin or residual chemicals present or antibiotics are required to maintain a low bioburden during processing. The technology has been successfully scaled to the 21,000L bioreactor offering the possibility to respond to late appearing influenza viruses and deal with mismatches in the vaccine that result in poor performance as again reported for the 2014/15 influenza vaccine [D’Mello et al. 2015; Skowronski et al. 2015]. The production capacity for recombinant proteins is in principle unlimited as any cell culture facility could be used for its manufacturing. At this moment there are two licensed facilities for the manufacturing of Flublok, one in Meriden, CT, USA with a production capacity of approximately 0.5 million doses and another in Pearl River, NY, USA with a production capacity of approximately 2 million doses. The third facility that will be submitted for licensure is located in Japan and offers an immediate 20-fold increase in capacity that could be further expanded threefold. This means that with established yields a production capacity exceeds 1 billion doses of 15 μg in a 6–9 month time period.
The commercial formulation of Flublok contains three times the amount of HA compared with the standard dose IIVs and consequently induces higher antibody titers, which may be of particular importance to those most at risk for influenza (for example, the elderly [Keitel et al. 1994, 1996, 2010] or immunologically compromised [Safdar et al. 2006]). The immunogenicity results for the B/strain in the elderly study PSC03 must be interpreted cautiously in the context of a lack of a direct antigen comparison. While Flublok contains 135 μg HA per dose, the total amount of protein (HA plus host cell proteins) contained within one dose of Flublok is roughly comparable to the total amount of protein contained in Fluzone (viral plus egg protein) [Renfrey and Watts, 1994; Hehme et al. 2003]. The vaccine was shown to be well tolerated and immunogenic in adults older than 18 years. When using standard dose Fluzone as a control to normalize safety data between Flublok and Fluzone HD it is apparent that solicited adverse events in Flublok recipients are generally much less frequent than those observed with Fluzone HD (Table 4). The immunogenicity data obtained for Flublok compare favorably with standard Fluzone and are similar for the H3 component of the vaccine between Flublok and Fluzone HD. The overall efficacy of Fluzone HD is higher than standard Fluzone as around 24% of the breakthrough illness in Fluzone recipients could be prevented by administration of Fluzone HD [DiazGranados et al. 2014].
Importantly, Flublok has demonstrated protective efficacy in a field efficacy trial against drifted influenza viruses [Treanor et al. 2011; Cox and Anderson 2007]. In a post hoc analysis of this efficacy study, we noted that the efficacy of Flublok was much higher when the efficacy analysis was restricted to the same reference period (21 January–8 February 2008) used in an interim analysis for IIV, with an estimated effectiveness of 44% (95% CI 17–65%) [Belongia et al. 2009]. The observed reduction of VE for the interval after 8 February, which declined to 9.0% (95% CI −62.9%, 49.3) for type A and 21.4% (95% CI −47.5%, 58.6%) for type B could not be ascribed to the number of drift variants isolated, which was essentially equivalent in both time periods. The observed decrease in efficacy late in the influenza season remains unexplained, and it is unclear whether this is a finding that might be generally applicable or whether this was unique to this vaccine or influenza season.
In general, comparison of the Flublok efficacy results with the results of other assessments of the protective efficacy of influenza vaccines is complicated by differences in methodologies, populations, and antigenic match between vaccine and circulating strains in the specific year that studies are carried out. Two published studies have evaluated egg-grown inactivated vaccines in healthy adults using a placebo-controlled design during the 2007–2008 influenza season. In one study, conducted primarily in Europe, the overall efficacy of egg-grown inactivated vaccine against culture confirmed illness was 63%, and the lower 95% CI was 46.7% [Frey et al. 2010]. The predominant influenza A isolates in that study were H1N1 viruses, which were mostly vaccine like. In another smaller study done on college campuses in Michigan [Monto et al. 2009], the protective efficacy of TIV against culture-confirmed illness was 73% (95% CI 51–85%). In that study, 90% of influenza isolates were influenza A (H3N2), but the antigenic characterization of isolates was not reported. In other randomized trials, the protective efficacy of TIV was 22.3% in the 2005–2006 influenza season predominated by influenza B viruses and with overall low attack rates [Beran et al. 2009], and 49.3% over two seasons, 2005–2007, with most cases due to antigenically variant viruses [Jackson, 2009].
Because of the difficulty in conducting placebo-controlled studies of influenza vaccine, especially as the target groups for vaccination have expanded, several recent assessments of influenza vaccine effectiveness have utilized the test-negative, case-control design. In this approach, individuals with a compatible illness are tested by a sensitive PCR diagnostic, and the vaccine histories compared between those with a positive PCR and those with negative diagnostic testing. Vaccine effectiveness is then calculated from the risk-adjusted odds ratio of vaccination. Estimates of overall inactivated vaccine effectiveness in these studies have ranged from 10% to 70% [Belongia et al. 2009; Skowronski et al. 2007, 2009] and are clearly impacted by antigenic differences between vaccine and circulating viruses, with the highest levels of effectiveness reported for H1 viruses [Skowronski et al. 2009] and the lowest levels for influenza B.
A large comparative efficacy study in approximately 9000 adults over 50 years old with a quadrivalent formulation is in progress during the 2014–2015 season to expand on this conclusion. Data from this double-blinded efficacy study will be available at the end of the influenza season and is expected to support approval of quadrivalent Flublok in time for the 2016–2017 influenza season. Furthermore, we anticipate this study to provide important insights regarding the role of mutations in the HA protein caused by adaptation to the egg-based manufacturing process in the low effectiveness of the 2014–2015 influenza vaccine. Mutations in the active site of the HA that occurred during adaptation to growth in eggs were reported in a previous season to be responsible for the ineffectiveness of the H3N2 component of the influenza vaccine [Skowronski et al. 2014]. Moreover, it has been previously reported that changes in the HA proteins in egg-grown influenza viruses when compared with primary isolates from infected individuals can result in a less effective vaccine [Katz et al. 1987; Wang et al. 1989; Rajakumar et al. 1990].
Recent studies suggest that the efficacy of influenza vaccine diminishes as the influenza season progresses [Eick-Cost et al. 2012]. This information should warn healthcare professionals about vaccinating individuals most at risk for influenza too early in the season.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors are all employees of Protein Sciences Corp, the manufacturer of flublok.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Study PSC011 has been funded with Federal funds from the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, Department of Health and Human Services, under Contract No. HHSO100200900106C.
Contributor Information
Manon M. J. Cox, Protein Sciences Corporation, 1000 Research Parkway, Meriden, CT 06450, USA.
Ruvim Izikson, Protein Sciences Corporation, 1000 Research Parkway, Meriden, CT, USA.
Penny Post, Protein Sciences Corporation, 1000 Research Parkway, Meriden, CT, USA.
Lisa Dunkle, Protein Sciences Corporation, 1000 Research Parkway, Meriden, CT, USA.
References
- Air G., Laver W., Webster R., Els M., Luo M. (1989) Antibody recognition of the influenza virus neuraminidase. Cold Spring Harb Symp Quant Biol 54: 247–255. [DOI] [PubMed] [Google Scholar]
- Baxter R., Patriarca P., Ensor K., Izikson R., Goldenthal K., Cox M. (2011) Evaluation of the safety, reactogenicity and immunogenicity of FluBlok® trivalent recombinant baculovirus-expressed hemagglutinin influenza vaccine administered intramuscularly to healthy adults 50–64 years of age. Vaccine 29: 2272–2278. [DOI] [PubMed] [Google Scholar]
- Belongia E., Kieke B., Donahue J., Greenlee R., Balish A., Foust A., et al. (2009) Marshfield influenza study group: effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004–2005 season to the 2006–2007 season. J Infect Dis 199: 159–167. [DOI] [PubMed] [Google Scholar]
- Beran J., Wertzova V., Honegr K., Kaliskova E., Havlickova M., Havlik J., et al. (2009) Challenge of conducting a placebo-controlled randomized efficacy study for influenza vaccine in a season with low attack rate and a mismatched vaccine B strain: a concrete example. BMC Infect Dis 7: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges C., Fukuda K., Uyeki T., Cox N., Singleton J. and Centers for Disease Control (CDC): ACIP (2002) Prevention and control of influenza, recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep 51: 1–31. [PubMed] [Google Scholar]
- Cox M., Anderson D. (2007) Production of a novel influenza vaccine using insect cells: protection against drifted strains. Influenza Respir Viruses 1: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M., Hashimoto Y. (2011) A fast track influenza virus vaccine produced in insect cells. J Invert Pathol 107: S31–S41. [DOI] [PubMed] [Google Scholar]
- Cox M., Patriarca P., Treanor J. (2008) FluBlok, a recombinant hemagglutinin influenza vaccine. Influenza Respir Viruses 2: 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiazGranados C., Dunning A., Kimmel M., Kirby D., Treanor J., Collins A., et al. (2014) Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 371: 635–645. [DOI] [PubMed] [Google Scholar]
- D’Mello T., Brammer L., Blanton L., Kniss K., Smith S., Mustaquim D., et al. (2015) Update: influenza activity – United States, September 28, 2014–February 21, 2015. Morb Mortal Wkly Rep 64: 206–212. [PMC free article] [PubMed] [Google Scholar]
- Eick-Cost A., Tastad K., Guerrero A., Johns M., Lee S., Macintosh V., et al. (2012) Effectiveness of seasonal influenza vaccines against influenza-associated illnesses among US military personnel in 2010–11: a case-control approach. PLoS One 7: e41435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (2007) Guidance for Industry: Clinical Data Needed to Support the Licensure of Seasonal Inactivated Influenza Vaccines. Rockville, MD: U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research. [Google Scholar]
- Frey S., Vesikari T., Szymczakiewicz-Multanowska A., Lattanzi M., Izu A., Groth N., et al. (2010) Clinical efficacy of cell culture–derived and egg-derived inactivated subunit influenza vaccines in healthy adults. Clin Infect Dis 51: 997–1004. [DOI] [PubMed] [Google Scholar]
- Hehme N., Engelmann H., Raderecht C., Porstmann T. (2003) Comparative laboratory results of different commercial influenza vaccines. Option for the control of influenza V, Okinawa, Japan. Poster W07P-41. [Google Scholar]
- Holtz K., Anderson D., Cox M. (2003) Production of a recombinant influenza vaccine using the baculovirus expression vector system. BioProcess J 2: 65–73. [Google Scholar]
- Huang R., Rott R., Wahn K., Klenk H., Kohama T. (1980a) The function of the neuraminidase in membrane fusion induced by myxoviruses. Virology 107: 313–319. [DOI] [PubMed] [Google Scholar]
- Huang R., Wahn K., Klenk H., Rott R. (1980b) Fusion between cell membrane and liposomes containing the glycoproteins of influenza virus. Virology 104: 294–302. [DOI] [PubMed] [Google Scholar]
- Huber V., Cullers J. (2008) FluBlok, a recombinant influenza vaccine. Curr Opin Mol Ther 10: 75–85. [PubMed] [Google Scholar]
- Jackson L. (2009) Using surveillance to evaluate influenza vaccine effectiveness. J Infect Dis 199: 155–158. Erratum in: J Infect Dis 199: 917. [DOI] [PubMed] [Google Scholar]
- Katz J., Naeve C., Webster R. (1987) Host cell-mediated variation in H3N2 influenza viruses. Virology 156: 386–395. [DOI] [PubMed] [Google Scholar]
- Keitel W., Cate T., Atmar R., Turner C., Nino D., Dukes C., et al. (1996) Increasing doses of purified influenza virus hemagglutinin and subvirion vaccines enhance antibody responses in the elderly. Clin Diagn Lab Immunol 3: 507–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitel W., Couch R., Cate T., Hess K., Baxter B., Quarles J., et al. (1994) High doses of purified influenza a virus hemagglutinin significantly augment serum and nasal secretion antibody responses in healthy young adults. J Clin Microbiol 32: 2468–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitel W., Treanor J., El Sahly H., Gilbert A., Meyer A., Patriarca P., et al. (2010) Comparative immunogenicity of recombinant influenza hemagglutinin (rHA) and trivalent inactivated vaccines (TIV) among persons ⩾65 years old. Vaccine 28: 379–385. [DOI] [PubMed] [Google Scholar]
- Kida H., Webster R., Yanagawa R. (1983) Inhibition of virus-induced hemolysis with monoclonal antibodies to different antigenic areas on the hemagglutinin molecule of A/seal/Massachusetts/1/80 (H7N7) influenza virus. Arch Virol 76: 91–99. [DOI] [PubMed] [Google Scholar]
- Kilbourne E., Christenson W., Sande M. (1968a) Antibody response in man to influenza virus neuraminidase following influenza. J Virol 2: 761–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourne E., Laver W., Schulman J., Webster R. (1968b) Antiviral activity of antiserum specific for an influenza virus neuraminidase. J Virol 2: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrosovich M., Matrosovich T., Gray T., Roberts N., Klenk H. (2004) Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J Virol 78: 12665–12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto A., Ohmit S., Petrie J., Johnson E., Trucson R., Teich E., et al. (2009) Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med 361: 1260–1267. [DOI] [PubMed] [Google Scholar]
- Rajakumar A., Swierkosz E., Schulze I. (1990) Sequence of an influenza virus hemagglutinin determined directly from a clinical sample. Proc Natl Acad Sci USA 87: 4154–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfrey S., Watts A. (1994) Morphological and biochemical characterization of influenza vaccines commercially available in the United Kingdom. Vaccine 12: 747–752. [DOI] [PubMed] [Google Scholar]
- Safdar A., Rodriguez M., Fayad L., Rodriguez G., Pro B., Wang M., et al. (2006) Dose-related safety and immunogenicity of baculovirus-expressed trivalent influenza vaccine: a double-blind, controlled trial in adult patients with non-Hodgkin B cell lymphoma. J Infect Dis 194: 1394–1397. [DOI] [PubMed] [Google Scholar]
- Sanofi-Pasteur (2014) Fluzone package insert. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM356094.pdf (accessed November 2014).
- Skowronski D., Chambers C., Sabaiduc S., de Serres G., Dickinson J., Winter A., et al. (2015) Interim estimates of 2014/15 vaccine effectiveness against influenza A(H3N2) from Canada’s Sentinel Physician Surveillance Network, January 2015. Eurosurveillance 20: 1–18. [DOI] [PubMed] [Google Scholar]
- Skowronski D., de Serres G., Dickinson J., Petric M., Mak A., Fonseca K., et al. (2009) Component-specific effectiveness of trivalent influenza vaccine as monitored through a sentinel surveillance network in Canada, 2006–2007. J Infect Dis 199: 168–179. [DOI] [PubMed] [Google Scholar]
- Skowronski D., Janjua N., de Serres G., Sabaiduc S., Eshaghi A., Dickinson J., et al. (2014) Low 2012–13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS One 9: e92153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowronski D., Masaro C., Kwindt T., Mak A., Petric M., Li Y., et al. (2007) Estimating vaccine effectiveness against laboratory-confirmed influenza using a sentinel physician network: results from the 2005–2006 season of dual A and B vaccine mismatch in Canada. Vaccine 25: 2842–2851. [DOI] [PubMed] [Google Scholar]
- Treanor J., El Sahly H., King J., Graham I., Izikson I., Kohberger R., et al. (2011) Protective efficacy of a trivalent, insect cell-expressed, recombinant hemagglutinin protein vaccine (FluBlok) against culture confirmed influenza in healthy adults: a randomized, placebo-controlled trial. Vaccine 29: 7733–7739. [DOI] [PubMed] [Google Scholar]
- Treanor J., Schiff G., Couch R., Cate T., Brady R., Hay C., et al. (2006) Dose-related safety and immunogenicity of a trivalent baculovirus-expressed influenza-virus hemagglutinin vaccine in elderly adults. J Infect Dis 193: 1223–1228. [DOI] [PubMed] [Google Scholar]
- Treanor J., Schiff G., Hayden F., Brady R., Hay C., Meyer A., et al. (2007) Safety and immunogenicity of a baculovirus-expressed hemagglutinin influenza vaccine: a randomized controlled trial. J Am Med Assoc 297: 1577–1582. [DOI] [PubMed] [Google Scholar]
- Wang K., Holtz K., Anderson K., Chubet R., Mahmoud W., Cox M. (2006) Expression and purification of an influenza hemagglutinin–one step closer to a recombinant protein-based influenza vaccine. Vaccine 24: 2176–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Katz J., Webster R. (1989) Extensive heterogeneity in the hemagglutinin of egg-grown influenza viruses from different patients. Virology 171: 275–279. [DOI] [PubMed] [Google Scholar]
- Wood J. (1998) Standardization of inactivated influenza vaccine. In: Nicholson K., Webster R., Hay A. (eds), Textbook of Influenza. London, UK: Blackwell Science Ltd, pp. 333–345. [Google Scholar]
- Yoden S., Kida H., Kuwabara M., Yanagawa R., Webster R. (1986) Spin-labeling of influenza virus hemagglutinin permits analysis of the conformational change at low pH and its inhibition by antibody. Virus Res 4: 251–264. [DOI] [PubMed] [Google Scholar]