Abstract
OBJECTIVE
Chikungunya virus (CHIKV) is an arthritogenic mosquito-transmitted alphavirus that spread to the Caribbean in 2013 and the United States in 2014. CHIKV-infected patients develop inflammatory arthritis that can persist for months to years, but little is known about the rheumatologic and immunologic features of CHIKV arthritis in humans, particularly as compared to rheumatoid arthritis (RA). Here, we describe these features in a group of 10 American travelers who were nearly simultaneously infected while visiting Haiti in June 2014.
METHODS
Patients were assessed by history, physical examination, and laboratory studies. All patients with CHIKV arthritis had detectable anti-CHIKV IgG. Using cytometry by time of flight (CyTOF), we analyzed peripheral blood mononuclear cells in CHIKV-infected patients, healthy controls, and patients with untreated, active RA.
RESULTS
Among ten CHIKV-infected individuals, eight developed persistent symmetric polyarthritis, who otherwise met the 2010 ACR/EULAR criteria for (seronegative) RA. CyTOF analysis revealed that RA and CHIKV-infected patients had greater percentages of activated and effector CD4+ and CD8+ T cells than healthy controls.
CONCLUSION
In addition to similar clinical features, patients with CHIKV infection and RA develop highly similar peripheral T cell phenotypes. These overlapping clinical and immunologic features highlight a need for rheumatologists to consider CHIKV infection when evaluating patients with new, symmetric polyarthritis.
INTRODUCTION
Chikungunya virus (CHIKV) is a mosquito-transmitted alphavirus that was first isolated in the 1950s from patients in Tanzania with fever and arthritis (1). Subsequent CHIKV outbreaks were regionally confined, but the virus began to spread widely over the last decade. Millions of people have been infected in La Reunion Island in the Indian Ocean and India (2, 3). In December 2013, CHIKV infections were reported in the Caribbean (4) and subsequently detected in the United States, including documented autochthonous infections in non-travelers in Florida (5). The Caribbean strain of CHIKV is spread by Aedes aegyptii, a mosquito found along the United States Gulf Coast. However, a single mutation in an envelope protein enhances virus spread by a second mosquito, Aedes albopictus, found throughout much of the continental US (6). Thus, there is great potential for CHIKV to spread quickly in North America, much like West Nile virus did more than a decade ago (7).
Acute CHIKV infection is characterized by viremia, fever, rash, arthralgia, arthritis, and myalgia. The fever and rash usually resolve within 7-10 days but arthralgia and inflammatory arthritis can persist in up to 60% of patients for up to three years (8). CHIKV has not been cultured from synovial fluid, but viral RNA can be detected in the synovium, suggesting that CHIKV may directly invade and persist within joints (9). CHIKV shares many clinical features with RA for which an etiology is unknown, including possible erosive disease (10). Thus, CHIKV-associated arthritis may present a unique challenge for rheumatologists in the differential diagnosis of chronic polyarthritis, but there have been few studies of CHIKV infection in patients from the Western Hemisphere.
Here, we describe a group of 10 Americans who traveled to Haiti within a 10-day period in June 2014 and became infected with CHIKV. This cohort allowed us to temporally assess the clinical and laboratory features, and immune cell phenotypes in nearly simultaneously infected individuals. There were potential immunologic similarities and differences between CHIKV-infected and newly diagnosed, untreated RA patients. To our knowledge, this report is the first rheumatologic description of nearly simultaneously CHIKV-infected travelers from the Western Hemisphere.
PATIENTS AND METHODS
Three groups from the Saint Louis, Missouri area traveled to Haiti between June 10th and June 19th, 2014 during a CHIKV outbreak (11). All travelers were similarly recruited regardless of symptoms. Most developed acute fever, rash, headache, and arthritis, including ten individuals who agreed to participate in this study. The age distribution was 18-57 years old, with younger and older patients being similarly affected. Several individuals presented to our rheumatology clinic in July 2014 after their arthritis failed to respond to NSAIDs. At 7-10 weeks post-infection, each patient gave informed consent and was examined by a rheumatology fellow and/or attending rheumatologist and completed a uniform questionnaire designed for this study in concordance with the Washington University Arthritis and Rheumatology-Tissue Procurement Facility IRB-approved protocol which included the ACR/EULAR criteria for RA. We isolated PBMCs from patients with CHIKV infection, healthy controls, and 6 newly diagnosed, untreated RA patients. All travelers underwent laboratory testing for routine CBC, CMP (comprehensive metabolic panel: total protein, albumin, liver enzymes, bilirubin, Ca++, BUN, Cr, electrolytes, glucose), ESR, CRP, CCP, RF, ANA, CK, and uric acid in a single laboratory. All patient sera were analyzed for anti-CHIKV IgG antibodies by ELISA using purified CHIKV E2 protein produced as previously described (12). For some samples, we independently confirmed anti-CHIKV IgG with a second ELISA using captured virions from an attenuated strain (CHIKV 181/25, see supplemental methods).
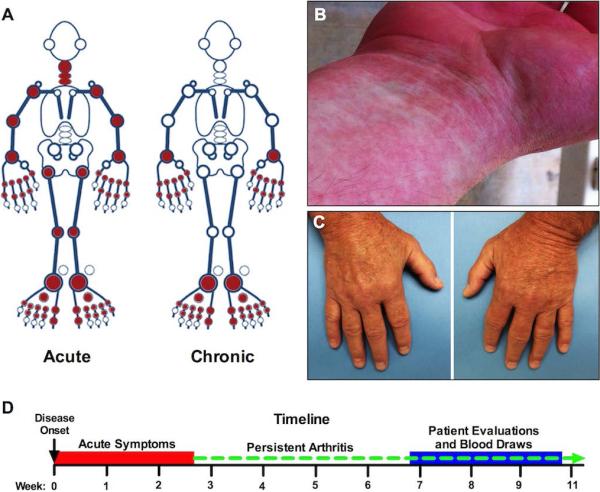
Patient #1 is a 33-year-old Caucasian woman with a history of polycystic ovarian syndrome who arrived in Haiti on June 19th. She reported having several mosquito bites within three days of arrival and on June 27th developed fever, diffuse arthritis (Figure 1A), and an erythematous, maculopapular rash over her entire body (Figure 1B). She measured a temperature of 102-103°F (38.9-39.4°C) for 2-to-3 days, associated with headache, neck pain, and severe symmetric polyarthritis involving the PIPs, MCPs, wrists, elbows, shoulders, neck, hips, knees, ankles, mid-foot, and MTPs, consistent with an acute pattern (Figure 1A). The acute rash and fever resolved within a few days, followed by desquamation. However, arthritis with morning stiffness persisted more than five months post infection, especially in the hands and feet. On July 14th, she presented to the emergency department for chronic MTP and mid-foot pain that made ambulation difficult and was referred to rheumatology. Foot radiographs were unremarkable, but synovitis in the left mid-foot was confirmed by musculoskeletal ultrasound. In addition to testing positive for anti-CHIKV IgG by both ELISA methods, the patient was also confirmed to have high-titer anti-CHIKV antibodies by the CDC. ANA was ≥1:320 (ref: ≤1:40), but anti-dsDNA was negative as were RF and CCP. Other labs included WBC 15.3 K/cumm (ref: 3.8-9.8 K/cumm) with a normal differential, CRP 9.56 mg/L (ref: 0.00-3.00 mg/L), and normal ESR. Her arthritis only slightly improved with ibuprofen 800 mg TID or naproxen 500 mg BID, which she took consistently. At 8 weeks post-infection, pain continued to interfere with her ability to work. A trial of prednisone 20 mg PO daily was initiated but it exacerbated her joint pain, so it was stopped out of concern that there could still be live virus within the joints. Five months after initial infection, she stopped taking naproxen 500mg BID because of GI upset. Within a few days, the arthritis in her feet acutely worsened, causing difficulty with ambulation. Her arthritis was still active at the time of this report.
Figure 1.
Clinical features of disease in patients with CHIKV infection. A) Distribution of joint involvement in the most severely affected patients (Patients #1 and #2), based on clinical history in the acute phase and physician examination at the chronic stage. B) Maculopapular rash during the acute infectious phase in Patient #1. In this patient, rash was distributed over the entire body, resolved after a few days, and was followed by desquamation. C) Active symmetric, synovitis in the MCPs and PIPs of a patient with active CHIKV-related arthritis during the persistent phase. D) Timeline (drawn to scale) depicting onset of acute symptoms, persistence of arthritis, and time of clinical evaluation with blood collection. Most patients' acute symptoms resolved within 7 days. The red box indicates the time period in which at least some patients were still having acute symptoms.
Patient #2 is a 57-year-old Caucasian man with a history of hyperlipidemia who entered Haiti on June 19th and noted multiple mosquito bites almost immediately. Within 10 days, he developed high fever, a diffuse maculopapular rash, joint pain, stiffness, and swelling consistent with an acute pattern (Figure 1A). The fever and rash resolved within two days, but the arthritis persisted. When he was evaluated by a rheumatology fellow and attending physician at 7 weeks post-infection, he was unable to make a fist and reported severe joint stiffness and pain that were worse in the morning or after inactivity. He ambulated with a limp due to ankle pain and swelling, and there was active synovitis in the MCPs, PIPs (Figure 1C), MTPs, and ankles bilaterally. Unlike RA, there was no synovial proliferation. RF, CCP, and ANA were negative, but high titer anti-CHIKV IgG was positive by both ELISA methods. ESR and CRP were normal. He was treated with NSAIDs but reported only minimal relief. Immunosuppression and joint injections were avoided because of concern of possible live virus in the synovium. His joint pain began to improve about 3 months after the initial infection, but he continued to have joint swelling 4 months later, at the time of this report.
Additional patient clinical courses are provided in the supplemental materials. In general, all patients had their potential exposure to CHIKV only during a visit to Haiti in June, 2014 and were evaluated approximately 7-10 weeks after disease onset (Figure 1D).
CyTOF analysis
PBMCs were isolated, frozen in 10% DMSO, and thawed for simultaneous parallel analysis. The cells were labeled with antibodies conjugated to transition element isotopes and analyzed on a CyTOF 2 mass cytometer (Fluidigm DVS Sciences; CA, USA) in the Center for Human Immunology and Immunotherapy Programs. Data were processed in Cytobank http://wustl.cytobank.org (Cytobank Inc.; CA, USA) and FlowJo 10.0.7 (FlowJO LLC.; OR, USA). See supplemental materials for more details.
RESULTS
A total of ten travelers to Haiti were recruited for this study; all were positive for anti-CHIKV IgG including eight patients with persistent arthritis (Table 1). Although there were additional travelers in the groups, not all could be contacted or agreed to participate for unclear reasons. Two CHIKV-seropositive patients had only mild symptoms (headache, fever, and/or rash) without arthritis and their initial fever and rash resolved within a week. However, all 8 individuals with arthritis (pain and swelling with or without redness) at onset also reported acute development (within hours to maximal intensity) of widespread myalgias, arthralgias and arthritis then persistent, symmetric polyarthritis lasting at least 6 weeks, especially involving the hands and feet (Figure 1A, 1D). Most arthritic patients still had joint pain and morning stiffness at least 8 weeks after initial infection; some reported gradual improvement of their symptoms. The arthritis was severe enough to cause some patients to miss work or seek urgent medical care for joint pain. Based on history, the acute arthritis tended to be more diffuse, whereas the chronic arthritis (assessed by physician) primarily affected the hands, wrists, feet, and ankles (Figure 1A). Two chronic patients had difficulty with ambulation due to pain in their feet or ankles. Even the patients with milder forms of CHIKV arthritis reported morning stiffness for a minimum of 1-2 hours, symmetric joint pain, and intermittent swelling lasting days, much like palindromic rheumatism. No patient had RF, anti-CCP antibodies, or personal or family history of rheumatologic disease. Three patients with CHIKV infection had positive ANAs. Two patients had an elevated CRP, one patient had a normocytic anemia, and there were no major abnormalities in the other laboratory parameters tested (see Methods). Joint aspirations were not performed since joint swelling generally involved the small joints of the hands and feet at the time of examination. Knee aspiration was considered for one patient, but she declined the procedure. Treatment with NSAIDs was marginally effective in some patients. Thus, if we had not been aware of their travel history to Haiti, all eight patients with CHIKV arthritis would have met the 2010 ACR/EULAR criteria for RA (13).
Table 1.
Clinical features of patients with confirmed CHIKV infection
| Pt. # | Gender | Initial Fever | Initial Rash | Symmetric Joint Pain | Joint Swelling | Morning Stiffness | RF | CCP | Elevated ESR | Elevated CRP | ANA | CHIKV IgG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | + | + | + | + | + | - | - | - | + | 1:320 | + |
| 2 | M | + | + | + | + | + | - | - | - | + | - | + |
| 3 | F | + | + | + | + | + | - | - | - | - | - | + |
| 4 | M | + | + | + | + | + | - | - | - | - | - | + |
| 5 | F | + | + | + | + | + | - | - | - | - | - | + |
| 6 | F | + | + | + | + | +/− | - | - | - | - | - | + |
| 7 | F | + | + | + | + | + | - | - | - | - | 1:2560 | + |
| 8 | F | +/− | - | - | - | - | - | - | - | - | - | + |
| 9 | F | + | + | - | - | - | - | - | - | - | 1:80 | + |
| 10 | F | + | + | + | + | + | ? | ? | - | - | ? | + |
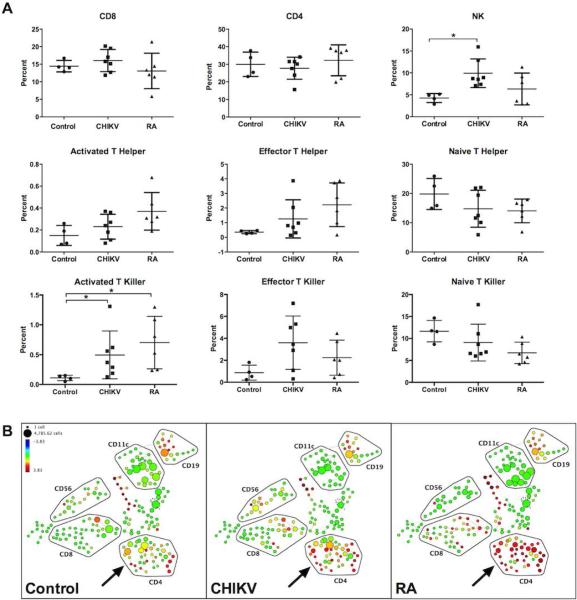
To better characterize the immunological parameters in CHIKV arthritis patients, we studied their PBMCs by CyTOF, a recently developed instrument akin to a fluorescence-based flow cytometer. Rather than cells stained with fluorochrome-conjugated antibodies, CyTOF analyzes cells stained with antibodies conjugated with rare earth metal isotopes, which are detected by mass spectrometry. Since metal isotopes have minimal background and do not require compensation across channels, many more markers can be quantified concurrently, allowing more complex, detailed analysis of immune cells than by conventional flow cytometry. Remarkably, we found that patients with CHIKV and RA had similar NK and T cell profiles including similar percentages of naïve, activated, and effector T killer and T helper populations (Figure 2A). Although PBMCs from CHIKV and RA were phenotypically similar to each other, there were only statistically significant differences in the activated T killer populations when these groups were compared to healthy controls and a significant increase in NK cell percentage in CHIKV compared with healthy controls. Although our sample size was small, spanning tree progression of density normalized events (SPADE) plots revealed a consistent trend toward higher L-selectin (CD62L) expression in CD4+ T cell subsets of RA patients when compared to healthy control and CHIKV-infected patients (Figure 2B). These data suggest that lymphocyte phenotypes in patients with CHIKV infection and RA are similar to each other, with subtle but distinct trends that could potentially distinguish these two groups from healthy controls, and from each other.
Figure 2.
Comparison of cell surface receptor and lymphocyte subset percentages in uninfected, CHIKV-infected, and untreated RA patients from two experiments. (A) Cell counts for each subset were derived from sequential manual gating of CyTOF data based on multiple cell surface marker expression. Representative data points from individual patients are shown with the mean percentage depicted by a line. One patient with CHIKV arthritis was unable to donate sufficient blood for PBMC collection. The results were validated by two independent CyTOF experiments performed using separate PBMC aliquots from patients with CHIKV arthritis (n=7), untreated RA (n=6; four seropositive and two seronegative RA), and healthy control subjects (n=4). Statistical significance was tested by one-way ANOVA followed by Bonferroni's post-test (*, p<0.05). (B) Representative SPADE plots from 3 different patient groups (left panel, control; middle panel, CHIKV; right panel, RA) showing expression of L-selectin (CD62L) are presented. Clustering of cell groups was performed manually. Relative fold-expression is indicated by the color scale. Each circle represents a cluster of cells expressing similar levels of all 22 markers and its relation to other cluster of cells expressing similar combination of 22 markers. The size of the circle correlates with the number of cells in each cluster.
DISCUSSION
The global spread of CHIKV to the Caribbean and the United States suggests that this disease is likely to become a diagnostic challenge for rheumatologists. We currently have a limited understanding of the immunologic and virologic mechanisms that create such diverse patient phenotypes, ranging from practically asymptomatic to persistent, debilitating arthritis. Furthermore, the pathogenesis of CHIKV-related arthritis remains poorly understood, but further study may provide insight into RA as well. Our report is the first to use CyTOF for analysis of immune cells in patients with either CHIKV infection or untreated RA. Since our cohort was almost simultaneously infected with CHIKV, we were able to assess PBMC phenotypes in CHIKV-infected individuals at a similar time in their clinical course, and identify similarities in their lymphocyte subpopulations to those from untreated RA patients. By conventional flow cytometry during acute CHIKV infection, one prior study documented T cell activation which resolved 6 months after infection (9). Longitudinal monitoring of our cohort may further detail the T cell phenotype and whether its resolution correlates with improvement in arthritic symptoms.
Although our conclusions are limited by a small sample size, our work underscores that CHIKV-related arthritis mimics seronegative RA. Eight of these patients would have met the 2010 ACR/EULAR criteria for RA if the initial fever, rash, and travel to the Caribbean had not been revealed. A confounding issue in prior studies is whether CHIKV arthritis and RA can be adequately distinguished from each other in CHIKV-endemic regions. For example, patients from the Eastern Hemisphere might have anti-CHIKV IgG because of prior, resolved infection and later developed bona fide RA. In such patients, CHIKV serologies may be less useful in distinguishing between persistent CHIKV arthritis and seronegative RA. However, our cohort had a defined temporal risk exposure to CHIKV, so we could reliably use the clinical history and serology to definitely diagnose CHIKV-related arthritis. Since we do not know how long to expect patients to remain seropositive, it will also be informative to continue monitoring their CHIKV serologies.
Whether immunosuppression with DMARDs or biologics used for RA is appropriate or effective remains controversial in the absence of randomized controlled trials for CHIKV-related arthritis. Our anecdotal experience with this cohort suggests that NSAIDs are marginally effective for persistent arthritis, and one study found that chloroquine was no more effective than NSAIDs (14). Furthermore, immunosuppression in CHIKV-infected patients could be deleterious because viral RNA and antigens can be found in target tissues in the chronic phase in humans and experimental animals (15).
Why some patients develop mild symptoms whereas others experience persistent, severe arthritis remains unclear though this could be related to viral inoculum dose as shown in experimental models (15). Our cohort had a high frequency (80%) of persistent CHIKV-related arthritis. However, there may have been a selection bias in our observational study, since symptomatic patients may have been more motivated to participate. Future prospective studies will be useful in determining the true incidence of persistent CHIKV-related arthritis.
Rheumatologists, even in non-CHIKV-endemic regions, need to consider CHIKV in their evaluation of symmetric polyarthritis lasting more than 6 weeks by obtaining a history of travel to CHIKV-endemic regions, which are likely to expand in the near future. The abrupt onset of arthritis as well as fever and rash of acute CHIKV infection may also be useful discriminating features. In patients who might have been exposed to CHIKV, serologic testing may be necessary before initiating immunosuppression. Also of concern is the possibility that RA patients could become infected with CHIKV and appear to be otherwise in the midst of a routine flare. Unfortunately, access to CHIKV testing is highly constrained at the current time as it is only available from the CDC and research laboratories. The CDC recommends that only symptomatic individuals who were potentially exposed to CHIKV should be tested at this time. Moving forward, the clinical history and validated, easily accessible laboratory tests for anti-CHIKV antibodies likely will be important tools for rheumatologists in order to distinguish CHIKV-related arthritis from seronegative RA.
Supplementary Material
ACKNOWLEDGMENTS
We thank Christopher Focht for helpful discussions regarding CyTOF statistical analysis and Lorraine Schwartz and Lacey Feigl for assistance in the collection of patient samples. Joint involvement in Figure 1 was depicted with permission using Discus Analytics' JointMan® homunculus.
Research Support : This study was supported by the Barnes-Jewish Hospital Foundation and the Howard Hughes Medical Institute.
Footnotes
Conflicts of Interest: There are no conflicts of interest to report.
REFERENCES
- 1.Ross R. The Newala epidemic. J Hyg (Lond) 1956;54(2):177–91. doi: 10.1017/s0022172400044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kariuki Njenga M, Nderitu L, Ledermann JP, Ndirangu A, Logue CH, Kelly CHL, et al. Tracking epidemic Chikungunya virus into the Indian Ocean from East Africa. Journal of General Virology. 2008;89(11):2754–60. doi: 10.1099/vir.0.2008/005413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staples JE, Breiman RF, Powers AM. Chikungunya Fever: An Epidemiological Review of a Re-Emerging Infectious Disease. Clinical Infectious Diseases. 2009;49(6):942–8. doi: 10.1086/605496. [DOI] [PubMed] [Google Scholar]
- 4.Enserink M. Crippling Virus Set to Conquer Western Hemisphere. Science. 2014;344(6185):678–9. doi: 10.1126/science.344.6185.678. [DOI] [PubMed] [Google Scholar]
- 5.Kuehn BM. Chikungunya virus transmission found in the united states: Us health authorities brace for wider spread. JAMA. 2014;312(8):776–7. doi: 10.1001/jama.2014.9916. [DOI] [PubMed] [Google Scholar]
- 6.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A Single Mutation in Chikungunya Virus Affects Vector Specificity and Epidemic Potential. PLoS Pathog. 2007;3(12):e201. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gubler DJ. The Continuing Spread of West Nile Virus in the Western Hemisphere. Clinical Infectious Diseases. 2007;45(8):1039–46. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- 8.Schilte C, Staikovsky F, Couderc T, Madec Y, Carpentier F, Kassab S, et al. Chikungunya Virus-associated Long-term Arthralgia: A 36-month Prospective Longitudinal Study. PLoS Negl Trop Dis. 2013;7(3):e2137. doi: 10.1371/journal.pntd.0002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoarau J-J, Jaffar Bandjee M-C, Krejbich Trotot P, Das T, Li-Pat-Yuen G, Dassa B, et al. Persistent Chronic Inflammation and Infection by Chikungunya Arthritogenic Alphavirus in Spite of a Robust Host Immune Response. The Journal of Immunology. 2010;184(10):5914–27. doi: 10.4049/jimmunol.0900255. [DOI] [PubMed] [Google Scholar]
- 10.Manimunda SP, Vijayachari P, Uppoor R, Sugunan AP, Singh SS, Rai SK, et al. Clinical progression of chikungunya fever during acute and chronic arthritic stages and the changes in joint morphology as revealed by imaging. Transactions of The Royal Society of Tropical Medicine and Hygiene. 2010;104(6):392–9. doi: 10.1016/j.trstmh.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Fischer M, Staples JE. Notes from the Field: Chikungunya Virus Spreads in the Americas — Caribbean and South America, 2013–2014. CDC Morbidity and Mortality Weekly Report. 2014 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6322a5.htm. [PMC free article] [PubMed]
- 12.Pal P, Dowd KA, Brien JD, Edeling MA, Gorlatov S, Johnson S, et al. Development of a Highly Protective Combination Monoclonal Antibody Therapy against Chikungunya Virus. PLoS Pathog. 2013;9(4):e1003312. doi: 10.1371/journal.ppat.1003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis & Rheumatism. 2010;62(9):2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 14.Chopra A, Saluja M, Venugopalan A. Effectiveness of Chloroquine and Inflammatory Cytokine Response in Patients With Early Persistent Musculoskeletal Pain and Arthritis Following Chikungunya Virus Infection. Arthritis & Rheumatology. 2014;66(2):319–26. doi: 10.1002/art.38221. [DOI] [PubMed] [Google Scholar]
- 15.Labadie K, Larcher T, Joubert C, Mannioui A, Delache B, Brochard P, et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. The Journal of Clinical Investigation. 2010;120(3):894–906. doi: 10.1172/JCI40104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.