Abstract
Herpes zoster (HZ) is primarily a disease of nerve tissue but the acute and longer-term manifestations require multidisciplinary knowledge and involvement in their management. Complications may be dermatological (e.g. secondary bacterial infection), neurological (e.g. long-term pain, segmental paresis, stroke), ophthalmological (e.g. keratitis, iridocyclitis, secondary glaucoma) or visceral (e.g. pneumonia, hepatitis). The age-related increased incidence of HZ and its complications is thought to be a result of the decline in cell-mediated immunity (immunosenescence), higher incidence of comorbidities with age and social-environmental changes. Individuals who are immunocompromised as a result of disease or therapy are also at increased risk, independent of age. HZ and its complications (particularly postherpetic neuralgia) create a significant burden for the patient, carers, healthcare systems and employers. Prevention and treatment of HZ complications remain a therapeutic challenge despite recent advances. This is an overview of the multidisciplinary implications and management of HZ in which the potential contribution of vaccination to reducing the incidence HZ and its complications are also discussed.
Keywords: herpes zoster, herpes zoster diagnosis, herpes zoster treatment, herpes zoster vaccination, multidisciplinary management, postherpetic neuralgia
Epidemiology of herpes zoster and its complications
Varicella zoster virus (VZV) causes a primary infection known as varicella (chicken pox). The virus then migrates from the skin lesions via nerve axons and, probably also by viremic spread, to spinal and cranial sensory ganglia where it becomes dormant. Later in life, in some individuals the virus is reactivated (usually within a single ganglion) to cause a secondary infection known as herpes zoster (HZ; shingles). Individuals with HZ can transmit VZV to their seronegative contacts, who may develop varicella, but not HZ [Bloch and Johnson, 2012; Viner et al. 2012]. The household transmission rate of HZ (to cause varicella) is 15%, making it significantly less contagious than varicella but nevertheless of relevance to at-risk contacts [Schmid and Juuman, 2010].
Over 95% of immunocompetent individuals aged at least 50 years are seropositive for VZV and are, therefore, at risk of developing HZ. VZV-specific cell-mediated immunity declines with age concomitantly with the rise in the incidence of HZ and its complications that occurs at about 50 years of age (Figure 1) [Burke et al. 1982; Helgason et al. 2000; Tanuseputro et al. 2011; Pinchinat et al. 2013; Yawn and Gilden, 2013]. The lifetime risk of developing HZ is between 25% and 30%, rising to 50% in those aged at least 80 years [Studahl et al. 2013; Yawn and Gilden, 2013; Chen et al. 2014a ]. The estimated average overall incidence of HZ is about 3.4–4.82 per 1000 person years which increases to more than 11 per 1000 person years in those aged at least 80 years (Figure 1). In Canada, the overall incidences of medically attended HZ and HZ-related outpatient visits and hospitalizations were reported to increase with age (Figure 2) [Tanuseputro et al. 2011]. Data from a general practitioner (GP) network in France showed that 1% of patients with HZ were hospitalized and the death rate was 0.2/100,000 [Gonzalez Chiappe et al. 2010]. HZ-associated mortality is rare, with reported incidence ranging from 0 to 0.47 per 100,000 person year, and the majority of deaths occur in those aged at least 60 years [Kawai et al. 2014; Bricout et al. 2015]. However, many studies use electronic or paper death certificates which can lead to underestimations or overestimates of the true mortality rate due to infections other than HZ and noninfectious diseases, particularly in older people.
Figure 1.
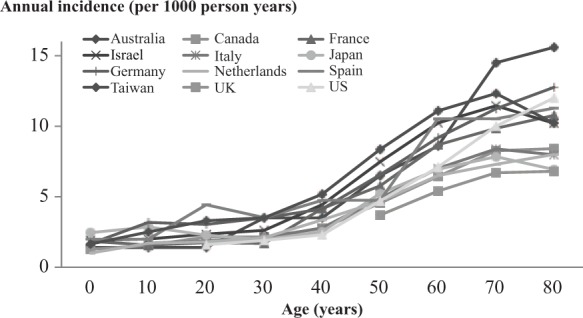
Results from 12 countries showing the risk of developing HZ increases with age, starting at about 50 years old [de Melker et al. 2006; Gauthier et al. 2009; Stein et al. 2009; Toyama and Shiraki, 2009; Gialloreti et al. 2010; Gonzalez Chiappe et al. 2010; Lin et al. 2010; Tanuseputro et al. 2011; Ultsch et al. 2013a; Weitzman et al. 2013; Yawn and Gilden, 2013; Esteban-Vasallo et al. 2014].
Figure 2.
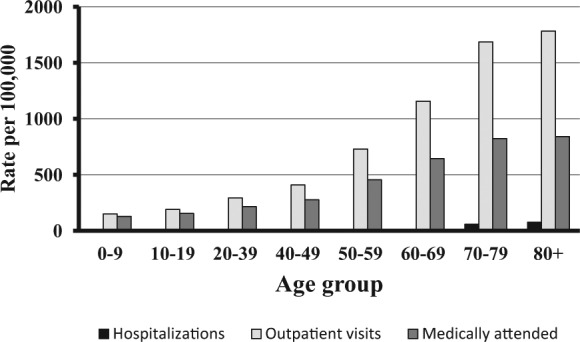
Overall incidence of herpes zoster (HZ)-related hospitalizations and outpatient visits and medically attended HZ as a function of age in Canada [Tanuseputro et al. 2011].
When reactivated, the virus travels along the affected sensory nerve, causing neuronal damage, to reach the corresponding dermatome in the skin where a vesicular rash develops. Prior to rash appearance, the frequent prodromal itching or pain can lead to erroneous and delayed diagnosis. The vesicles pustulate and then scab, usually within 2–4 weeks, but residual scarring is common. There are three phases of HZ pain: acute pain phase (up to a month), subacute pain phase (any pain 30–90 days after rash healing) and post herpetic neuralgia (PHN) phase (pain for more than 90 days after the onset of rash). Acute pain, largely inflammatory, may progress to persistent neuropathic pain resulting from peripheral and central nerve damage and secondary sensitization.
Postherpetic neuralgia
Pain present for 90 days or more after HZ rash onset is known as PHN [Sampathkumar et al. 2009]. PHN is the most common complication of HZ. Although the pain often resolves within a few weeks, it can last for several months or even years. It is a debilitating complication that is challenging to treat and is responsible for most of the HZ-related burden of disease [Johnson and Rice, 2014].
Factors associated with the development of PHN include greater severity of acute pain, older age, greater rash severity, immuno compromised patients and restricted activities of daily living prior to HZ [Opstelten et al. 2002; Johnson, 2007; Drolet et al. 2010; O’Connor and Paauw, 2013; Johnson and Rice, 2014]. Prodromal pain, female sex, diabetes and presence of herpes zoster ophthalmicus (HZO) have also been reported to play a role [Gnann and Whitley, 2002; Johnson and Rice, 2014].
Other complications
Other complications, both in the primarily affected dermatome and elsewhere, may occur, although these are less common [Oxman, 2000; Harpaz et al. 2008; Drolet et al. 2013; Kawai et al. 2014]. Dermatomal complications include secondary bacterial infections, ophthalmic complications and various degrees of segmental paresis, which can affect extraocular muscles, limbs, abdominal wall or diaphragm, depending on the dermatome involved.
Viral reactivation in the first branch of the trigeminal nerve (i.e. the ophthalmic or V1 division) results in HZO [Ragozzino et al. 1982; Arvin, 1996; Gnann and Whitley, 2002; Wang et al. 2005]. HZO accounts for about 10–20% of cases of HZ. The lifetime risk of developing HZO is approximately 1% [Liesegang, 2008]. Ocular complications typical of HZO can occur even when there is no visible rash (i.e. zoster sine herpete) [Liesegang, 2008]. Polymerase chain reaction (PCR) analysis has shown that VZV may be present in the cornea and tears, even in the absence of ocular symptoms or skin rash [Pitkaranta et al. 2000; van Gelderen et al. 2000]. Hence VZV reactivation in the ocular neuronal pathways is frequently misdiagnosed and, therefore, its incidence underestimated. Between 20% and 70% of patients with HZO develop complications that can include blepharitis, keratoconjunctivitis, iritis, scleritis and acute retinal necrosis. Neurological complications are less frequent than ocular complications and may include ophthalmoplegia, optic neuritis and ptosis. Patients presenting with HZO should be treated with oral antiviral drugs, preferably within 72 h after rash onset; however, if new lesions are present, treatment may still be useful after this time [Cobo et al. 1986; Hoang-Xuan et al. 1992; Severson et al. 2003; Liesegang, 2004]. Treatment is usually for 7 days; there is no evidence that longer treatment is beneficial, except in older or immunocompromised patients in whom viral activity has been shown to be ongoing for up to 34 days [Harding, 1993; Wenkel et al. 1998; Zaal et al. 2001; Liesegang, 2008; Hu et al. 2010]. Despite antiviral treatment, chronic disease can persist in 30% of patients with HZO, increasing to 70% in patients over 80 years old. A prominent cause of persistent or chronic disease is vasculitis, perivasculitis, neuritis and perineuritis; the neuronal damage begins before the characteristic dermatomal rash appears and therefore before antiviral treatment is initiated [Liesegang, 2008].
Disseminated zoster occurs mainly in immunocompromised patients and, in the case of visceral location, can lead to pneumonia, encephalitis (with associated cognitive impairment and sensory or motor deficits) and hepatitis with a 5–10% fatality rate, despite antiviral drug treatment [De Broucker et al. 2012]. Primary VZV encephalitis is not necessarily associated with immunodeficiency. In a French study, HZ encephalitis was identified as the second most frequent cause of death (15%) after herpes simplex virus (HSV) [Mailles and Stahl, 2009].
Dermatological complications that occur include disseminated HZ usually associated with immune suppression and characterized by vesicles spreading beyond the distribution of the affected dermatome, with the potential to affect other organs (e.g. encephalitis) making the condition potentially lethal [Rommelaere et al. 2012]. Others include hemorrhagic HZ, a rare condition also correlated with immune suppression, coagulopathy, thrombocytopenia and Ramsay Hunt syndrome, defined as peripheral facial nerve palsy accompanied by an erythematous vesicular rash in the ear (zoster oticus), resulting from involvement of the geniculate ganglion by HZ reactivation. Chronic varicella zoster is defined as an atypical mucocutaneous wart-like or ulcerative VZV infection, persisting for at least 1 month, mainly occurring in patients who are HIV positive [Wauters et al. 2012].
Patients with cancers and other diseases that alter immune function, such as autoimmune inflammatory rheumatic disease (AIRD: rheumatoid arthritis, systemic lupus erythematosus, granulomatosis with polyangiitis) and patients taking immunosuppressants or chemotherapy have a higher risk of HZ [Smitten et al. 2007; Hata et al. 2011; Yawn et al. 2011; Blank et al. 2012; Habel et al. 2013; Long et al. 2013; Chen et al. 2014b; Forbes et al. 2014]. It has been suggested that in patients with AIRD the drugs used for treatment (e.g. steroids, antitumor necrosis factor α agents and some disease-modifying drugs) add to the risk [Winthrop et al. 2013; Che et al. 2014]. When HZ is diagnosed, the possibility of stopping immunosuppressive drugs should be considered. Patients who are HIV positive with low CD4 counts also have a higher risk than the general population; the lower the count, the greater the risk [Blank et al. 2012; Shearer et al. 2014]. In addition, in the first 3 months of HIV treatment there is a higher incidence of HZ, probably as a consequence of immune activation by antiretroviral drug treatment. HZ incidence is lower in patients receiving Highly-active antiretroviral treatment (HAART) but it remains higher than the general population [Blank et al. 2012; Shearer et al. 2014].
HZ and its complications have been shown to have an adverse impact on quality of life (QoL) and activities of daily living. Some patients, particularly older patients, lose their independence and many patients require help from their families or paid caregivers [Weinke et al. 2010, 2014; Bouhassira et al. 2012; Gater et al. 2014]. In particular, sleep and social activities are most severely affected. Although individuals can recover some of their loss of independence, they rarely recover to the same level as they had before the HZ episode [McElhaney, 2010]. People who have had HZ or know people who have had HZ are more aware of the risks, associated pain, consequences and impact on QoL of the disease [Álvarez-Pasquín et al. 2011; Mortensen, 2011]. An individual’s attitude towards HZ prevention is related to their knowledge of HZ and views on vaccination in general.
Impact of varicella vaccination on HZ epidemiology
Hope-Simpson was the first to postulate that re-exposure to circulating VZV, a phenomenon known as exogenous boosting, could prevent reactivation of VZV [Hope-Simpson, 1965; Arvin, 1996; Brisson et al. 2002]. Vaccination of children against varicella, which is established in some countries, could have two effects on the epidemiology of HZ. The first is that there will be fewer adults carrying dormant wild type VZV as vaccinated children grow older, thus reducing the incidence of HZ. The second is that, in the shorter term, adults with dormant VZV will have less contact with children with varicella and therefore less opportunity for exogenous boosting [Ogunjimi et al. 2013]. Since exogenous boosting is thought to inhibit VZV reactivation, this could result in a temporary increased incidence of HZ and could reduce the age of HZ onset. Mathematical models predict that this increased incidence could last for more than 30 years [Schuette and Hethcote, 1999].
Since the introduction of widespread childhood varicella vaccination, no impact has been observed on the incidence or age distribution of HZ [Tanuseputro et al. 2011]. Other studies have reported an increasing trend in the general population as well as in immunocompromised populations, but this trend preceded the implementation of childhood varicella vaccination [Leung et al. 2011; Hales et al. 2013]. Long-term surveillance will be necessary to establish if there will be an increased incidence of HZ [Leung et al. 2011].
Diagnosis
HZ is generally diagnosed clinically, once the rash has appeared. However, prior to the rash occurring and for atypical cases, diagnosis can require laboratory confirmation, using PCR analysis which can detect VZV DNA rapidly and accurately [Schmader, 2006; Cohen, 2013]. HZ can sometimes be confused with HSV or a number of other conditions.
Treatment options
The management of HZ with antiviral drugs and analgesics frequently reduces acute rash and pain and may prevent some complications [Johnson et al. 2010; Cohen, 2013]. Antiviral drugs have been shown to reduce acute pain and rash severity, accelerate rash resolution and reduce duration of pain. However, many patients suffer from PHN despite antiviral drug use [Rabaud et al. 2013; Li et al. 2014]. The prodrugs valacilovir and famciclovir have a more convenient dosing schedule and more constant blood concentrations than acyclovir. Paracetamol alone or in combination with a weak opioid (e.g. codeine) is frequently used as analgesia. Addition of drugs active against neuropathic pain (e.g. tricyclic antidepressants such as amitryptyline, α-2-δ ligands such as gabapentin or pregabalin, strong opioids such as oxycodone) are used for resistant pain but older adults often suffer from adverse effects. Successful PHN treatment needs to not only be effective but to have an acceptable side-effect profile. Generally, systemic drugs are poorly effective for the treatment of PHN (pain reduced by 50% in 50% of patients at best) and they have significant side effects. Topical application of lidocaine patches and treatment with 8% capsaicin patches may provide relief for some patients and avoids systemic side effects [Johnson and Rice, 2014; Finnerup et al. 2015].
Multidisciplinary perspectives of clinical manifestations and management of HZ
Patients who develop HZ can be seen by a variety of medical specialists depending on healthcare organization where they live and the evolution of their HZ episode, particularly the occurrence of complications. For example, in Italy a survey reported that HZ was managed by GPs in the majority of cases, with only 18% of cases being referred to specialists, mainly pain specialists, ophthalmologists or dermatologists (mean: 0.2 visits/patient); the number of specialist referrals increased with age [Gialloreti et al. 2010]. Only 1.3% were hospitalized; 92% of those hospitalized for HZ or PHN were over 50 years old [Gialloreti et al. 2010]. Among the 7.4% patients who developed PHN, 74% were referred to a specialist (mean: 3.5 visits/patient) and 2% were hospitalized. Costs for outpatient care from GPs and specialists and for hospitalization for HZ-related complications are high [Kawai et al. 2014].
Although individual GPs do not generally see many patients with HZ, it often affects their most vulnerable patients who are at risk of severe complications. These patients have an increased iatrogenic risk as they often take several drugs for chronic diseases and are at high risk of decompensation and functional decline [Bruckenthal and Barkin, 2013; Sacks, 2013]. Recommendations are important to provide guidance to GPs as they may have little personal experience due to the low incidence among their patients. Current guidelines recommend that antiviral drugs should not be administered beyond a 72 h window, although most state that this window can be extended when disease is severe or if there is evidence of ongoing viral activity (e.g. new lesions) and for HZO. GPs may refer patients with complications, such as HZO or PHN, to specialists.
The type of patients with HZ seen by geriatricians varies between healthcare systems. In some systems, where older people are regularly seen by geriatricians, they will see most cases, while in others, only ‘complicated’ cases will be referred to geriatricians or will be hospitalized under their care. Geriatricians are often faced with older patients who have longer and more severe HZ-associated pain and who, on average, can have about five comorbidities at 65 years increasing to almost seven at 85 years [Lopes et al. 2013; Miranda et al. 2013]. PHN and its treatment can affect the cardiovascular system (increased heart rate and blood pressure, hypercoagulation leading to an increased risk of deep vein thrombosis and pulmonary embolism), the gastrointestinal system (delayed gastric emptying and reduced bowel motility), respiratory dysfunction (leading to hypoxia and cardiac complications), retention of sodium and water (resulting in urine retention, increased cardiac workload and hypertension) [Bruckenthal et al. 2013; Lopes et al. 2013; Miranda et al. 2013; Sacks, 2013]. The immune system can also be depressed, thus predisposing patients to wound and respiratory infections. Poorly treated pain can affect QoL, interfering with sleep, diet and exercise, increase anxiety and give rise to cognitive impairment (disorientation, confusion, concentration difficulties) [Johnson et al. 2010; Pickering and Leplege 2011; Pickering et al. 2014a, 2014b].
Infectious disease specialists can advise GPs, treat severe cases (e.g. neurological manifestations), complications and recurrences. They are also involved in prevention (prophylaxis, preorgan transplantation consultation, risk of transmission). They are solicited for preventive measures for immunocompromised patients, usually on a case-by-case basis. They generally see ‘complicated’ HZ as only severe acute cases, complicated cases and patients with debilitating PHN are hospitalized in infectious disease units. Rheumatologists should be aware that their patients with AIRD have a higher risk of HZ and complications, and should interrupt immunosuppressive drugs when HZ is diagnosed.
Patients can be referred to pain specialists and dermatologists to seek advice on the most effective management of pain and dermatologic complications when they occur. An ophthalmic examination should be offered to patients who present with at least one obvious ocular symptom (ocular redness or pain, blurred vision), even if HZO occurred several weeks previously, since inflammatory complications are frequently delayed [Harding et al. 1987]. Patients with Hutchinson’s sign, defined as skin lesions at the tip or side of the nose (nasociliary skin lesions) should also be checked for intraocular lesions (incidence rate of 80% versus 50% in the absence of Hutchinson’s sign) [Ragozzino et al. 1982; Yamada et al. 1990; Zaal et al. 2003; Opstelten and Zaal, 2005]. The risk of necrotizing retinitis, a sight-threatening complication, is common in immunocompromised patients with HZO [Batisse et al. 1996; Vafai and Berger, 2001].
Prevention of HZ
Prevention of HZ and its complications by vaccination would improve the life of older people and also reduce the societal impact of HZ [Gater et al. 2014]. A live-attenuated VZV vaccine against HZ (Zostavax (manufactured by Merck & Co., Inc, NJ, USA and commercialized in Europe by Sanofi Pasteur MSD, Lyon, France)), which has been licensed in a number of countries worldwide for administration to adults aged over 50 years, has been shown to reduce the incidence of HZ, PHN and other complications in immunocompetent adults (Table 1) [Oxman et al. 2005; Schmader et al. 2012]. Although live-attenuated vaccines are not generally recommended for immunocompromised individuals, in 2012 the Canadian National Advisory Committee on Immunization stated that individuals on low-dose immunosuppressive therapy, including anti-TNF biologics, could receive the HZ vaccine, after review with an expert in immunodeficiency, on a case by case basis [Public Health Agency of Canada, 2014]. Vaccination recommendations should target at-risk groups, including older individuals, since age is a known risk factor. In addition, there is no way to predict which patients will develop HZ, when and how severe the disease will be [Harpaz et al. 2008; Oxman, 2009; Weaver, 2009]. However, individuals need to be adequately informed about the disease and its complications to enable them to make a personal risk assessment, in consultation with their GP, before deciding to accept HZ vaccination.
Table 1.
Summary of vaccine efficacy and 95% confidence intervals from two pivotal efficacy studies by age: Zostavax Efficacy and Safety Trial (ZEST) in individuals aged 50–59 years and Shingles Prevention Study (SPS) in individuals aged ⩾60 years.
| ZEST |
SPS |
|||||
|---|---|---|---|---|---|---|
| 50–59 years | ⩾60 years | 60–69 years | ⩾70 years | 70–79 years | ⩾80 years | |
| Vaccinated/placebo N | 11,211/11,228 | 19,254/19,247 | 10,370/10,356 | 8884/8891 | 7621/7759 | 1263/1332 |
| HZ incidence | 70% (54%; 81%) | 51% (44%; 58%) | 64% (56%; 71%) | 38% (25%; 48%) | 41% (28%; 52%) | 18% (<0%; 48%) |
| PHN incidence | Not evaluated | 67% (48%; 79%) | 66% (20%; 37%) | 67% (43%; 81%) | 74% (49%; 87%) | 40% (<0%; 67%) |
| % PHN among HZ | Not evaluated | 39% (7%; 59%) | 5% (<0%; 56%) | 47% (13%; 67%) | 55% (18%; 52%) | 26% (<0%; 68%) |
| BOI* | 73% (53%; 85%) | 61% (51%; 69%) | 66% (52%; 76%) | 55% (40%; 67%) | 59% (43%; 71%) | 38% (<0%; 67%) |
BOI: burden of illness; incidence, severity and duration of acute and chronic HZ-associated pain. In ZEST D0-D21, in SPS D0-D182.
HZ, herpes zoster; PHN, postherpetic neuralgia.
HZ vaccination reduces the burden of HZ-related interference with daily life activities in vaccinated subjects, particularly older people, who develop HZ. The vaccine attenuates the severity of HZ and PHN when they occur in individuals of all ages, and thus contributes to a reduction of disease burden. The vaccine has been shown to be effective for reducing the risk of HZO and other complications in those aged over 60 and if HZO occurs, despite vaccination, the risk of PHN is significantly lower [Oxman et al. 2005; Gelb, 2008; Tseng et al. 2011].
HZ vaccination does not induce herd protection (as HZ is not transmitted between individuals) and protection may persist for up to 10 years, although efficacy decreases with the recipients’ age. After its introduction in the US, HZ vaccination has been reported to be associated with lower HZ incidence in vaccinated individuals [Tseng et al. 2011; Zhang et al. 2012; Langan et al. 2013].
Age at vaccination, duration of vaccine protection and vaccine efficacy against PHN are all important variables for determining vaccine cost effectiveness. The results from modeling studies performed with European data suggest that the optimal age for HZ vaccination is 65 or 70 years (Table 2) [van Hoek et al. 2009; Annemans et al. 2010; Moore et al. 2010; van Lier et al. 2010; Szucs et al. 2011; Bresse et al. 2013; de Boer et al. 2013; Ultsch et al. 2013b]. Cost effectiveness decreases after this age because vaccine efficacy declines with age and life expectancy is shorter. Cost effectiveness is also reduced for vaccination before this age due to uncertainties about the duration of vaccine protection. Therefore, in the light of current evidence, HZ vaccination offers significant clinical benefit to older adults and economic benefit to healthcare systems.
Table 2.
Summary of results from cost-effectiveness studies of herpes zoster vaccination in Europe.
| Study reference | Year of publication | Country | Perspective | Age of vaccination | Incremental cost effectiveness/QALY |
|---|---|---|---|---|---|
| Van Hoek et al. [2009] | 2006 | England and Wales | Provider | 65 years | £20,412 |
| Moore et al. [2010] | 2006 | United Kingdom | Society | ⩾50 years | £11,417 |
| ⩾65–69 years | £10,033 | ||||
| Provider | ⩾50 years | £13,077 | |||
| ⩾65–69 years | £10,275 | ||||
| Annemans et al. [2010] | 2007 | Belgium | Society | ⩾50 years | €7137 |
| Provider | ⩾60 years | €6799 | |||
| Van Lier et al. [2010] | 2008 | The Netherlands | Society | 60 years | €38,519 |
| Provider | 70 years | €21,716 | |||
| Szucs et al. [2011] | 2011 | Switzerland | Society | 70–79 years | CHF28,544 |
| Provider | 70–79 years | CHF25,528 | |||
| De Boer et al. [2013] | 2013 | The Netherlands | Society | 60 years | €35,555 |
| 70 years | €29,664 | ||||
| Ultsch et al. [2013b] | 2013 | Germany | Society | 60 years | €30,212 |
| Provider | 60 years | €28,146 | |||
| Bresse et al. [2013] | 2013 | France | Provider | 70–79 years | €14,198 |
QALY, quality-adjusted life year.
Attitudes and knowledge about HZ disease and vaccination
The results from a survey carried out on the general public in Spain showed that 75% of those questioned knew what shingles was but only 10% knew that a vaccine exists [Álvarez-Pasquín et al. 2011]. Other surveys have shown knowledge that long-term pain may follow HZ is low, although those who had a close friend or relative who had suffered from HZ were more aware [Paek and Johnson, 2010].
Two-thirds of healthcare workers considered HZ vaccination to be an important clinical priority but half reported that the vast majority remained unvaccinated [Paek and Johnson, 2010]. Half of patients questioned stated that they would have the HZ vaccine if it was recommended by their GP and a third would if it was recommended in the media. The main reasons for not getting vaccinated were that patients did not know that the vaccine existed, did not consider themselves to be at risk and did not know the cost of vaccination [Paek and Johnson, 2010]. HZ vaccine is mainly prescribed by GPs but when prescribed by a specialist, the patients generally said that they wanted to discuss it with their GP first. Therefore, GPs need to be informed about HZ and the efficacy and safety of the vaccine so that they can effectively recommend it and be able to help the patient to evaluate the risks and benefits.
Remaining challenges
The remaining challenges for HZ management include improving knowledge and awareness of physicians and the general public about HZ, establishing programs for HZ prevention and attenuation by vaccination, and improving treatments for HZ and its complications. In particular, prompt consultation and diagnosis would allow treatment to be initiated earlier with improved antiviral drugs to limit neuronal damage. Additionally, more effective treatments for PHN with fewer adverse effects are needed, particularly for older people. Moreover, there is a need for evidence identifying which categories of immunocompromised patients can safely and effectively receive the currently available live-attenuated HZ vaccine and for development of other vaccines to address the needs of this population. The duration of protection afforded by HZ vaccine needs to be established and vaccination programs should take this into account.
Conclusion
HZ is a common disease with the highest burden in older adults who frequently have at least one chronic disease. The risk of drug–drug interactions is higher in this population when treatment for HZ and PHN is required, making management challenging, with the risk of unsatisfactory pain relief, adverse events, decompensation of comorbidities and functional decline. HZ and its complications represent a significant burden on the patients, caregivers, the healthcare economy and employers. Acute disease treatment does not significantly prevent the most common long-term complication, PHN. Treatments for PHN that provide higher levels of pain relief with fewer adverse effects are needed. Less common, but often serious, ophthalmic locations (HZO) and related complications can have permanent detrimental effects.
Future goals include developing new vaccines for specific groups, such as immunocompromised individuals, and developing an evidence base on which type of immunocompromised individuals can be safely and effectively vaccinated with a live-attenuated vaccine. Prevention and attenuation of HZ and its complications are now feasible for most patients and a rational and economically acceptable integrated vaccination policy is a desirable goal. Improved knowledge about HZ and its complications for the public and physicians is needed so that individuals can make informed decisions about HZ vaccination.
Acknowledgments
We are grateful to 3E Pharma Consultancy and Margaret Haugh (MediCom Consult) for their valuable support during the preparation and finalization of this manuscript and to Sanofi Pasteur MSD for their financial support for these activities and for the organization of the related meeting.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors received writing assistance from 3E Pharma Consultancy and MediCom Consult funded by Sanofi Pasteur MSD. Robert W. Johnson (lead author): has consulted and lectured for Sanofi Pasteur MSD and consulted for GSK, received writing assistance from 3E Pharma Consultancy and MediCom Consult and received travel expenses and subsistence to attend a meeting with the authors of this paper but no honorarium or consultancy fee was received. Marie-José Alvarez-Pasquin: received fees for participation at a workshop as an expert from Sanofi Pasteur MSD. Marc Bijl: received fees for participation at a workshop as an expert from Sanofi Pasteur MSD, a research grant from Sanofi Pasteur MSD. Elisabetta Franco: has consulted and lectured for Sanofi Pasteur MSD, GSK, Novartis and Pfizer, and received reimbursement of expenses but no honorarium or consultancy fee. Jacques Gaillat: received fees for participation at a workshop as an expert from Sanofi Pasteur MSD. João Gorjão Clara: received fees for participation in Advisory Board Meeting as an expert in Geriatrics from Sanofi Pasteur MSD, Speaker fees for Menarinni and Sanofi Pasteur MSD. Marc Labetoulle: Reimbursement of expenses and fees for advisory boards and speakers bureau from Alcon, Allergan, Bausch & Lomb, Sanofi Pasteur MSD, Santen, Thea. Jean-Pierre Michel: has been an invited speaker for Merck, Pfizer vaccines and SPMSD. Luigi Naldi: received fees for participation at a workshop as an expert from Sanofi Pasteur MSD. Luis Salleras Sanmarti: received fees for participation in round tables sponsored by Sanofi Pasteur MSD. Thomas Weinke: received fees for advisory boards or as a speaker from GSK, Novartis Vaccines, Sanofi Pasteur MSD.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for profit sectors.
Contributor Information
Robert W. Johnson, Senior Research Fellow, Clinical Sciences, University of Bristol, 9 Ridgeway Road, Long Ashton, Bristol, BS41 9EX, UK.
Marie-José Alvarez-Pasquin, Primary Care, Servicio Madrileño de Salud, Madrid, Spain.
Marc Bijl, Department of Internal Medicine and Rheumatology, Martini Hospital, Groningen, The Netherlands.
Elisabetta Franco, Department of Biomedicine and Prevention, University Tor Vergata, Rome, Italy.
Jacques Gaillat, Annecy-Genevois Hospital, Infectious Diseases Department, Annecy, France.
João G. Clara, Lisbon Faculty of Medicine, Lisbon University, Lisbon, Portugal
Marc Labetoulle, Service d’Ophtalmologie, Hôpital Bicêtre, APHP, Université Paris-Sud, France Département de Virologie, Institute for Integrative Biology of the Cell (I2BC), CNRS, Gif/Yvette, France.
Jean-Pierre Michel, Department of Geriatrics, University Hospitals of Geneva, Belle Idée, Geneva, Switzerland.
Luigi Naldi, Department of Dermatology, Azienda Ospedaliera papa Giovanni XXIII, Bergamo, Italy.
Luis S. Sanmarti, School of Medicine, University of Barcelona, Barcelona, Spain
Thomas Weinke, Klinikum Ernst von Bergmann, Klinik für Gastroenterologie und Infekiologie, Potsdam, Germany.
References
- Álvarez-Pasquín M., Morató M., Sampedro A., San-Martín M. (2011) Perception of herpes zoster in the general population. Vacunas 12: 86–94. [Google Scholar]
- Annemans L., Bresse X., Gobbo C., Papageorgiou M. (2010) Health economic evaluation of a vaccine for the prevention of herpes zoster (Shingles) and post-herpetic neuralgia in adults in Belgium. J Med Econ 13: 537–551. [DOI] [PubMed] [Google Scholar]
- Arvin A. (1996) Varicella-zoster virus. Clin Microbiol Rev 9: 361–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batisse D., Eliaszewicz M., Zazoun L., Baudrimont M., Pialoux G., Dupont B. (1996) Acute retinal necrosis in the course of aids: study of 26 cases. AIDS 10: 55–60. [DOI] [PubMed] [Google Scholar]
- Blank L., Polydefkis M., Moore R., Gebo K. (2012) Herpes zoster among persons living with HIV in the current antiretroviral therapy era. J Acquir Immune Defic Syndr 61: 203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch K., Johnson J. (2012) Varicella zoster virus transmission in the vaccine era: unmasking the role of herpes zoster. J Infect Dis 205: 1331–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhassira D., Chassany O., Gaillat J., Hanslik T., Launay O., Mann C., et al. (2012) Patient perspective on herpes zoster and its complications: an observational prospective study in patients aged over 50 years in general practice. Pain 153: 342–349. [DOI] [PubMed] [Google Scholar]
- Bresse X., Annemans L., Preaud E., Bloch K., Duru G., Gauthier A. (2013) Vaccination against herpes zoster and postherpetic neuralgia in France: a cost-effectiveness analysis. Expert Rev Pharmacoecon Outcomes Res 13: 393–406. [DOI] [PubMed] [Google Scholar]
- Bricout H., Haugh M., Olatunde O., Gil Prieto R. (2015) Herpes zoster-associated mortality in Europe: a systematic review. BMC Public Health 15: 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson M., Gay N., Edmunds W., Andrews N. (2002) Exposure to varicella boosts immunity to herpes-zoster: implications for mass vaccination against chickenpox. Vaccine 20: 2500–2507. [DOI] [PubMed] [Google Scholar]
- Bruckenthal P., Barkin R. (2013) Options for treating postherpetic neuralgia in the medically complicated patient. Ther Clin Risk Manag 9: 329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke B., Steele R., Beard O., Wood J., Cain T., Marmer D. (1982) Immune responses to varicella-zoster in the aged. Arch Intern Med 142: 291–293. [PubMed] [Google Scholar]
- Che H., Lukas C., Morel J., Combe B. (2014) Risk of herpes/herpes zoster during anti-tumor necrosis factor therapy in patients with rheumatoid arthritis. Systematic review and meta-analysis. Joint Bone Spine 81: 215–221. [DOI] [PubMed] [Google Scholar]
- Chen N., Li Q., Yang J., Zhou M., Zhou D., He L. (2014a) Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev 2: CD006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Suaya J., Li Q., Galindo C., Misurski D., Burstin S., et al. (2014b) Incidence of herpes zoster in patients with altered immune function. Infection 42: 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobo L., Foulks G., Liesegang T., Lass J., Sutphin J., Wilhelmus K., et al. (1986) Oral acyclovir in the treatment of acute herpes zoster ophthalmicus. Ophthalmology 93: 763–770. [DOI] [PubMed] [Google Scholar]
- Cohen J. (2013) Clinical practice: herpes zoster. N Engl J Med 369: 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer P., Pouwels K., Cox J., Hak E., Wilschut J., Postma M. (2013) Cost-effectiveness of vaccination of the elderly against herpes zoster in the Netherlands. Vaccine 31: 1276–1283. [DOI] [PubMed] [Google Scholar]
- De Broucker T., Mailles A., Chabrier S., Morand P., Stahl J. (2012) Acute varicella zoster encephalitis without evidence of primary vasculopathy in a case-series of 20 patients. Clin Microbiol Infect 18: 808–819. [DOI] [PubMed] [Google Scholar]
- De Melker H., Berbers G., Hahne S., Rumke H., van den Hof S., de Wit A., et al. (2006) The epidemiology of varicella and herpes zoster in the Netherlands: implications for varicella zoster virus vaccination. Vaccine 24: 3946–3952. [DOI] [PubMed] [Google Scholar]
- Drolet M., Brisson M., Schmader K., Levin M., Johnson R., Oxman M., et al. (2010) Predictors of postherpetic neuralgia among patients with herpes zoster: a prospective study. J Pain 11: 1211–1221. [DOI] [PubMed] [Google Scholar]
- Drolet M., Oxman M., Levin M., Schmader K., Johnson R., Patrick D., et al. (2013) Vaccination against herpes zoster in developed countries: state of the evidence. Hum Vaccin Immunother 9: 0–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban-Vasallo M., Gil-Prieto R., Dominguez-Berjon M., Astray-Mochales J., Gil de Miguel A. (2014) Temporal trends in incidence rates of herpes zoster among patients treated in primary care centers in Madrid (Spain), 2005–2012. J Infect 68: 378–386. [DOI] [PubMed] [Google Scholar]
- Finnerup N., Attal N., Haroutounian S., McNicol E., Baron R., Dworkin R., et al. (2015) Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 14: 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes H., Bhaskaran K., Thomas S., Smeeth L., Clayton T., Langan S. (2014) Quantification of risk factors for herpes zoster: population based case-control study. BMJ 348: g2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gater A., Abetz-Webb L., Carroll S., Mannan A., Serpell M., Johnson R. (2014) Burden of herpes zoster in the UK: findings from the zoster quality of life (ZQOL) study. BMC Infect Dis 14: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier A., Breuer J., Carrington D., Martin M., Remy V. (2009) Epidemiology and cost of herpes zoster and post-herpetic neuralgia in the United Kingdom. Epidemiol Infect 137: 38–47. [DOI] [PubMed] [Google Scholar]
- Gelb L. (2008) Preventing herpes zoster through vaccination. Ophthalmology 115: S35–S38. [DOI] [PubMed] [Google Scholar]
- Gialloreti L., Merito M., Pezzotti P., Naldi L., Gatti A., Beillat M., et al. (2010) Epidemiology and economic burden of herpes zoster and post-herpetic neuralgia in Italy: a retrospective, population-based study. BMC Infect Dis 10: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnann J., Jr, Whitley R. (2002) Clinical practice: herpes zoster. N Engl J Med 347: 340–346. [DOI] [PubMed] [Google Scholar]
- Gonzalez Chiappe S., Sarazin M., Turbelin C., Lasserre A., Pelat C., Bonmarin I., et al. (2010) Herpes zoster: burden of disease in France. Vaccine 28: 7933–7938. [DOI] [PubMed] [Google Scholar]
- Habel L., Ray G., Silverberg M., Horberg M., Yawn B., Castillo A., et al. (2013) The epidemiology of herpes zoster in patients with newly diagnosed cancer. Cancer Epidemiol Biomarkers Prev 22: 82–90. [DOI] [PubMed] [Google Scholar]
- Hales C., Harpaz R., Joesoef M., Bialek S. (2013) Examination of links between herpes zoster incidence and childhood varicella vaccination. Ann Intern Med 159: 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding S. (1993) Management of ophthalmic zoster. J Med Virol 41(Suppl. 1): 97–101. [DOI] [PubMed] [Google Scholar]
- Harding S., Lipton J., Wells J. (1987) Natural history of herpes zoster ophthalmicus: predictors of postherpetic neuralgia and ocular involvement. Br J Ophthalmol 71: 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpaz R., Ortega-Sanchez I., Seward J. (2008) Prevention of herpes zoster: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep 57: 1–30; quiz CE32–34. [PubMed] [Google Scholar]
- Hata A., Kuniyoshi M., Ohkusa Y. (2011) Risk of herpes zoster in patients with underlying diseases: a retrospective hospital-based cohort study. Infection 39: 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgason S., Petursson G., Gudmundsson S., Sigurdsson J. (2000) Prevalence of postherpetic neuralgia after a first episode of herpes zoster: prospective study with long term follow up. BMJ 321: 794–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang-Xuan T., Buchi E., Herbort C., Denis J., Frot P., Thenault S., et al. (1992) Oral acyclovir for herpes zoster ophthalmicus. Ophthalmology 99: 1062–1070; discussion 1070–1061. [DOI] [PubMed] [Google Scholar]
- Hope-Simpson R. (1965) The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med 58: 9–20. [PMC free article] [PubMed] [Google Scholar]
- Hu A., Strauss E., Holland G., Chan M., Yu F., Margolis T. (2010) Late varicella-zoster virus dendriform keratitis in patients with histories of herpes zoster ophthalmicus. Am J Ophthalmol 149: 214–220.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. (2007) Zoster-associated pain: what is known, who is at risk and how can it be managed? Herpes 14(Suppl. 2): 30–34. [PubMed] [Google Scholar]
- Johnson R., Bouhassira D., Kassianos G., Leplege A., Schmader K., Weinke T. (2010) The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med 8: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R., Rice A. (2014) Clinical practice: postherpetic neuralgia. N Engl J Med 371: 1526–1533. [DOI] [PubMed] [Google Scholar]
- Kawai K., Gebremeskel B., Acosta C. (2014) Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 4: e004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan S., Smeeth L., Margolis D., Thomas S. (2013) Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med 10: e1001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J., Harpaz R., Molinari N., Jumaan A., Zhou F. (2011) Herpes zoster incidence among insured persons in the United States, 1993–2006: evaluation of impact of varicella vaccination. Clin Infect Dis 52: 332–340. [DOI] [PubMed] [Google Scholar]
- Li W., Peng W., Zhou J., Liu Z. (2014) Acupuncture for postherpetic neuralgia: a systematic review protocol. Brit Med J 4: e005725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesegang T. (2004) Herpes zoster virus infection. Curr Opin Ophthalmol 15: 531–536. [DOI] [PubMed] [Google Scholar]
- Liesegang T. (2008) Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology 115: S3–S12. [DOI] [PubMed] [Google Scholar]
- Lin Y., Huang L., Chang I., Tsai F., Lu C., Shao P., et al. (2010) Disease burden and epidemiology of herpes zoster in pre-vaccine Taiwan. Vaccine 28: 1217–1220. [DOI] [PubMed] [Google Scholar]
- Long M., Martin C., Sandler R., Kappelman M. (2013) Increased risk of herpes zoster among 108 604 patients with inflammatory bowel disease. Aliment Pharmacol Ther 37: 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes F., Dias A., Miranda A., Alves M., Narciso M., Fonseca T., et al. (2013) Claim for a new paradigm of hospital physicians: evolution along 13 years in an acute internal medicine ward in Portugal. Eur Geriatr Med 4(Suppl. 1): S25. [Google Scholar]
- Mailles A., Stahl J. (2009) Infectious encephalitis in France in 2007: a national prospective study. Clin Infect Dis 49: 1838–1847. [DOI] [PubMed] [Google Scholar]
- McElhaney J. (2010) Herpes zoster: a common disease that can have a devastating impact on patients’ quality of life. Expert Rev Vaccines 9: 27–30. [DOI] [PubMed] [Google Scholar]
- Miranda A., Alves M., Lopes F., Dias A., Narciso M., Fonseca T., et al. (2013) Polypathology in Portuguese old patients – performance of three comorbidity scales and their (Un)ability to predict outcome. Eur Geriatr Med 4(Suppl. 1): S20. [Google Scholar]
- Moore L., Remy V., Martin M., Beillat M., McGuire A. (2010) A health economic model for evaluating a vaccine for the prevention of herpes zoster and post-herpetic neuralgia in the UK. Cost Eff Resour Alloc 8: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen G. (2011) Perceptions of herpes zoster and attitudes towards zoster vaccination among 50–65-year-old danes. Dan Med Bull 58: A4345. [PubMed] [Google Scholar]
- O’Connor K., Paauw D. (2013) Herpes zoster. Med Clin North Am 97: 503–522, ix. [DOI] [PubMed] [Google Scholar]
- Ogunjimi B., Van Damme P., Beutels P. (2013) Herpes zoster risk reduction through exposure to chickenpox patients: a systematic multidisciplinary review. PLoS One 8: e66485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opstelten W., Mauritz J., de Wit N., van Wijck A., Stalman W., van Essen G. (2002) Herpes zoster and postherpetic neuralgia: incidence and risk indicators using a general practice research database. Fam Pract 19: 471–475. [DOI] [PubMed] [Google Scholar]
- Opstelten W., Zaal M. (2005) Managing ophthalmic herpes zoster in primary care. Brit Med J 331: 147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxman M. (2000) Clinical manifestations of herpes zoster. In: Arvin A., Gershon A. (eds), Varicella Zoster Virus: Virology and Clinical Management. Cambridge, UK: Cambridge University Press, pp. 246–275. [Google Scholar]
- Oxman M. (2009) Herpes zoster pathogenesis and cell-mediated immunity and immunosenescence. J Am Osteopath Assoc 109: S13–S17. [PubMed] [Google Scholar]
- Oxman M., Levin M., Johnson G., Schmader K., Straus S., Gelb L., et al. (2005) A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 352: 2271–2284. [DOI] [PubMed] [Google Scholar]
- Paek E., Johnson R. (2010) Public awareness and knowledge of herpes zoster: results of a global survey. Gerontology 56: 20–31. [DOI] [PubMed] [Google Scholar]
- Pickering G., Leplege A. (2011) Herpes zoster pain, postherpetic neuralgia, and quality of life in the elderly. Pain Pract 11: 397–402. [DOI] [PubMed] [Google Scholar]
- Pickering G., Pereira B., Clere F., Sorel M., de Montgazon G., Navez M., et al. (2014a) Cognitive function in older patients with postherpetic neuralgia. Pain Pract 14: E1–E7. [DOI] [PubMed] [Google Scholar]
- Pickering G., Pereira B., Dufour E., Soule S., Dubray C. (2014b) Impaired modulation of pain in patients with postherpetic neuralgia. Pain Res Manag 19: e19–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinchinat S., Cebrian-Cuenca A., Bricout H., Johnson R. (2013) Similar herpes zoster incidence across Europe: results from a systematic literature review. BMC Infect Dis 13: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkaranta A., Piiparinen H., Mannonen L., Vesaluoma M., Vaheri A. (2000) Detection of human herpesvirus 6 and varicella-zoster virus in tear fluid of patients with Bell’s Palsy by PCR. J Clin Microbiol 38: 2753–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health Agency of Canada (2014) Statement on Seasonal Influenza Vaccine for 2014–2015. Canada: Public Health Agency of Canada. [Google Scholar]
- Rabaud C., Rogeaux O., Launay O., Strady C., Mann C., Chassany O., et al. (2013) Early antiviral treatment fails to completely prevent herpes-related pain. Med Mal Infect 43: 461–466. [DOI] [PubMed] [Google Scholar]
- Ragozzino M., Melton L., 3rd, Kurland L., Chu C., Perry H. (1982) Population-based study of herpes zoster and its sequelae. Medicine (Baltimore) 61: 310–316. [DOI] [PubMed] [Google Scholar]
- Rommelaere M., Marechal C., Yombi J., Goffin E., Kanaan N. (2012) Disseminated varicella zoster virus infection in adult renal transplant recipients: outcome and risk factors. Transplant Proc 44: 2814–2817. [DOI] [PubMed] [Google Scholar]
- Sacks G. (2013) Unmet need in the treatment of postherpetic neuralgia. Am J Manag Care 19: S207–S213. [PubMed] [Google Scholar]
- Sampathkumar P., Drage L., Martin D. (2009) Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clinic Proc 84: 274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmader K. (2006) Treatment and prevention strategies for herpes zoster and postherpetic neuralgia in older adults. Clin Geriat 14: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmader K., Levin M., Gnann J., Jr., McNeil S., Vesikari T., Betts R., et al. (2012) Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50–59 years. Clin Infect Dis 54: 922–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid D., Jumaan A. (2010) Impact of varicella vaccine on varicella-zoster virus dynamics. Clin Microbiol Rev 23: 202–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuette M., Hethcote H. (1999) Modeling the effects of varicella vaccination programs on the incidence of chickenpox and shingles. Bull Math Biol 61: 1031–1064. [DOI] [PubMed] [Google Scholar]
- Severson E., Baratz K., Hodge D., Burke J. (2003) Herpes zoster ophthalmicus in Olmsted county, Minnesota: have systemic antivirals made a difference? Arch Ophthalmol 121: 386–390. [DOI] [PubMed] [Google Scholar]
- Shearer K., Maskew M., Ajayi T., Berhanu R., Majuba P., Sanne I., et al. (2014) Incidence and predictors of herpes zoster among antiretroviral therapy-naive patients initiating HIV treatment in Johannesburg, South Africa. Int J Infect Dis 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smitten A., Choi H., Hochberg M., Suissa S., Simon T., Testa M., et al. (2007) The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum 57: 1431–1438. [DOI] [PubMed] [Google Scholar]
- Stein A., Britt H., Harrison C., Conway E., Cunningham A., Macintyre C. (2009) Herpes zoster burden of illness and health care resource utilisation in the Australian population aged 50 years and older. Vaccine 27: 520–529. [DOI] [PubMed] [Google Scholar]
- Studahl M., Petzold M., Cassel T. (2013) Disease burden of herpes zoster in Sweden–predominance in the elderly and in women - a register based study. BMC Infect Dis 13: 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szucs T., Kressig R., Papageorgiou M., Kempf W., Michel J., Fendl A., et al. (2011) Economic evaluation of a vaccine for the prevention of herpes zoster and post-herpetic neuralgia in older adults in Switzerland. Hum Vaccin 7: 749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanuseputro P., Zagorski B., Chan K., Kwong J. (2011) Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine 29: 8580–8584. [DOI] [PubMed] [Google Scholar]
- Toyama N., Shiraki K. (2009) Epidemiology of herpes zoster and its relationship to varicella in Japan: a 10-year survey of 48,388 herpes zoster cases in Miyazaki prefecture. J Med Virol 81: 2053–2058. [DOI] [PubMed] [Google Scholar]
- Tseng H., Smith N., Harpaz R., Bialek S., Sy L., Jacobsen S. (2011) Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. J Am Med Assoc 305: 160–166. [DOI] [PubMed] [Google Scholar]
- Ultsch B., Koster I., Reinhold T., Siedler A., Krause G., Icks A., et al. (2013a) Epidemiology and cost of herpes zoster and postherpetic neuralgia in Germany. Eur J Health Econ 14: 1015–1026. [DOI] [PubMed] [Google Scholar]
- Ultsch B., Weidemann F., Reinhold T., Siedler A., Krause G., Wichmann O. (2013b) Health economic evaluation of vaccination strategies for the prevention of herpes zoster and postherpetic neuralgia in Germany. BMC Health Serv Res 13: 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafai A., Berger M. (2001) Zoster in patients infected with HIV: a review. Am J Med Sci 321: 372–380. [DOI] [PubMed] [Google Scholar]
- Van Gelderen B., Van der Lelij A., Treffers W., van der Gaag R. (2000) Detection of herpes simplex virus type 1, 2 and varicella zoster virus DNA in recipient corneal buttons. Br J Ophthalmol 84: 1238–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoek A., Gay N., Melegaro A., Opstelten W., Edmunds W. (2009) Estimating the cost-effectiveness of vaccination against herpes zoster in England and Wales. Vaccine 27: 1454–1467. [DOI] [PubMed] [Google Scholar]
- Van Lier A., van Hoek A., Opstelten W., Boot H., de Melker H. (2010) Assessing the potential effects and cost-effectiveness of programmatic herpes zoster vaccination of elderly in the Netherlands. BMC Health Serv Res 10: 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viner K., Perella D., Lopez A., Bialek S., Newbern C., Pierre R., et al. (2012) Transmission of varicella zoster virus from individuals with herpes zoster or varicella in school and day care settings. J Infect Dis 205: 1336–1341. [DOI] [PubMed] [Google Scholar]
- Wang K., Lau T., Morales M., Mont E., Straus S. (2005) Laser-capture microdissection: refining estimates of the quantity and distribution of latent herpes simplex virus 1 and varicella-zoster virus DNA in human trigeminal ganglia at the single-cell level. J Virol 79: 14079–14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauters O., Lebas E., Nikkels A. (2012) Chronic mucocutaneous herpes simplex virus and varicella zoster virus infections. J Am Acad Dermatol 66: e217–e227. [DOI] [PubMed] [Google Scholar]
- Weaver B. (2009) Herpes zoster overview: natural history and incidence. J Am Osteopath Assoc 109: S2–S6. [PubMed] [Google Scholar]
- Weinke T., Edte A., Schmitt S., Lukas K. (2010) Impact of herpes zoster and post-herpetic neuralgia on patients’ quality of life: a patient-reported outcomes survey. Z Gesundh Wiss 18: 367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinke T., Glogger A., Bertrand I., Lukas K. (2014) The societal impact of herpes zoster and postherpetic neuralgia on patients, life partners, and children of patients in Germany. Scientific World J 2014: 749698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman D., Shavit O., Stein M., Cohen R., Chodick G., Shalev V. (2013) A population based study of the epidemiology of herpes zoster and its complications. J Infect 67: 463–469. [DOI] [PubMed] [Google Scholar]
- Wenkel H., Rummelt V., Fleckenstein B., Naumann G. (1998) Detection of varicella zoster virus DNA and viral antigen in human eyes after herpes zoster ophthalmicus. Ophthalmology 105: 1323–1330. [DOI] [PubMed] [Google Scholar]
- Winthrop K., Baddley J., Chen L., Liu L., Grijalva C., Delzell E., et al. (2013) Association between the initiation of anti-tumor necrosis factor therapy and the risk of herpes zoster. J Am Med Assoc 309: 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Hayasaka S., Yamamoto Y., Setogawa T. (1990) Cutaneous eruption with or without ocular complications in patients with herpes zoster involving the trigeminal nerve. Graefes Arch Clin Exp Ophthalmol 228: 1–4. [DOI] [PubMed] [Google Scholar]
- Yawn B., Gilden D. (2013) The global epidemiology of herpes zoster. Neurology 81: 928–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawn B., Wollan P., Kurland M., St Sauver J., Saddier P. (2011) Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc 86: 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaal M., Völker-Dieben H., D’Amaro J. (2003) Prognostic value of Hutchinson’s sign in acute herpes zoster ophthalmicus. Graefe’s Arch Clin Exp Ophthalmol 241: 187–191. [DOI] [PubMed] [Google Scholar]
- Zaal M., Volker-Dieben H., Wienesen M., D’Amaro J., Kijlstra A. (2001) Longitudinal analysis of varicella-zoster virus DNA on the ocular surface associated with herpes zoster ophthalmicus. Am J Ophthalmol 131: 25–29. [DOI] [PubMed] [Google Scholar]
- Zhang J., Xie F., Delzell E., Chen L., Winthrop K., Lewis J., et al. (2012) Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. J Am Med Assoc 308: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]


