Abstract
Background
Survival rates for children with medulloblastoma have risen over the past decade, in part due to the addition of cisplatin-containing adjuvant chemotherapy. Total dose of cisplatin required for optimal treatment is unknown. The purpose of this study was to evaluate the survival outcomes based on cumulative cisplatin doses (CCD) in children with newly diagnosed average-risk medulloblastoma.
Procedure
CCD data were reviewed for 363 patients in a prospective study evaluating patients between 3 and 21 years with a newly diagnosed average-risk medulloblastoma and treated with craniospinal radiation and post-radiation cisplatin based adjuvant chemotherapy.
Results
Eight-year event-free survival (EFS) and overall survival (OS) estimates were 78.2±2.6% and 83.9±2.4%, respectively. Only 73 patients received the protocol specified CCD of 600 mg/m2, primarily due to mandated cisplatin toxicity-related dose reductions. The median CCD given to those without relapse or death on treatment was 487.5 mg/m2. CCD, as a time-dependent covariate, was not associated with EFS (P=0.54) or OS (P=0.11). The 343 patients who completed chemotherapy failure-free were categorized into four groups according to CCD (n=10; 75–150 mg/m2), (n=26; 151–300 mg/m2), (n 113; 301–450 mg/m2), and (n=194; 451–600 mg/m2). There were no statistically significant differences in distributions of EFS (P=0.53) or OS (P=0.49) among these four groups.
Conclusion
CCD is not associated with EFS or OS suggesting that lower doses of cisplatin may be incorporated into future medulloblastoma trials, thereby limiting its toxicity profile without affecting survival. If ototoxicity is encountered, more stringent cisplatin dose modification/cessation rules seem warranted.
Keywords: average-risk medulloblastoma, cumulative cisplatin dose, survival outcomes
INTRODUCTION
Medulloblastoma is the most common malignant brain tumor of childhood and accounts for 13% and 3.9% of all pediatric brain tumors in the 0–14 and 15–19 years age groups, respectively [1]. There is a bimodal peak in incidence with first peak between 3 and 4 years and second peak between 8 and 9 years of age and for those children diagnosed at 3 years of age or later the 5 year survival rate is 60–70% [2,3]. The survival rates have increased over the years likely due to improved treatment strategies which include radiation therapy and the addition of cisplatin containing adjuvant chemotherapy [4–6].
However, these therapeutic regimens agents carry with them a significant risk for acute as well as long-term sequelae, impacting the quality of life in long-term survivors [7]. Cisplatin is often associated with irreversible sensorineuronal hearing loss related to the total cumulative dose [8–11]. The long-term impact of hearing loss is greater in younger children whose language is still not completely developed. In children, even minimal high frequency hearing loss can affect academic performance and social development [12,13].
The optimal dose of cisplatin for the treatment of medulloblastoma is currently unknown. This retrospective study evaluated the association of survival outcomes and cumulative cisplatin dose in children with newly diagnosed average-risk medulloblastoma.
PATIENTS AND METHODS
Patients
Cumulative cisplatin dose data were reviewed on patients enrolled in the Childrens Cancer Group/Pediatric Oncology Group (CCG/POG A9961) trial, a Phase III prospective study evaluating effectiveness of treatment in patients with a newly diagnosed average-risk medulloblastoma. Patients with a histologically confirmed medulloblastoma, between the ages of 3 and 21 years at the time of diagnosis, were eligible for this study. Patients were to have <1.5 cm2 of residual tumor on postoperative neuroimaging performed within 21 days, preferably, within 72 hours of surgery. Patients were to have no evidence of disseminated disease on magnetic resonance imaging (MRI) of the entire brain and spine performed pre- or postoperatively and on cytologic examination of lumbar cerebrospinal fluid (CSF). Patients with brainstem involvement were eligible. Patients were ineligible if they had any previous anticancer treatment, other than corticosteroids, and treatment must have begun within 31 days of definitive surgery. Patients also had to have adequate physiologic function, including a nuclear glomerular filtration rate (GFR) or creatinine clearance > 70 ml/min/1.73 m2 [4]. All institutions participating in the study had received approval from their institutional review boards and age-appropriate informed consent/assent was obtained by each patient/parent/guardian.
Of the 379 patients enrolled between December 1996 and December 2000 in the study, 12 patients who were removed from the study prior to receiving any cisplatin (n=3 due to relapse/progressive disease prior to starting chemotherapy, n=9 due to parental/physician choice) and four patients with incomplete submission of dosing data were excluded from this analysis.
Treatment
Chemo-radiotherapy
A dose of 2,340 cGy of craniospinal radiation therapy with a posterior fossa boost of 3,240 cGy (total dose 5,580 cGy) was prescribed in fractions of 180 cGy per day, 5 days per week. The whole-brain treatment volume extended to the entire frontal lobe and cribriform plate region. The spinal treatment volume extended laterally to cover the recesses of the entire vertebral bodies with at least a 1 cm margin at either side and inferiorly 1–2 cm below the termination of the thecal sac. The boost volume included the entire posterior fossa with a 1 cm margin around the tentorium. All patients received weekly vincristine during radiotherapy (dose: 1.5 mg/m2, maximum dose of 2 mg and a total of 8 doses).
Maintenance chemotherapy
Following surgery, patients were randomized to receive 8 cycles of one of two cisplatin based post-radiation adjuvant chemotherapy regimens (Table I). Chemo-therapy was to begin 6 weeks after completion of radiation therapy.
TABLE I.
Treatment Regimens
| Regimen | Drugs (8 cycles) | Dose |
|---|---|---|
| Regimen A | ||
| Day 0 | CCNU | 75 mg/m2 |
| Day 0 | Cisplatin | 75 mg/m2 |
| Day 0, 7, 14 | VCR | 1.5 mg/m2; max 2 mg |
| Regimen B | ||
| Day 0 | Cisplatin | 75 mg/m2 |
| Day 0, 7, 14 | VCR | 1.5 mg/m2; max 2 mg |
| Day 21, 22 | Cyclophosphamide | 1 gm/m2 |
CCNU, lomustine; VCR, vincristine; max, maximum.
Cisplatin dose modifications
The Children's Cancer Group toxicities and complications criteria were followed for grading of toxicities (Table II). The site, measure, and grade for all toxicities (grades 3 and 4) were collected.
TABLE II.
Children's Cancer Group Toxicity and Complications Criteria
| Grade 1 (mild) | Grade 2 (moderate) | Grade 3 (severe) | Grade 4 (unacceptable) | |
|---|---|---|---|---|
| Hearing loss | ||||
| Objective | 20–40 db loss >4 kHz | >40 db loss >4 kHz | >40 db loss 2 kHz | >40 db loss <2 kHz |
| Subjective | Loss on audiometry only | Tinnitus, soft speech | Loss correctable with hearing aid | Deafness not correctable |
| Creatinine clearance | 75% | 50–74% | 25–49% | <25% |
Nephrotoxicity
If the creatinine clearance or GFR was <50% of baseline value, then the cisplatin was to be held until creatinine clearance rose above 50% of baseline value. Following transient renal dysfunction, the cisplatin was to be reinstituted at 50% dosage until the creatinine clearance or GFR was maintained above 50% of baseline value for two cycles of chemotherapy. After two cycles of therapy with acceptable creatinine clearance, cisplatin could be given at full doses. If the creatinine clearance or GFR was <75% of baseline value, then the cisplatin dose was reduced by 50% of the calculated dose. If the creatinine clearance or GFR did not rise above 30 ml/min/1.73 m2, the cisplatin was omitted.
Ototoxicity
For a decrease in auditory acuity of ≥30 decibels at 4,000–8,000 Hz, a 50% reduction in cisplatin dosage was required. For a ≥20 decibel loss at 500–3,000 Hz, a 50% reduction in cisplatin dosage was made. A more than 40 decibel hearing loss at 2,000 Hz (grade 3 toxicity) required a 50% reduction in cisplatin dosage. A more than 40 decibel hearing loss at <2,000 Hz (grade 4 toxicity) required omitting cisplatin and was not restarted unless follow-up audiograms showed an improvement in hearing function.
Statistical Analysis
Cumulative cisplatin dose data were collected on patients enrolled on study who were fully eligible. The 363 patients who had received at least one cycle of cisplatin and did not have significant missing dosing data are included in this analysis.
Event-free survival (EFS) is defined as time from date of study enrollment to the date of first occurrence of relapse or progression of disease, death, or secondary malignancy or date of last follow-up. Overall survival (OS) is measured as time from date of study enrollment to date of death or last follow-up. Kaplan-Meier estimates of event-free and overall survival distributions were calculated. Cox proportional hazards models were used to investigate the association of cumulative cisplatin dose with outcome.
A failure while on chemotherapy was defined as either relapse or death without relapse while receiving chemotherapy. A subset analysis of the 343 patients who did not experience a failure while on chemotherapy was conducted by categorizing these patients into four groups according to the cumulative cisplatin dose received [(n=10; 75–150 mg/m2), (n 26; 151–300 mg/m2), (n 113; 301–450 mg/m2), and (n=194; 451–600 mg/m2)]. A log-rank test was used to compare survival distributions among the four cumulative cisplatin dose groups after stratifying for treatment regimens.
RESULTS
Patient Characteristics
Median follow-up for the 306 patients alive at last follow-up is 8.9 years (range; 0.67–12.6 years) and 88% of patients have been followed for at least 5 years. The patient characteristics of the 343 patients who did not experience a failure while on chemotherapy are listed in Table III.
TABLE III.
Patient Demographics and Treatment Characteristics
| Cummulative cisplatin dose groupa |
||||
|---|---|---|---|---|
| Characteristic | 75–150 mg/m2 (n = 10) | 151–300 mg/m2 (n = 26) | 301–450 mg/m2 (n=113) | 451–600 mg/m2 (n = 194) |
| Sex | ||||
| Male | 7 (70) | 11 (42) | 62 (55) | 122 (63) |
| Female | 3 (30) | 15 (58) | 51 (45) | 72 (37) |
| Race and ethnicity | ||||
| White | 8 (80) | 22 (84) | 96 (85) | 147 (76) |
| Hispanic | 1 (10) | 1 (4) | 7 (6) | 24 (12) |
| Black | 0 (0) | 1 (4) | 3 (2.5) | 14 (7) |
| Other | 1 (10) | 2 (8) | 4 (4) | 7 (4) |
| Unknown | 0 (0) | 0 (0) | 3 (2.5) | 2 (1) |
| Age (years) | ||||
| 3–4 | 2 (20) | 3 (11.5) | 24 (21) | 26 (14) |
| 5–9 | 3 (30) | 14 (54) | 52 (46) | 107 (55) |
| 10–14 | 5 (50) | 6 (23) | 30 (27) | 49 (25) |
| 15–19 | 0 (0) | 3 (11.5) | 7 (6) | 12 (6) |
| Treatment regimen | ||||
| Regimen A | 3 (30) | 16 (62) | 57 (50) | 97 (50) |
| Regimen B | 7 (70) | 10 (38) | 56 (50) | 97 (50) |
| Grade 3/4 objective hearing loss | 3/8 patients (38) | 14/26 patients (54) | 40/113 patients (35) | 28/194 patients (14) |
Values are number (percentage) unless otherwise specified.
For 343 patients who did not experience a failure while on chemotherapy.
Overall Outcomes
Eight-year EFS and OS for all 363 patients were 78.2±2.6% and 83.9±2.4% respectively. Eight-year EFS was 74.9±3.8% and 81.6±3.6% for treatment regimen A and B, respectively. Eight-year overall survival was 81.9±3.4% and 86.0±3.3% for treatment regimen A and B, respectively. There were no statistically significant differences in EFS based on age, gender or treatment regimen (P=0.80, 0.30, and 0.19, respectively).
Treatment related toxicities for patients enrolled on this study have been previously reported [4]. Grade 3/4 objective hearing loss was noted in 86 (24%) of the 358 patients with sufficient hearing evaluation data. Of the 343 patients who did not experience any failure while on chemotherapy, two patients did not have sufficient hearing evaluation data. Grade 3/4 objective hearing loss for the remaining patients in each of the four groups categorized by cumulative cisplatin dose is listed in Table III. There was no significant association between hearing loss and gender or treatment regimen. However, age was found to be a significant factor (P=0.046) with grade 3/4 objective hearing loss observed more frequently in younger children.
Cumulative Cisplatin Dose and Survival Outcomes
The protocol specified maximum cumulative cisplatin dose was 600 mg/m2 (75 mg/m2 per cycle for 8 cycles). Only 73 patients received the maximum cumulative cisplatin dose. This was mainly due to mandated dose reductions secondary to cisplatin related toxicities. Cox proportional hazards model showed that cumulative cisplatin dose as a time-dependent covariate was not associated with EFS (hazard ratio: 0.96 (0.82–1.10) with a P-value of 0.54) or OS (hazard ratio: 0.875 (0.74–1.03) with a P-value of 0.11).
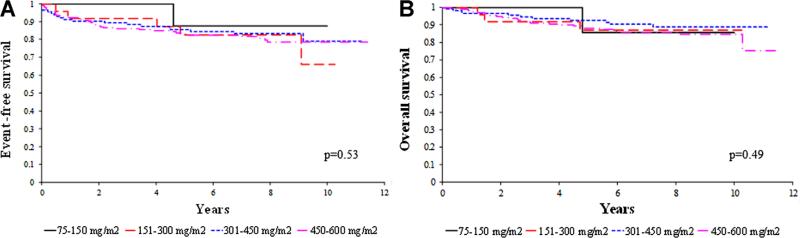
Of the 363 patients, 20 patients experienced a failure while on chemotherapy which included relapse (n=17) and death without relapse (n=3). The median cumulative cisplatin dose for the 343 patients who did not experience a failure while on chemotherapy was 487.5 mg/m2. These patients were further categorized into four groups based on the cumulative cisplatin dose received. There were no statistically significant differences in EFS (P=0.53, Fig. 1A) or OS (P=0.49, Fig. 1B) among these four groups after stratifying for treatment regimen.
Fig. 1.
A: Event-free survival and (B) overall survival by cumulative cisplatin dose for patients without relapse or death while on chemotherapy. Pediatr Blood Cancer DOI 10.1002/pbc
Similarly, if the analysis is restricted to the subset of these 343 patients who had centrally reviewed imaging scans (n=303), there is no statistically significant differences in distributions of EFS (P=0.33) or OS (P=0.23) among the four groups after stratifying for treatment regimen. The 40 excluded patients had imaging scans that were incomplete or not adequate for central review.
DISCUSSION
The survival rates of medulloblastoma continue to improve as therapeutic modalities have progressed over the years. This has led to multiple pediatric brain tumors survivor studies which underscore the negative impact of specific aspects of treatment and the significant risk of acute and long-term morbidity that medulloblastoma survivors experience [7,14]. With improving survival rates a major goal is to attempt minimizing therapy related acute and long-term toxicity sequelae which can impair quality of life, academic performance and socioeconomic status.
Cisplatin is one of the most active chemotherapy agents in the treatment of medulloblastoma, especially when used in combination with vincristine and lomustine [15,16]. One of the limitations of cisplatin is the associated ototoxicity. Cisplatin causes dose-dependent cochlear damage [17] which results in hearing loss at higher frequencies which progressively affects hearing at lower frequencies. Sixty percent of children treated with cisplatin develop permanent bilateral hearing loss [18,19] and the incidence and severity is thought to be dose-dependent [17] and is higher in younger children [9] and children who received prior radiation therapy [20]. In our study about one quarter of patients had developed grade 3/4 ototoxicty and younger age was associated with greater incidence and severity of hearing loss. In order to limit the toxicity, mandated audiometric evaluations before and during treatment and strict mandated dose-reductions based on toxicity are currently followed. The dose of cisplatin required in the treatment of medulloblastoma is not known and this is the first study that has been undertaken to evaluate the association between cumulative cisplatin dose and EFS and OS in patients with newly diagnosed average-risk medulloblastoma.
This study shows that the cumulative cisplatin dose is not associated with EFS or OS. Only about 20% of patients received the maximum cisplatin dose of 600 mg/m2. When patients were categorized based on the cumulative cisplatin dose received there were no significant differences in the survival outcomes among the four dose groups. These results suggest that lower doses of cisplatin can be used in the treatment of medulloblastoma without affecting survival outcomes. Given the very small number of patients who received <300 mg/m2, it is hard to assess the effect of cumulative cisplatin dose in those dose groups. However, one third of this cohort received between 301 and 450 mg/m2 of cumulative cisplatin dose and their overall survival did not differ from those who received more than 450 mg/m2. These results are reinforced by those from another prospective, multicenter study (St. Jude Medulloblastoma-96) utilizing risk-adapted radiotherapy followed by a cisplatin containing dose intense chemotherapy regimen with peripheral stem cell rescue which showed a 5 year overall and event free survival of 85% (95% CI 75–94) and 83% (95% CI 73–93), respectively, in patients with newly diagnosed average-risk medulloblastoma. The protocol specified cumulative cisplatin dose was 300 mg/m2 in their study [5] with the cumulative dose of cyclophosphamide similar to the treatment Regimen B of this study.
Thus, based on our study results and currently available literature, dosing between 300 and 450 mg/m2 of cisplatin seems a reasonable dose for future medulloblastoma clinical trials. The cumulative cisplatin dose used in the current Children's Oncology Group Phase III study for patients with newly diagnosed average-risk medulloblastoma is 450 mg/m2. Also, this study is looking at reduction in the radiation dose to the cochlea as an additional approach to reducing the rate of severe hearing loss. Given the results of this study, more stringent guidelines for reduction or discontinuation of cisplatin, if any significant ototoxicity is encountered, seems warranted. There should be strict audiometric surveillance during treatment and discontinuation of cisplatin for any grade 3 or 4 ototoxicity should be strongly considered. In addition, at higher frequencies, any hearing loss greater than 20 decibels, should likely result in cisplatin dose reduction.
Additional approaches that are being evaluated include the addition of otoprotective agents such as sodium thiosulfate and amifostine which may help prevent ototoxicity while allowing us to use these chemotherapy agents for their antitumor activity [19,21]. A recent prospective study showed that the incidence of severe hearing toxicity was significantly reduced in average-risk medulloblastoma patients treated with Amifostine pre and at hour 3 of a 6 hour cisplatin infusion (35% incidence rate in the control group vs. 17% in the amifostine treated group; P=0.048) [21].
Several pharmacogenomics studies have identified genetic polymorphisms that maybe associated with cisplatin induced ototoxicity. Genetic polymorphisms in genes encoding glutathione S-transferases [22] and megalin [23] and more recently, genetic variants in TPMT (thiopurine S-methyltransferase) and COMT (catechol-O-methyl transferase) [24] are thought to be associated with cisplatin induced ototoxicity. Identification of such genetic polymorphisms may potentially improve long-term outcomes by helping identify patients at risk for cisplatin induced ototoxicity and incorporating earlier dose stoppage or reduction rules.
Current advances in molecular biology continue to enhance our understanding of medulloblastoma [25]. Identification of molecular subgroups may also help us better stratify medulloblastoma and may potentially allow us to modify current treatment modalities and thus help improve survival and help reduce long-term msorbidity.
Footnotes
Conflict of interest: Nothing to declare.
REFERENCES
- 1.CBTRUS. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2004–2007. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crawford JR, MacDonald TJ, Packer RJ. Medulloblastoma in childhood: New biological advances. Lancet Neurol. 2007;6:1073–1085. doi: 10.1016/S1474-4422(07)70289-2. [DOI] [PubMed] [Google Scholar]
- 3.Packer RJ, Vezina G. Management of and prognosis with medulloblastoma: Therapy at a crossroads. Arch Neurol. 2008;65:1419–1424. doi: 10.1001/archneur.65.11.1419. [DOI] [PubMed] [Google Scholar]
- 4.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 5.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): Long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 6.Rood BR, Macdonald TJ, Packer RJ. Current treatment of medulloblastoma: Recent advances and future challenges. Semin Oncol. 2004;31:666–675. doi: 10.1053/j.seminoncol.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Turner CD, Rey-Casserly C, Liptak CC, et al. Late effects of therapy for pediatric brain tumor survivors. J Child Neurol. 2009;24:1455–1463. doi: 10.1177/0883073809341709. [DOI] [PubMed] [Google Scholar]
- 8.Brock P, Pritchard J, Bellman S, et al. Ototoxicity of high-dose cis-platinum in children. Med Pediatr Oncol. 1988;16:368–369. doi: 10.1002/mpo.2950160517. [DOI] [PubMed] [Google Scholar]
- 9.Kushner BH, Budnick A, Kramer K, et al. Ototoxicity from high-dose use of platinum compounds in patients with neuroblastoma. Cancer. 2006;107:417–422. doi: 10.1002/cncr.22004. [DOI] [PubMed] [Google Scholar]
- 10.Brock PR, Bellman SC, Yeomans EC, et al. Cisplatin ototoxicity in children: A practical grading system. Med Pediatr Oncol. 1991;19:295–300. doi: 10.1002/mpo.2950190415. [DOI] [PubMed] [Google Scholar]
- 11.Cohen BH, Zweidler P, Goldwein JW, et al. Ototoxic effect of cisplatin in children with brain tumors. Pediatr Neurosurg. 1990;16:292–296. doi: 10.1159/000120545. [DOI] [PubMed] [Google Scholar]
- 12.Bess FH, Dodd-Murphy J, Parker RA. Children with minimal sensorineural hearing loss: Prevalence, educational performance, and functional status. Ear Hear. 1998;19:339–354. doi: 10.1097/00003446-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Gurney JG, Tersak JM, Ness KK, et al. Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: A report from the children's oncology group. Pediatrics. 2007;120:e1229–e1236. doi: 10.1542/peds.2007-0178. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong GT, Stovall M, Robison LL. Long-term effects of radiation exposure among adult survivors of childhood cancer: Results from the childhood cancer survivor study. Radiat Res. 2010;174:840–850. doi: 10.1667/RR1903.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lefkowitz IB, Packer RJ, Siegel KR, et al. Results of treatment of children with recurrent medulloblastoma/primitive neuroectodermal tumors with lomustine, cisplatin, and vincristine. Cancer. 1990;65:412–417. doi: 10.1002/1097-0142(19900201)65:3<412::aid-cncr2820650306>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Packer RJ, Sutton LN, Elterman R, et al. Outcome for children with medulloblastoma treated with radiation and cisplatin, CCNU, and vincristine chemotherapy. J Neurosurg. 1994;81:690–698. doi: 10.3171/jns.1994.81.5.0690. [DOI] [PubMed] [Google Scholar]
- 17.Laurell G, Bagger-Sjoback D. Dose-dependent inner ear changes after i.v. administration of cisplatin. J Otolaryngol. 1991;20:158–167. [PubMed] [Google Scholar]
- 18.Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: Underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005;23:8588–8596. doi: 10.1200/JCO.2004.00.5355. [DOI] [PubMed] [Google Scholar]
- 19.Brock PR, Knight KR, Freyer DR, et al. Platinum-induced ototoxicity in children: A consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J Clin Oncol. 2012;30:2408–2417. doi: 10.1200/JCO.2011.39.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker DA, Pillow J, Waters KD, et al. Enhanced cis-platinum ototoxicity in children with brain tumours who have received simultaneous or prior cranial irradiation. Med Pediatr Oncol. 1989;17:48–52. doi: 10.1002/mpo.2950170110. [DOI] [PubMed] [Google Scholar]
- 21.Fouladi M, Chintagumpala M, Ashley D, et al. Amifostine protects against cisplatin-induced ototoxicity in children with average-risk medulloblastoma. J Clin Oncol. 2008;26:3749–3755. doi: 10.1200/JCO.2007.14.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oldenburg J, Kraggerud SM, Cvancarova M, et al. Cisplatin-induced long-term hearing impairment is associated with specific glutathione S-transferase genotypes in testicular cancer survivors. J Clin Oncol. 2007;25:708–714. doi: 10.1200/JCO.2006.08.9599. [DOI] [PubMed] [Google Scholar]
- 23.Riedemann L, Lanvers C, Deuster D, et al. Megalin genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Pharmacogenomics J. 2008;8:23–28. doi: 10.1038/sj.tpj.6500455. [DOI] [PubMed] [Google Scholar]
- 24.Ross CJ, Katzov-Eckert H, Dube MP, et al. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat Genet. 2009;41:1345–1349. doi: 10.1038/ng.478. [DOI] [PubMed] [Google Scholar]
- 25.Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: The current consensus. Acta neuropathol. 2012;123:465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]