The MauiDerm annual educational meeting, sponsored by Advances in Cosmetic and Medical Dermatology, aims to increase physician knowledge in the areas of both medical and cosmetic dermatology. MauiDerm’s world-renowned experts have discussed significant advances in the diagnosis, management, and treatment of both medical and cosmetic dermatologic conditions at a very high scientific level. This supplement to The Journal of Clinical and Aesthetic Dermatology is based upon the proceedings from the cutaneous oncology, psoriasis, and psoriatic arthritis sessions that took place at the 2015 MauiDerm Meeting.
Psoriasis Update
Psoriasis is an immunologically mediated systemic disease involving both the skin and joints. A growing understanding of immunologic pathways has created interest in treating psoriasis with targeted biologics, such as the well-known tumor necrosis factor alpha (TNF-α) inhibitors etanercept, infliximab, and adalimumab. Two other TNF inhibitors, golimumab and certolizumab pegol, have been shown effective against plaque psoriasis in clinical studies of psoriatic arthritis.1 An important new small molecule for treating both psoriasis and psoriatic arthritis is apremilast, approved for commercial release by the United States Food and Drug Administration (FDA) in September 2014.
Apremilast is a phosphodiesterase-4 (PDE4) enzyme inhibitor that blocks leukocyte production of interleukin (IL)-12, IL-23, TNF-α, and interferon (INF)-y and suppresses the immune responses mediated by Th1 and Th17.2 Apremilast was approved for both psoriatic arthritis and psoriasis.
In a phase 3, multicenter, double-blind, placebo-controlled study, 844 patients were randomized in a 2:1 ratio to receive apremilast or placebo for the treatment of moderate-to-severe plaque psoriasis.3 Patients were treated with apremilast 30mg twice a day with or without topicals and ultraviolet-B phototherapy. Doses of apremilast were titrated during the first week of administration and again at Week 16 when placebo patients were switched to apremilast. At 16 weeks, more patients in the apremilast group achieved PASI-75 than placebo (33.1 vs. 5.3%, p<0.001).
A second phase 3 clinical study, ESTEEM 2, has not yet been published, but examines the use of apremilast in nail, scalp, and palmoplantar psoriasis.4 Adverse events in ESTEEM 1 were mild to moderate. In the placebo-controlled portion of the study, 55.7 versus 69.3 percent of placebo versus apremilast patients, respectively, reported at least one adverse event.3 It should be noted that no new adverse events were reported after Week 16, and the incidence of serious adverse events was 2.8 percent for apremilast versus 2.1 percent for placebo. The most frequently reported adverse events based on pooled data from ESTEEM 1 during the first 16 weeks were diarrhea (18.8 vs. 7.1% for apremilast vs. placebo patients, respectively) and nausea (15.7 vs. 6.7%, respectively). Other adverse events reported in ESTEEM 1 include upper respiratory tract infection, nasopharyngitis, and headache. The discontinuation rates owing to adverse events were low (1.8% in apremilast group and 0.4% in placebo group). Apremilast was shown in another study to produce a slightly higher response rate in patients who had received no prior systemic or biologic therapy.5
Apremilast was associated with transient abnormal laboratory parameters, which investigators deemed as not clinically significant. Further studies will examine the effect of apremilast versus placebo on pruritus, weight loss, and psychiatric disorders. According to product labeling, in the 0- to 16-week placebo-controlled period of three controlled clinical studies, 1.3 percent of apremilast patients versus 0.4 percent of placebo patients reported depression.6 Product labeling also warns about weight decrease,6 the mechanism of action for which remains unknown. Patients with both high and low body mass index (BMI) may lose as much as five percent of total body weight.
With psoriasis, durable results remain a clinical challenge. Data extracted from a prospective registry in the Netherlands were analyzed for one-year survival with adalimumab, etanercept, and ustekinumab for so-called “happy drug survival,” defined as a score on the Dermatology Life Quality Index (DLQI) >5 at 3, 6, 9, and 12 months.7 At baseline, 73 percent of patients could be considered “unhappy.” The percentage of treatment episodes with “happy” patients taking drugs increased to 79 percent after one year. Ustekinumab showed better overall drug survival than etanercept and a trend toward better overall survival than adalimumab.7
PSOLAR is a large, ongoing, international, observational registry of more than 12,000 psoriasis patients treated with biologics (infliximab, ustekinumab, adalimumab, and etanercept) and other agents. Cumulative rates are 0.46 per 100 patient years for death, 0.26 per 100 patient years for a major adverse cardiovascular event (MACE), 0.68 per 100 patient years for malignancy, and 1.50 per 100 patient years for a serious infection.8 Notable in these findings is that age was a significant predictor for all adverse events of interest. Compared to nonbiologic treatments for psoriasis, the use of biologics is not a significant predictor of death, MACE, or malignancy and the PSOLAR data through 2013 revealed no new safety concerns.8 A more recent analysis of PSOLAR data found a higher risk for serious infections with adalimumab and infliximab (but not ustekinumab or etanercept) versus nonbiologic and non-methotrexate therapies.9
Interpatient pharmacokinetic variability, differences in therapeutic response, drug immunogenicity, and the natural fluctuations in the course of a chronic condition, such as psoriasis, may lead to off-label dosing (both dose escalation and reduction). Using the Spanish Registry for Systemic Treatments in Psoriasis (BIOBADERM), which includes data from approximately 2,000 moderate-to-severe psoriasis patients translating to 5,383 person-years, dose reduction, usually achieved by extending the dosing interval, occurred mainly in patients treated with adalimumab (41.3%), infliximab (33.3%), ustekinumab (30.9%) and infliximab (29.4%).10
Using statistical regression, the risk of dose reduction increased by eight percent for every five percent improvement in Psoriasis Area and Severity Index (PASI) at the cutoff date. On the other hand, dose escalation occurred with infliximab (13.7%), ustekinumab (10.4%), etanercept (7.9%), and adalimumab (2.2%), accomplished primarily by truncating the dosing interval. There were 2,209 discontinuations over the course of the study, the main reasons for which were lack of efficacy (36.4%) and disease remission (27.2%). Further, for each additional biologic agent, the odds increased 85 percent for dose escalation. The BIOBADERM analysis found that about 40 percent of moderate-to-severe psoriasis patients on biologic therapy were receiving an off-label dose; dose escalation was more frequent for ustekinumab and infliximab.10
Principles of Topical Therapy: Maintenance Strategies and Future Directions
Despite the growing academic interest in biologics, most psoriasis patients today are treated topically, and emerging topical therapies may offer important advantages in psoriasis treatment.11 Some of these new molecules and new formulations are discussed below, along with strategies to maintain remission in topical treatment of psoriasis.
When a patient is cleared with topical therapy, the prescriber must arrive at a strategy for long-term maintenance, such as a “drug holiday.” In a placebo-controlled study of psoriasis patients treated with betamethasone dipropionate in an optimized vehicle twice daily, 38/59 patients had 85-percent improvement or greater versus baseline.12 Of these patients, 74 percent could maintain remission with treatment on weekends only, compared to 21 percent who maintained remission with vehicle only. In an open-label, multicenter study of 316 psoriasis patients treated with clobetasol propionate intermittently over 14 days, 62 percent achieved clearance.13 When 132 of the cleared patients were put on a twice-weekly maintenance schedule, 75 percent of patients remained clear.
Patient compliance is crucial to good results with topical therapy. In a randomized trial of 885 scalp psoriasis patients, patients were maintained on a formulation of calcipotriol (50ug.g) and betamethasone dipropionate (0.5mg/kg) either by using it twice weekly (Group A) or by using it on demand (Group B).14 After two weeks and at evaluations on 4, 8, and 12 weeks, both groups showed significant improvement over baseline, but at 8 and 12 weeks, Group A had a significantly improved clinical response compared to Group B (p<0.05). Group A patients had a significantly lower relapse rate compared to Group B (19.5 vs. 41.7%, p<0.001).14 Thus, even well-maintained patients may benefit from fixed scheduling.
The vehicle can have a substantial impact on how well and deeply the topical agent penetrates the skin. In fact, changing the vehicle may change the product’s potency, as is the case with mometasone furoate, a high-potency Class II topical when prepared in a 0.1% ointment, but is a mid-potency Class IV in a 0.1% cream product. Desoximetasone is another example, which is more potent as a new spray product (Class I) than previous formulations.15
A number of new molecules for topical therapy are on the horizon; in some cases these “new” molecules are established drugs being reformulated for potential topical treatments. Data are not yet available for a new fixed combination of tazarotene and halobetasol for psoriasis. Topical halobetasol propionate 0.5% ointment is a Class I corticosteroid with demonstrated efficacy as monotherapy against plaque psoriasis.16 It may be combined with tazarotene without affecting the stability of halobetasol and may offer synergistic efficacy.16,17
Calcipotriene combined with betamethasone dipropionate for pediatric scalp psoriasis was shown effective in a prospective observational study, including 84 treatment episodes.18 At 12 weeks, the Psoriasis Scalp Severity Index (PSSI) showed significant improvement with treatment (18.7%±11.8 to 12.7±9.4) and this result could be maintained over 48 weeks of follow-up. This combination of calcipotriene and betamethasone dipropionate had previously been shown to be more effective in the treatment of adult scalp psoriasis than either agent in monotherapy.19,20 In an older multicenter study, combination therapy of halobetasol plus calcipotriene was tested against these two agents as monotherapies for treating psoriasis in adults.21 Patients used calcipotriene ointment 0.005% mornings and halobetasol propionate ointment 0.05% in the evening versus either calcipotriene or halobetasol ointment (as monotherapy) applied twice daily in a study of 127 patients with moderate plaque psoriasis. Efficacy was better for combination treatment (71% for combination vs. 57% for halobetasol alone and 30% for calipotriene alone), and combination therapy resulted in fewer cutaneous side effects compared to calcipotriene alone.21
In a study of 86 plaque psoriasis patients treated with twice-daily clobetasol foam plus calcipotriene ointment versus these agents in monotherapy, combination patients achieved significantly lower psoriasis scores (p<0.001) at two weeks compared to monotherapy patients.22 Adjusted trunk lesion scores were 0.67 for combination therapy, 1.40 for calcipotriene alone, and 1.13 for clobetasol foam alone. Combination therapy patients who remitted received weekday calcipotriene therapy and on weekends used clobetasol foam or vehicle for six months; during this phase, clobetasol foam trended toward greater maintenance of remission versus vehicle (92% improvement of trunk lesions vs. 62%, respectively).22
Calcipotriene is available commercially as an ointment, a solution, a cream, a fixed-dose combination ointment product with betamethasone dipropionate, and most recently as an aqueous-based foam. In two identical, randomized, double-blind, vehicle-controlled, eight-week studies, 659 plaque psoriasis patients were randomized to receive calcipotriene 0.005% foam or vehicle, twice daily.23 Based on intention-to-treat analysis, treatment success in the first of the two studies occurred in 14 percent of calcipotriene foam versus seven percent of vehicle patients at eight weeks (P=0.058). In the last-observation-carried-forward (LOCF) analysis, calcipotriene foam was effective in 15 versus 7 percent of patients (P=0.034). In the second study, calcipotriene foam patients had significantly better results than vehicle foam patients (27 vs. 16%, respectively, p=0.016); using LOCF analysis, results were 28 versus 16 percent, respectively, p=0.010). Adverse events rates were similar between groups. Calcipotriene foam 0.005% was also more effective than vehicle foam on scalp psoriasis at eight weeks.24
Janus kinase (JAK) inhibitors are an important new class of drugs with potential indications for psoriasis as well as rheumatic disorders and several types of cancer.25,26 The Janus family kinases include JAK1, JAK2, JAK3, and TYK2, involved in cell growth, cell survival, as well as cell development and differentiation.27 JAK inhibitors block the barrage of cytokines bombarding a given cell in an effort to have their unique signaling cascade impact genetic transcription of that cell. These bombarding cytokines include, but are not limited to, TNF, immune complexes, and T-cell antigens.28 JAK inhibitors block those bombarding cytokines; signal transducer and activator transcription (STAT) signaling pathways are activated by IL-2-produced cytokines,29 but STAT transcription factors make less promising targets for drug development as they lack enzymatic activity.30 Genetic mutations in the JAK-STAT pathway have been implicated in many autoinhibitory dysfunctions, including malignancies. Since psoriasis is associated with a plethora of pro-inflammatory cytokines, JAK inhibitors may offer promising new treatment options.31 For example, tofacitinib is a new small molecule for treatment of psoriasis and rheumatic disorders that primarily inhibits JAK1/JAK3.29,32 Originally developed as a selective JAK3 inhibitor for use in immunosuppression secondary to transplantations, tofacitinib was found to also inhibit JAK1 and was approved for treatment of active rheumatoid arthritis in 2012.30 A selective JAK3 inhibitor is in development for psoriasis treatment.33
A novel JAK inhibitor (ASP015K, peficitinib) demonstrated dose-dependent improvements in moderate-to-severe psoriasis at six weeks with no serious adverse events (n=124).34 Ruxolitinib, a selective JAK1 and JAK2 inhibitor is in development and may be useful in the treatment of psoriasis if formulated as a topical product.35 A potential benefit of JAK inhibition is that they can block signals from multiple (rather than single) cytokines on a cellular level.
Phosphodiesterase-4 (PDE4) inhibitors, such as apremilast, are small molecules that may benefit patients with psoriasis and psoriatic arthritis.36 A novel compound (AN-2728) containing boron is currently being studied for its use in a topical formulation in treating psoriasis and atopic dermatitis.37 AN-2728 inhibits PDE4 activity, and, in so doing, suppresses the release of TNF-α, IL-12, and IL-23. Selective PDE4 inhibitors reduce inflammation in almost all inflammatory cells and inhibit TNF-α.
What’s New in Systemic Psoriasis Treatments?
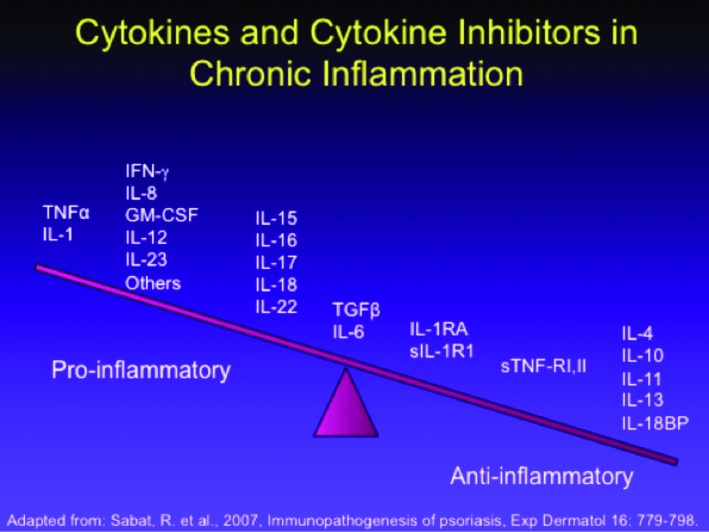
Psoriasis is a T-cell-driven inflammatory cutaneous disease,38,39 but there is differentiation between resident T-cells and T-cells recruited from the circulation.40,41 Visually, nonlesional skin in the psoriasis patient may appear devoid of inflammation, but often contains an abnormally high number of resident T-cells in the dermis and epidermis, and these resident T-cells may have a TH-1 cytokine secretion bias.40 Preclinical studies have suggested the existence of an inflammatory axis in which IL-17 and IL-23 play important roles. This notion is supported by the observation that an agent targeting the p40 antibody (shared by IL-12 and IL-23) improves psoriatic lesions.42,43 A summary of the cytokine and cytokine inhibitors associated with chronic inflammation appears in Figure 1. Second-generation biologic agents, such as adalimumab, etanercept, infliximab,and certolizumab, for psoriasis treatment all target TNF-α.
Figure 1.
The cytokine and cytokine inhibitors relevant in chronic inflammation associated with psoriasis
Ustekinumab may be considered a third-generation biologic; it antagonizes IL-12 and IL23 by way of its unique p19 subunit, leading to downstream effects. Secukinumab, an IL-17 antagonist, was shown effective against psoriasis in two large randomized clinical trials.44 A summary of remarks on new drug development appears in Table 1.
TABLE 1.
A summary of new systemic drug therapies for psoriasis
| DRUG | CURRENT STATUS AND OBSERVATIONS | COMMENTS |
|---|---|---|
| Certolizumab-Pegol | Developed for treating Crohn’s disease | |
| Ustekinumab | Antagonizes IL-23 by targeting the p19 subunit (not shared) | Pure IL-23 antagonist; adverse events include risk of serious infection |
| Guselkumab | Phase 2 dose-ranging studies resulted in comparisons to adalimumab | |
| Tidrakizumab | Phase 2 dose-ranging studied, good PASI-90 scores at 12 weeks. Antagonizes IL-23 by targeting p19 subunit | Well-tolerated |
| Boehringer-Ingelheim 655066 | Novel monoclonal antibody, phase 2 proof-ofconcept study with 58% achieving PASI-90 and remaining clear at 66 weeks | Subcutaneous injection, may need dosing only every four months |
| Secukinumab, ixekizumab, and brodalumab | IL-17 antagonists | Secukinumab approved in U.S. for psoriasis in May 2015 |
Secukinumab’s efficacy was related directly to its serum concentration, an observation with particular clinical relevance with regard to predicting therapeutic response. Recommended doses are 150 and 300mg, but the FDA recommended 450mg for higher-BMI patients, even though the 450mg dose had not been tested.45
In a phase 2, double-blind, placebo-controlled study, 142 patients with moderate-to-severe plaque psoriasis received subcutaneous ixekizumab (10, 25, 75, or 150mg) or placebo at 0, 2, 4, 8, 12, and 16 weeks.46 At 12 weeks, PASI scores of >75% were significantly greater for ixekizumab patients at all but the 10mg dose: 82.1, 82.8, 76.7 percent for 150, 75, and 25mg, respectively, versus 7.7 percent placebo (p<0.001 for each). PASI 100% was achieved by 39.3 and 37.9 percent of ixekizumab 150 and 75mg patients, respectively, versus 0 patients in the placebo group (p<0.001 for both). Significant differences emerged by the first week and were durable through Week 20; adverse event rates were similar among all groups, including placebo, and no serious adverse events or MACE occurred. A subsequent analysis of study data explored whether achieving a PASI-50 score was predictive for achieving PASI-75 at 12 weeks and found a PASI-50 had 90 percent specificity and 83 percent sensitivity in this analysis.
In a 96-week, phase 2 study of brodalumab, 198 psoriasis patients were randomized to receive brodalumab 70, 140, or 210mg at Day 1 and again on Weeks 1, 2, 4, 6, 8, and 10 or 280 mg/month or placebo.47 The mean percentage improvements in PASI scores were 45.0 (70mg), 85.9 (140mg), 86.3 (210mg), and 76.0 percent (280mg/month) compared to 16.0 percent placebo patients (p<0.001 for all versus placebo). By Week 12, an improvement of at least 75 and 90 percent occurred in 77 and 72 percent of all patients, respectively, compared with 0 in the placebo group (p<0.001 for all). Adverse events included nasopharyngitis (8%), upper respiratory tract infection (8%), and injection-site erhythema (6%).47 As these drugs are also still standard drugs for the treatment of psoriatic arthritis, this finding is particularly relevant as both conditions arise in about one-third of those with plaque psoriasis.
The number-needed-to-treat (NNT) can be a valuable real-world metric in appropriate prescribing, as it helps convey effect size. NNT may be defined as the average number of patients who need to be treated in order to achieve one additional good outcome; as such, it is the inverse of absolute risk reduction.48 Since many systemic psoriasis treatments offer evident results in about four weeks, there is little to no need for biomarkers to predict response.
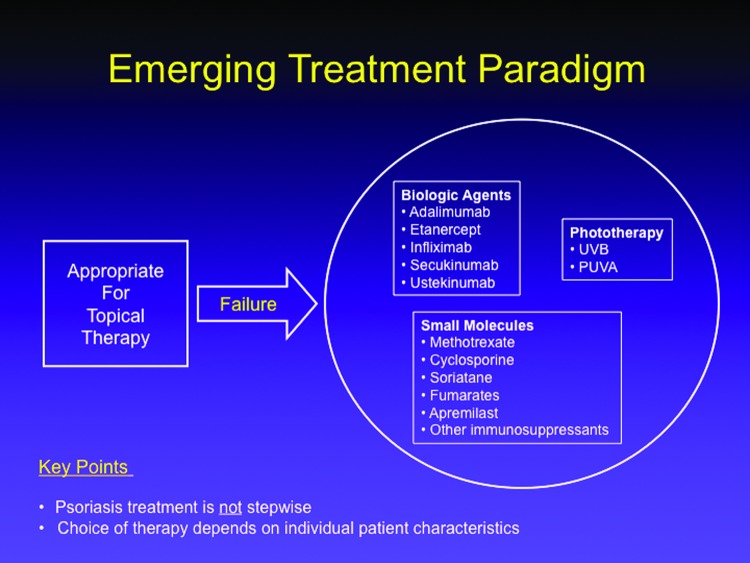
The “stepwise approach” to psoriasis treatment was replaced by individualized care. In the old model, patients initiated psoriasis therapy with over-the-counter (OTC) products and gradually progressed, step by step, to prescription agents, phototherapy, and finally systemic therapy. Each new step was only taken if the patient failed the prior step. The emerging treatment paradigm still recognizes two categories of psoriasis patients (i.e., those who achieve good results with a topical treatment versus those who clearly need more), but rather than proceeding step-by-step through increasingly more aggressive therapies, the choice of therapy following topical treatment failure is based on the patient’s individual characteristics. Thus, it may be appropriate to move a particular patient from failed topical therapy directly to a biologic with no intervening steps (Figure 2).
Figure 2.
The emerging treatment paradigm for psoriasis versus the older stepwise model.
Despite novel therapies, even in the year 2000, clearance was not considered a reasonable goal for psoriasis treatment. Fortunately, the attainable and reasonable goals for psoriasis treatment have changed drastically in the last 15 years. More small molecules and biologics for psoriasis are in the pipeline. Dermatologists will need a greater facility working with multiple drugs and managing more complex pharmacological treatments; patient expectations about therapeutic goals and product safety must evolve as well. In terms of systemic treatments for psoriasis, we are on the cusp of a renaissance.
Psoriatic Arthritis: Key Developments in 2014
The prevalence of psoriatic arthritis in psoriasis patients appears to be about 30 percent based on the PREPARE study, of whom about 41 percent had not been previously diagnosed with psoriatic arthritis.49 Extrapolated from psoriasis rates, the overall prevalence of psoriatic arthritis may be as high as one percents of the population.50 In patients with both psoriasis and psoriatic arthritis, the cutaneous symptoms may precede musculoskeletal symptoms by 10 years.51 Since joint damage may occur within two years of disease onset,52 timely and accurate diagnosis and prompt treatment are essential to maintain optimal function. Yet in a large, population-based survey of patients with psoriasis and/or psoriatic arthritis in Europe and North American (n=3,426 of whom 712 had psoriatic arthritis), the average time between onset of psoriatic arthritis signs and symptoms and its appropriate diagnosis was five years.53 Of those with known psoriatic arthritis, 15 percent in North America and 22 percent in Europe had not seen a healthcare provider in the past year for that condition. Only about a third of patients (38% in North America and 37% in Europe) reported their psoriatic arthritis was most often treated by a rheumatologist.53
Thus, there is an urgent need to shorten the time from onset of symptoms to diagnosis and treatment of psoriatic arthritis, because delayed diagnosis has been associated with poorer outcomes.54
Polyarticular disease in psoriatic arthritis can be as severe as rheumatoid arthritis, although it is often not treated as aggressively. Powerful new treatments for psoriatic arthritis are available, although 31 percent of those with known psoriatic arthritis receive only topical treatments and another 28 percent receive no treatment at all.53
A number of classification systems exist to help categorize psoriatic arthritis symptoms. The CASPAR Classification System requires that a patient must present with inflammatory articular disease (joint, spine, or entheseal) and have a score of >3 on the following: current psoriasis (score 2), family history of psoriasis, RF negativity, current dactylitis or history of dactylitis documented by a rheumatologist, radiographic evidence of juxta-articular new bone formation, or typical psoriatic nail dystrophy.55 The CASPAR Classification System is 91-percent sensitive and 99-percent specific for psoriatic arthritis, including early disease, and across various populations. Other screening tools include Psoriatic Arthritis Screening and Evaluation (PASE), Toronto Psoriatic Arthritis Screening Questionnaire (ToPAS), and Psoriasis Epidemiology Screening Tool (PEST). The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) has published treatment recommendations for psoriatic arthritis that are based on a multidisciplinary paradigm.56
TNF-inhibitors have revolutionized the treatment of psoriatic arthritis. In this regard, it is important to note that biosimilars will also change how psoriatic arthritis is treated.57 Biosimilars have been studied in diseases such as rheumatoid arthritis, but not psoriatic arthritis, yet in Europe, biosimilar approval encompassed psoriatic arthritis, although they had not been tested in this setting.58 This may very well have less to do with medical science and more to do with healthcare economics, but dermatologists in North America may soon encounter biosimilars arriving here in similar fashion.59
In determining appropriate dosing for TNF-inhibitors, the relationship of trough serum biologic concentrations and therapeutic response has been explored. In a prospective study of 103 consecutive psoriatic arthritis patients, patients were treated with adalimumab and their serum concentrations of antidrug antibodies (ADA) were recorded.60 ADA concentrations were significantly lower at 28 and 52 weeks in patients with detectable ADA levels compared to those with no detectable ADA levels. Patients with detectable ADA had lower adalimumab concentrations and significantly poorer outcomes compared to patients in whom no ADA could be detected. An important therapeutic topic with possible public health and even political ramifications involves treatment discontinuation for patients in remission. For example, in a study of 76 psoriatic arthritis patients, 53 remitted and had their doses halved; at 29 months, 88.6 percent were still in remission.61 However, in a prospective observational study of 26 psoriatic arthritis patients treated with methotrexate or a TNF-inhibitor, treatment was stopped upon remission and 77 percent experience rapid flares (mean 74.5±52 days).62 Should a decision be reached to taper or discontinue biologic therapy upon achievement of treatment goals, the clinical team should develop a tapering plan, establish metrics for clinical success and failure, and account for motivations for discontinuing therapy (for example, whether this strategy was a payer-drive decision or a patient preference).63,64 It is unclear how to identify the subset of patients in remission who will do well even after treatment stops, and it is also not established if and for how long they should be followed once treatment is discontinued.
In FUTURE1, a placebo-controlled study of 606 psoriatic arthritis patients treated with intravenous (IV) secukinumab 10mg/kg up to a maximum dose of 150mg, IV secukinumab 10mg/kg up to a maximum dose of 75mg, or placebo, 50.0 and 50.5 percent of the IV secukinumab 150 and 75mg maximum groups, respectively, met the study’s primary endpoint of an ARC20 score (placebo 17.3%) at 24 weeks. Scores for ARC50 were 34.7, 30.7, and 7.4 percent, respectively, and scores for ARC70 were 18.8, 16.8, and 2.0 percent, respectively.65 The FUTURE1 study then grouped patients by prior exposure to a TNF-inhibitor and found that TNF-inhibitor-naive patients overall had better results than those who had previously taken TNF inhibitors. Futhermore, PASI scores were good in the FUTURE1 study with 76.9 and 65.7 percent of patients taking IV secukinumab up to 150 or 75mg, respectively, achieving PASI75 scores and 59.3 and 48.1 percent, respectively, achieving PASI90.65
Ustekinumab is increasingly prescribed for psoriatic arthritis, with the unexpected finding in the PSUMMIT 1 and 2 studies that it reduced joint damage as evidenced in radiographs of hands and feet.66
The PALACE1 studies evaluated apremilast in psoriatic arthritis patients and found results for aprimelast 30mg twice daily, 20mg twice daily, and placebo were 45, 36, and 13 percent, respectively, for ARC20; 22, 16, and 4 percent, respectively, for ARC50; and 12, 6, and 1 percent, respectively, for ARC70.67 Data from that study and the subsequent PALACE2 and PALACE3 studies, found adverse events with apremilast occurred in about a quarter of all patients, with nausea and diarrhea the most frequently reported side effects. Unintentional weight loss of up to 5 or 10 percent of body weight occurred in about 10 percent of patients. Depression as a possible treatment-emergent adverse event occurred in one percent of aprimelast and 0.8 percent of placebo patients.68
Update on Psoriasis Comorbidities
There is a large and growing body of literature linking psoriasis to metabolic syndrome, atherosclerosis, and myocardial infarction.69-73 Obesity, an independent risk factor for psoriasis, has been associated with more severe psoriasis. Psoriasis is further associated with diabetes, major cardiovascular events, and chronic kidney disease independent of the traditional risk factors for these conditions.69,70,74 Although these comorbidities represent diverse phenotypes, they share the following pathological elements: chronic inflammation, angiogenesis, oxidative stress, and selected genetic components. Severe psoriasis is associated with an increased risk of mortality that culminates in approximately five years of life lost.75,76
Emerging data has compared cardiometabolic outcomes in psoriasis patients treated with systemic agents and phototherapy to rheumatoid arthritis patients receiving disease-modifying antirheumatic drugs (DMARDs). Psoriasis is associated with an increased risk of diabetes that is independent of traditional risk factors, whereas RA is not associated with diabetes.77 Alarmingly, emerging pediatric data suggest that metabolic syndrome develops early. The prevalence of metabolic syndrome in pediatric psoriasis patients was found in one study to be 30 versus 7.4 percent control (p<0.05).78
Moreover, the risk of cardiovascular mortality and all cause mortality in moderate-to-severe psoriasis is quite similar to the risk of these outcomes in patients with moderate-to-severe RA.79
The risk of cardiometabolic disease is so pronounced in patients with more severe psoriasis that patients with severe psoriasis are 30 times more likely to experience a MACE attributable to psoriasis than to develop a melanoma. Their 10-year risk of a MACE attributable to psoriasis is six percent, and psoriasis reduces their life expectancy by five years.76,80-83
In a KC-Tie2 psoriasis skin-specific murine skin model, aortic inflammation and thrombosis could be modeled.84,85 Skin-specific inflammation may drive vascular disease with aortic inflammation associated with psoriasis equivalent to about 10 years of aging.
Despite a robust body of literature in support of the metabolic and cardiovascular risks associated with psoriasis, particularly in its more severe manifestation, clinicians may under-screen and under-manage these risks in their psoriasis patients. For example, psoriasis patients are at elevated risk for hypertension and the prevalence of uncontrolled hypertension (among patients with high blood pressure) is 51.6 percent of patients in the general population, 53.9 percent for psoriasis patients in general, and 59.5 percent of severe psoriasis patients.86 Yet dermatologists do not routinely screen severe psoriasis patients for hypertension.87
Whether dermatologists should treat psoriasis as aggressively as possible to reduce cardiovascular risk remains an open question, and the relationship between greater psoriasis control and improved cardiovascular outcomes is the focus of several planned and ongoing studies. Some observational data suggest that methotrexate and TNF inhibitors for psoriasis may reduce cardiovascular events, but data do not exist for other treatment modalities.88-91
The following emerging comorbidities associated with psoriasis may seem unexpected: sleep apnea, nonalcoholic steatohepatitis (NASH), chronic obstructive pulmonary disease (COPD), infections, chronic and end-stage renal disease, and peptic ulcers.92-96 Patients with moderate-to-severe psoriasis have a twofold risk of chronic kidney disease and a fourfold risk of dialysis, independent of hypertension and diabetes.97 In fact, severe psoriasis patients are at a greater risk for chronic kidney disease than patients with diabetes and hypertension.97 Psoriasis is also associated with mental health conditions, such as anxiety, depression, and suicidal ideation.98 Restless leg syndrome and atrial fibrillation have recently been implicated as potential psoriasis comorbidities.99,100
These findings challenge the old notion that psoriasis is “just a skin disease.” Comorbidity research suggests that psoriasis is part of a larger systemic disease that confers substantial risk. Clinicians treating psoriasis patients should expand their standard screenings to include regular evaluations for hypertension, diabetes, and cardiovascular risks, and patients should be encouraged to keep up with regular cancer screenings, especially when considering immune modulating treatments. Clinicians should screen for infections, and human immunodeficiency virus (HIV) screenings should be performed in those with severe psoriasis. Psoriasis patients undergoing immune suppressive treatment should be vaccinated against influenza, pneumonia, and hepatitis B, and young psoriasis patients (ages 9-26) should be vaccinated against the human papilloma virus (HPV).101 Zoster vaccine should also be considered, especially in older patients, but it is a live vaccine and thus should be given prior to initiating an immune suppressive treatment. Finally, it is well-known that a large subset of psoriasis patients will develop psoriatic arthritis. Psoriasis patients should be regularly screened for psoriatic arthritis and educated about its symptoms.
Cutaneous Oncology Cutaneous Manifestations of Systemic Malignancy and Disease
Systemic malignancies often have a cutaneous presentation, presenting dermatologists with the unique opportunity to identify early clinical presentations of paraneoplastic dermatoses as well as other skin conditions associated with systemic disease processes. Disease categorization should be based on pathology. A categorization scheme might include papulosquamous disorders, interface dermatitides, reactive erythemas, neutrophilic dermatoses, dermal proliferative disorders, deposition disorders, and other (miscellaneous) dermatoses.
Papulosquamous disorders may routinely be seen in clinical practice, although there are also rare forms, such as acanthosis nigricans, acquired ichthyosis, tripe palms, Leser-Trelat sign, and acrokeratosis paraneoplastica (also known as Bazex syndrome). Interface dermatitides include dermatomyositis, paraneoplastic derma-tomyositis, and paraneoplastic pemphigus. Reactive erythemas and neutrophilic dermatoses include erythema gyratum repens, necrolytic migratory erythema, Sweet’s Syndrome, and pyoderma gangrenosum. Sweet’s Syndrome is relatively common, but the underlying malignancies associated with it are less well-known. Dermal processes infiltrate the skin, but the following conditions are rarely seen in the clinic: multicentric reticulohitiocytosis, necrobiotic xantho-granuloma, scleromyxedema, cutaneous amyloidosis, and hypertrichosis lanuginosa acquisita. Certain more common dermatologic conditions suggest systemic disease and deserve greater elucidation.
Acquired ichthyosis. It is not unusual to treat ichthyosis in clinical practice, but a sudden onset of a new case in elderly patients (>50 years), particularly with a distribution pattern similar to ichthyosis vulgaris (that is, sparing palms, soles, folds, or flexures) suggests either an inflammatory response or an underlying malignancy. Acquired ichthyosis may present before or after a cancer diagnosis. About 70 to 80 percent of cancer associated with acquired ichthyosis is Hodgkin’s lymphoma. Acquired ichthyosis may also occur in non-Hodgkin’s lymphoma, multiple myelomas, and leukemia.102
Acanthosis nigricans. Acanthosis nigricans lesions may appear on the neck, axillae, groin, hands, feet, abdomen, nipples, or lips. They are associated with a variety of malignancies, including gastric, esophageal, ovarian, endometrial, gallbladder, lung, and bladder cancers, nearly all of which are adenocarcinomas and 70 to 90 percent are intra-abdominal (50-60% gastric). Acanthosis nigricans in conjunction with tripe palms is most typically associated with gastric cancers. Unfortunately, cancer is usually well-established by the time cutaneous signs appear. Successful treatment of the cancer may result in resolution of the skin lesion.103,104
Tripe palms. Although not commonly seen in clinical practice, tripe palms have an association with lung cancer. Tripe palms typically present either slightly before or around the same time as tumor diagnosis. When tripe palms occur together with acanthosis nigricans, they are associated with gastric tumors.105
Acrokeratosis paraneoplastica (Bazex syndrome). Older patients with no history of skin disease who present with sudden-onset psoriasis-like plaques may benefit from a biopsy, because what appears as psoriasis may, in fact, be psoriasiform epidermal hyperplasia, which will not respond to the psoriasis treatments. Untreated psoriasiform epidermal hyper-plasia may appear on palmar surfaces instead of the dorsal surfaces more typical of psoriasis. A typical presentation involves symmetrically distributed lesions over hands, feet, knees, nails, elbows, and ears. The three clinical stages of the disease are:
Erythrosquamous eruption that typically involves fingers and toes and may spread to nails
Violaceous keratoderma on lateral aspects of fingers and toes
Eventual involvement of trunk, extremities, and scalp.
Bazex syndrome is most commonly associated with squamous cell carcinoma of the aerodigestive tract, but also with adenocarcinoma. Most skin conditions (60%) will occur a year before the tumor is diagnosed, but in about 20 percent of cases the tumor and the skin condition appear at the same time. Since the cutaneous condition often appears before the tumor is detectable, Bazex syndrome offers the dermatologist a “window of opportunity” to refer the patient for timely and potentially life-saving treatment. Bazex syndrome may resolve when the tumor is irradiated.102
Scleromyxedema. Scleromyxedema is characterized by an abrupt onset of waxy, erythemous skin-colored papules and nodules, sometimes in linear distribution, on the face, neck, chest, and limbs.106 A biopsy may be needed to differentiate scleromyxedema from plaque-like cutaneous mucinosis.
Dermatomyositis. Idiopathic dermatomyositis is a prevalent skin condition, and it may be associated with malignancies in elderly patients (>50 years). The skin condition precedes the malignancy; tumors are typically diagnosed in a year. Older female patients with sudden dermatomyositis are predisposed to ovarian carcinoma, a cancer that otherwise often defies early detection. Thus, this skin condition may offer another “window of opportunity” for dermatologists. In many cases, treatment of the malignancy helps to resolve the skin changes. 102
Paraneoplastic pemphigus. Paraneoplastic pemphigus is often seen in clinical practice and may include lip erosion, papules on the tongue, and involvement of the ocular orbits. The typical presentation involves pruritic polymorphous papules and patches on the trunk, leading to mucosal erosions, which may include the conjunctiva and genitalia. Severe palmoplantar involvement may occur with painful erosions at the fingertips. This condition typically afflicts the elderly, with an average age of onset of 59 years. In 75 percent of cases, this cutaneous condition may be associated with lymphoproliferative diseases (42% non-Hodgkin’s lymphoma, 29% chronic lymphocytic leukemia, and Castleman’s disease). The skin condition will not resolve if there is underlying malignancy, even if it is aggressively treated.102,107
Sweet’s syndrome. Sweet’s syndrome is a neutrophilic dermatosis with cutaneous and often painful lesions. It may be associated with underlying malignancy, infection, systemic inflammatory disorders, or the use of certain medications. When Sweet’s syndrome is associated with cancer, it is typically a hematologic cancer, but it may also be associated with solid tumors in the genitourinary tract. In a retrospective study of 77 Sweet’s syndrome patients evaluated at the Mayo Clinic from 1992 to 2010, 35 percent had a malignancy.108 Of malignancy-associated Sweet’s syndrome patients, about 40 percent will be diagnosed with cancer within a month.102,109 Patients with Sweet’s syndrome should be screened for malignancies (blood counts, peripheral smear) and biopsies repeated, if needed.
Sweet’s syndrome has also been associated with chronic inflammatory conditions, such as inflammatory bowel diseases (including Crohn’s disease) and ulcerative colitis (UC).110 In contrast to UC and Sweet’s syndrome, Crohn’s disease with Sweet’s syndrome may skip areas on the mucosal wall. In fact, Crohn’s disease with Sweet’s syndrome will present with lesions typically in the perianal region in only about 10 to 30 percent of patients. These lesions or fistulae may be ulcerated and show a granulomatous pattern of inflammation. The lymph nodes may be involved and there may be other systemic sequelae. Metastatic Crohn’s disease is rare, but when it presents, its lesions may mimic those of erythema nodosum. Granulomatous colitis occurs around colostomy sites; this may lead to metastatic or localized Crohn’s disease.
In general, Crohn’s disease is associated with greater cutaneous symptomology than UC. UC presents with diffuse colon-rectal inflammation that is more likely to lead to serious complications, such as colon cancer, than Crohn’s disease. UC patients typically present with more overt and more severe gastrointestinal complaints, including bloody diarrhea, compared to patients with Crohn’s disease.
The pattern of inflammation with UC is usually observable throughout the colon, starting at the rectum and spreading proximally. With a typical onset in early adulthood, UC may be linked to underlying pathology, for example, inflammatory bowel disease. Cutaneous manifestations associated with ulcerative colitis include erythema nodosum, pyoderma gangrenosum, apthous stomatitis, aseptic pustular eruptions, and pyoderma vegetans (rare).111,112 Apthous stomatitis associated with either UC or Crohn’s disease may be substantially larger, more painful, and more challenging to treat than other forms of apthous stomatitis. Apthous stomatitis will flare or remit based on bowel involvement. Erythema nodosum may occur in patients with either Crohn’s disease or UC (more common). Erythema nodosum is the most frequently observed skin manifestation of bowel disease in pediatric patients. Systemic steroid treatment may be appropriate with nonsteroidal anti-inflammatory drugs (NSAIDs) for pain control.
Pyoderma gangrenosum is three times more common in UC than Crohn’s disease; about half of patients with pyoderma gangrenosum have UC. It usually initiates with a pustule that becomes a nodule, ulcerates, and then produces exudate. Pyoderma gangrenosum may appear at any site on the body, but favors acral sites. Treatment for pyoderma gangrenosum includes systemic steroids (such as prednisone 1-2mg/kg per day) along with cleaning, but not debriding the lesions. Patients can then be transitioned to dapsone, sulfasalazine, methotrexate, and other drugs, including biologics.
Biologics may be able to address both the pyoderma gangrenosum and gastrointestinal symptoms.113 Healed pyoderma gangrenosum may scar with a unique almost “shiny” appearance.114
Pyostomatitis vegetans. Pyostomatitis vegetans involves annular, pustular skin lesions with a “snail track” appearance, which may accompany, precede, or follow extensive vegetating oral disease. The cutaneous and oral lesions are manifestations of the same disease and are associated with UC.115
Erythema gyratum repens. In this rare condition, patients present with polycyclic, geometric, and arciform plaques with a “wood grain” appearance. Erythema gyratum repens occurs twice as often in men as women and is one of the most specific paraneoplastic cutaneous conditions, in that it has an 82-percent association with malignancy, most commonly bronchial cancer, followed by esophageal cancer. In 80 percent of cases, cutaneous manifestations precede the tumor by as much as two years, offering dermatologists a “window of opportunity” for early detection.116 This rare, but clinically distinctive, skin condition may sometimes be associated with drug intake.117
Necrolytic migratory erythema. Necrolytic migratory erythema may be considered an obligatory paraneoplastic syndrome, usually associated with a glucagon-secreting tumor of the alpha-islet cells of the pancreas.118 Cutaneous symptoms, which typically precede the tumor, include erythematous scaly lesions which typically appear on the perineum, distal extremities, lower abdomen, and face. The clinical syndrome includes diabetes, macroglossia, anemia, unintentional weight loss, and diarrhea. While the cutaneous symptoms usually appear in advance of the tumor, patients often do not present for medical care until their symptoms progress. 118
Cutaneous metastases. The most common sites for cutaneous metastases are the trunk and scalp, and the site is often close to the site of the primary tumor. For men, the most common underlying disease is lung cancer followed by colon carcinoma; for women, it is breast cancer and colon carcinoma. The overall incidence of such cutaneous metastases is low (about 2-6%), and it may be considered in general as a poor prognostic indicator.119
The International League of Dermatological Societies Guidelines for Treatment of Actinic Keratosis
Spearheaded by Dr. Eggert Stockfleth, Dr. Alexander Nast, and Ricardo Werner, the International League of Dermatological Societies (ILDS) guidelines were created by an expert panel of 15 dermatologists, three dermatopathologists (histopathologists), and one patient, with the goals of advancing a more widely accepted definition of actinic keratosis (AK), raising awareness among other disciplines about AK (for example, primary care physicians, internists, Ob-Gyn, plastic and ENT surgeons), publishing systematic assessments of the safety and efficacy of the available treatments, and improving the quality of care, including reducing the number of patients whose AKs progress to invasive squamous cell carcinomas (SCCs). The Cochrane review of AK interventions was evaluated,120 updated, and the Cochrane “risk of bias” tool was applied followed by use of the evidence-based GRADE system. Evidence was rigorously assessed using a forest plot. Results from the literature were presented to the expert committee, who, in turn, consulted in an online conference to offer their treatment recommendations. Recommendations were based on the scheme presented in Table 2. The steering committee drafted the recommendations, which were reviewed internally by the expert committee members and then subjected to an external review. The finalized manuscript has been submitted for publication, with the hope that this will become an international guideline.
TABLE 2.
The ILDS treatment guidelines for AK used this verbiage in its recommendations. Note that strength of recommendation and expert consensus (implications) are two different categories and not necessarily equivalent
| STRENGTH | WORDING | SYMBOL | IMPLICATIONS | |
|---|---|---|---|---|
| Strong recommendation FOR the use of intervention | “We recommend…” | ↑↑ | We believe that all or almost all informed people would make that choice. Clinicians will have to spend less time on the process of decision making and may devote that time to overcome barriers to implementation and adherence. In most clinical situations, the recommendation may be adopted as policy. | |
| Weak recommendation FOR the use of intervention | “We suggest…” | ↑ | We believe that most informed people would make that choice, but a substantial number would not. Clinicians and healthcare providers will need to devote more time on the process of share decision making. Policy makers will have to involve many stakeholders and policy making requires substantial debate. | No recommendation with respect to an intervention |
| “We cannot make a recommendation with respect to…” | 0 | At the moment, a recommendation in favor or against an intervention cannot be made due to certain reasons, e.g., no evidence data available, conflicting outcomes, etc. | ||
| Weak recommendation AGAINST the use of an intervention | “We suggest not to…” | ↓ | We believe that most informed people would make a choice against that intervention, but a substantial number would not. | |
| Strong recommendation AGAINST the use of an intervention | “We recommend not to…” | ↓↓ | We believe that all or almost all informed people would make a choice against that intervention. This recommendation can be adopted as a policy in most clinical situations. |
As an example, consider a patient with a single AK. More than 75 percent suggested treatment with 0.5% fluorouracil and gave it a T recommendation. A patient with multiple AK lesions or field cancerization >50 percent was given a TT recommendation for 0.5% fluorouracil.
As with any guidelines, it is important that we recognize the value of professional expertise, clinical judgment, and individualized care. Guidelines should not be taken as inflexible mandates, but as guidance based on the best available evidence to date to aid clinical judgment.
New and Novel Uses for “Field Therapies”
AK is often considered strictly as a field disease and is treated with field-directed therapies. However, subclinical AK may present with cells genetically similar to AK and SCC.121 Advanced imaging technology, such as cross-polarized light, fluorescence, and dermoscopy, may be helpful in identifying different types of subclinical AK lesions. Reflectance confocal microscopy and high-definition optical coherence tomography can provide high-resolution skin imaging and have the capacity to detect subclinical lesions noninvasively.122 The toolkit is robust and useful, but these technologies have some disadvantages as well, particularly in terms of cost.
Photodynamic Therapy
Photodynamic therapy (PDT) requires oxygen, a photosensitizer, and an activating wavelength of light. Cutaneous application of pro-drugs 5-aminolevulinic acid (5-ALA) or methylaminolevulinic acid (MAL; no longer commercially available in the United States, but widely used in other parts of the world) bypasses the rate limiting step in the heme synthesis pathway. This results in the preferential accumulation of the photosensitizer protoporphyrin IX (PpIX) within AKs, which can be targeted with activating wavelengths of blue or red light.
The pivotal phase 3 ALA PDT trial utilized a 14- to 18-hour ALA incubation period in order to maximize PpIX buildup within AKs, which peaks at approximately 12 hours. This prolonged incubation is impractical for the practicing clinician and patient. In a recent phase 2 study evaluating shorter incubation periods, patients were divided into the following five groups:
Group 1: Broad area (BA) application of ALA one hour prior to blue light
Group 2: BA application of ALA two hours prior to blue light
Group 3: BA application of ALA three hours prior to blue light
Group 4: Spot application of ALA two hours prior to blue light
Group 5: Vehicle group.
Study results found that the one-, two-, and three-hour incubation periods resulted in 35- to 57-percent clearance at eight weeks, 78 to 80 percent at 12 weeks, and 64 to 75 percent at 24 weeks, respectively, demonstrating that multiple treatments with shorter
incubation times per treatment were effective. To achieve around 80-percent clearance, at least two of these shorter incubation treatments are needed at least eight weeks apart.
Patient discomfort during PDT remains an important issue, with more than 60 percent of patients in the previously mentioned short incubation study reporting moderate-to-severe pain during treatment. A new approach employing 30 minutes of MAL incubation prior to 1.5 to 2.5 hours of exposure to daylight, which serves as the activating wavelength of light, has vastly improved tolerability while achieving individual AK lesion clearance rates of approximately 75 percent.123 In a report on three clinical studies, 92 percent of daylight-mediated PDT patients reported mild to no pain (defined as 0-3 on a 10-point analog scale), 7.5 percent said they had moderate pain (score 4-7), and one patient reported severe pain (8-10). There was no correlation between pain scores and efficacy.123 Unfortunately, these promising results are subject to variations in seasonal weather patterns and hence ambient activating light, so efforts were made to develop an indoor form of painless PDT (George Martin, MD, personal experience, submitted for publication). The concept involved applying ALA to the patient’s face about 15 minutes before the patient was exposed to one hour of standard blue light (BLU-U, Dusa Pharmaceuticals, Inc.) light, reversing the old paradigm of long incubation with short light exposure. Using a split-face study, painless indoor PDT (15-minute incubation/60-minute blue light) was compared to standard PDT (75-minute incubation, 1000-second blue light) in three patients. Both treatments were similarly effective and reduced AK count around 50 percent (painless indoor PDT had 27 AK lesions at baseline, 13 post-treatment, 52% reduction; standard PDT had a baseline count of 32 AKs, 18 post-treatment, 44% reduction). Pain scores on a scale of 0 to 5 (with 0 being no pain) showed the “painless” indoor PDT lived up to its name with a score of 0 (range 0-0) while standard PDT had an average pain score of 3.5 (range 3-4). While large, randomized, controlled trials are needed for this new painless PDT treatment model, initial evidence provided by this split-face study and experience in more than 100 patients utilizing the protocol as monotherapy or in conjunction with pretreatments using 5-fluorouracil or imiquimod suggests this type of therapy can be readily reproduced in the clinic.
Most AK studies compare treatments to a placebo rather than head-to-head. A recent meta-analysis (25 studies, n=5,562 total patients) attempted to evaluate the most effective AK treatments by comparing them directly using a complicated statistical model.124 With the exception of diclofenac 3%, all treatment options achieved at least 40-percent clearance and only one treatment offered 70-percent clearance (ALA-PDT gel). Thus, it is reasonable to say that many AK treatments are effective, but ALA-PDT seems superior.
There is a rationale for using PDT to prevent basal cell carcinoma (BCC), which can develop in older patients treated with X-ray therapy for acne, who are at risk for developing numerous facial BCC, or middle-aged patients with a history of multiple facial BCC. Thus, “preventative PDT” might decrease the risk of further BCC development. In case reports on six patients with basal-cell nevus syndrome, 20% ALA was applied to up to 22 percent of the body surface area for 24 hours under occlusion and then patients were exposed to red light for one to three treatments.125 The decision to use red rather than blue light was based on the idea that the longer wavelength of red light would penetrate better (penetration is about 2.0mm with blue light and >5mm with red). Responses were durable up to six years (two patients had clearance for an average of five years). This study suggests that PDT destroys clinically visible BCC but also subclinical lesions as well. Localized PDT induces a systemic immune response involving the production of antibodies to HIP1 (BCC-specific associate antigen). In a study comparing PDT to surgery for BCC, these antibodies were produced within 7 to 10 days post PDT, but not in the surgical group.126 This supports the argument favoring preventative PDT therapy for patients predisposed toward multiple BCC.
Imiquimod
Most patients see AK lesions partially or completely cleared following a 2 week on-2 week off-2 week on daily application of 3.75% imiquimod (IM) to the face and scalp.127 An alternative and effective approach to limiting downtime and maintaining long-term efficacy involves daily application of 3.75% IM to the face for seven days followed by a two-week rest period and then continuing with a once-weekly application for over a year (George Martin, MD, unpublished observatioin).
Ingenol Mebutate 0.015% and 0.05%
Ingenol mebutate has dual mechanisms of action—it causes controlled cell death within a matter of hours and an IL-8 neutrophilic immune response over the course of the next days. Phase 3 trials treating only areas about 25cm2 have resulted in good safety data with no scarring or hyperpigmentation issues.128 Since patients must self-administer this agent, it is very important for clinicians to set realistic expectations. The initiation of ingenol mebutate over larger areas than the approved protocol, while apparently safe and efficacious, may result in a temporary, but marked, exacerbation of symptoms. Patients should be educated to expect results along the lines of a “bad sunburn” particularly if large areas of the chest are treated, and be provided analgesics, if necessary.
Combination therapy. Ingenol mebutate may be combined with cryosurgery for treating AK.129,130 In these studies, combination therapy was more effective than cryosurgery alone at 11 weeks (60.5 vs. 49.4%, respectively) and at 52 weeks (30.5 vs. 18.5%). Thus, these cryosurgery results are not as effective in clearing AKs as combination therapy results.
High-risk Basal Cell Carcinoma and Squamous Cell Carcinoma: Recognition and Treatment
Basal cell carcinoma. Basal cell carcinoma (BCC) is the most common cancer in humans, and its incidence is on the rise. Although there are no official registries maintained, it is estimated that 2 to 4 million new cases occur per year in the United States.131 The incidence increases with age (median age of patient is 68 years, but younger patients are increasingly affected) and a disproportionate relative increase has been noted in women. Skin type and exposure to UV radiation remain the predominant risk factors leading to a mutation of the PTCH gene.
Certain BCC may be considered a low-risk cancer. In these cases, the histology of BCC is superficial, nodular, small in size, and with well-defined margins. Most BCC lesions appear on the trunk and extremities, rather than the face. These include superficial BCC, BCC with atypical basaloid islands, and micronodular BCC. When dealing with these BCC subtypes, clinicians should bear in mind that biopsies may be too small to be representative of the cancer. A “thin shave” might miss deeper growth in certain nodular or micronodular forms of BCC. Furthermore, there can be “sampling errors” in that the sample may be taken from less aggressive regions of the cancer.
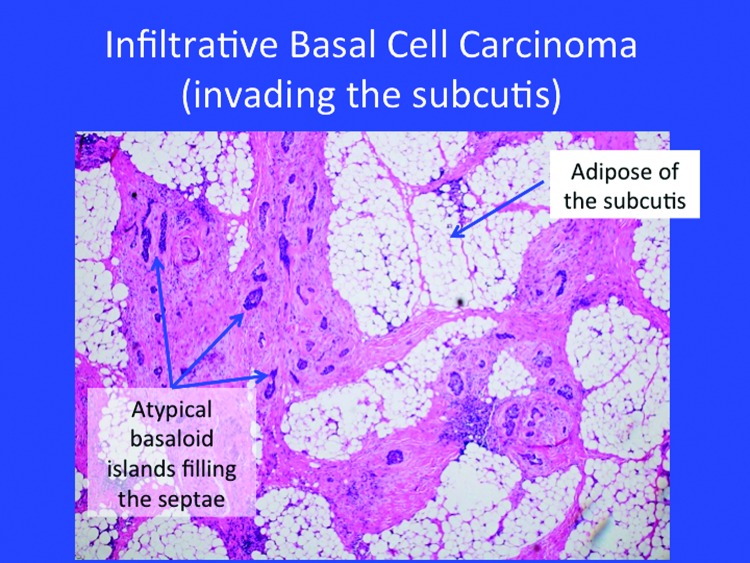
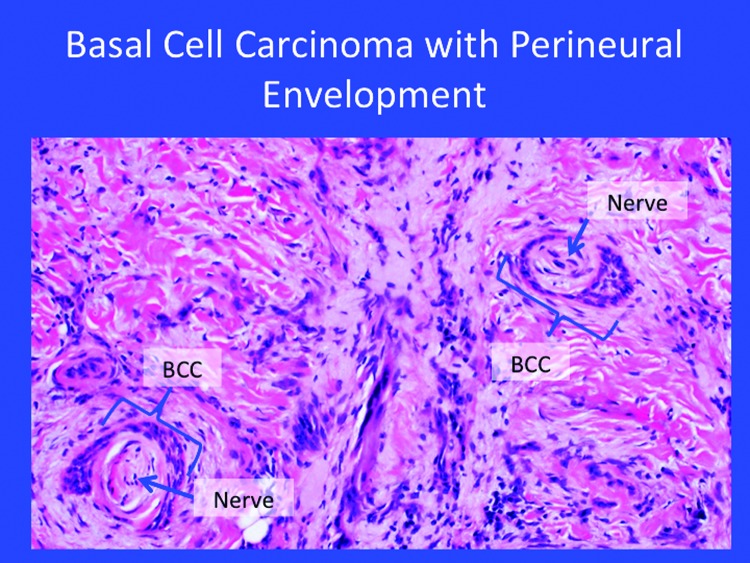
High-risk BCC exhibits an aggressive growth pattern, frequently recurs, tends to be larger, and may appear on the mask or “H-zone” of the face along the embryonic fusion planes. High-risk BCC often exhibits ill-defined clinical margins and may be associated with immunosuppression. It is not unusual to see these high-risk BCC occur at sites of prior radiation (such as the sites for X-ray therapy in acne patients). The histology of invasive forms of BCC appears in Figure 3. Tumor size may help define risk—high-risk growths may be considered those >6mm in the mask area of the face; >1cm on cheek, forehead, scalp, or neck; and >2cm on the trunk or extremities.
Figure 3.
The histology of certain invasive forms of high-risk basal cell carcinoma.
When treating patients with BCC, dermatologists should consider patients holistically, recognizing that not all patients are appropriate candidates for aggressive treatments. Since significant morbidity may be involved, all likely treatment options should be evaluated and discussed with the patient. In this connection, it should be noted there are no absolute indications for Mohs surgery.
Squamous cell carcinoma. Squamous cell carcinoma (SCC) is the second most common form of cancer with over half a million new cases diagnosed each year. Men are about twice as likely to be diagnosed with SCC as women, and the incidence of SCC increases sharply with advanced age. In fact, among patients over 80 years of age, skin cancer deaths are more likely to be attributable to SCC than melanoma.131 Risks for developing SCC include chronic, long-term exposure to UV light; tanning; or ionizing radiation with a latency period that may exceed 20 years. Organ transplant recipients and other immunocompromised patients are at increased risk. Certain strains of HPV may also be considered risk factors along with exposure to certain chemicals, nonhealing wounds, lupus, genetic syndromes, and burns. In basic terms, anything that causes skin cells to turn over at an accelerated rate will increase the chance of replication errors and, in that way, raise the risk of SCC. Surgical excision is the first-line treatment for most forms of skin SCCs, but other treatments are available as well.132
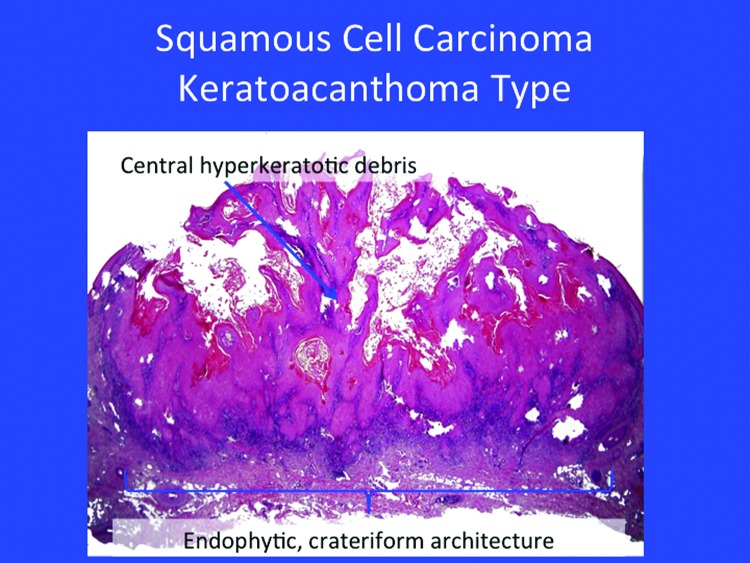
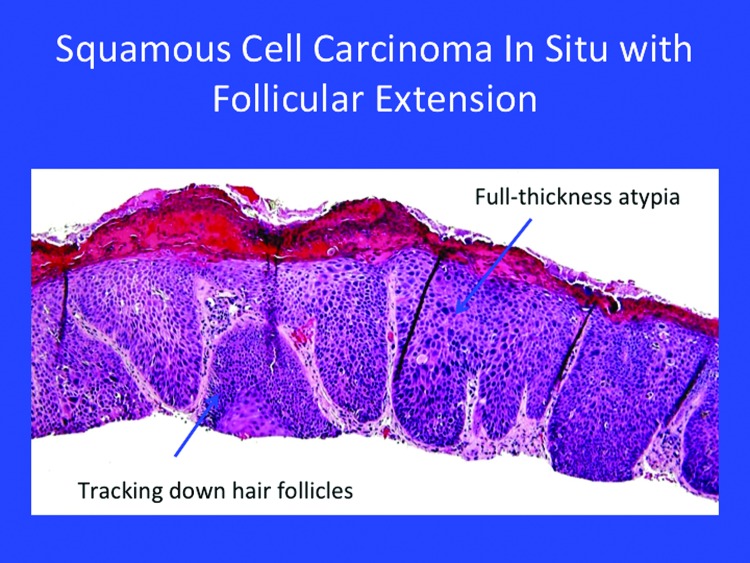
Certain in-situ SCC may be considered low risk, such as Bowen’s disease or keratoacanthoma (Figure 4). Most of these lesions may track down to the follicle (Figure 5).
Figure 4.
The classical appearance of a keratoacanthoma. Note the endophytic, crateriform architecture, hyperkeratotic debris, and squamous islands with the glassy atypia to the keratinocytes.
Figure 5.
Squamous cell carcinoma in situ with follicular extension
When treating these SCC lesions, curetting is helpful before performing the first Mohs layer. In a small, but significant subset, the curette will “dive” a bit deeper than anticipated, will not “scoop out” cleanly, or will encounter a gelatinous bottom. In such cases, a biopsy may be conducted. Note that shave biopsies may not detect poorly or moderately differentiated histology near the base of the lesion. Histology may change as depth increases.
More invasive forms of SCC may occur in the ear, lip, and genitalia. Poorly differentiated areas may require greater scrutiny, in that atypical single cancer cells may be “hiding” in areas of inflammation. Immunostains may be necessary with poorly differentiated SCC; multiple stains may be required to confirm spindle-cell SCC. The histology of these more aggressive SCC may include perineural invasion and they may be acantholytic, adenoid squamous, or desmoplastic. Perineural envelopment occurs when the SCC in the field, the nerve, and the area around the nerve are surrounded by keratinocytes. While not all SCCs are painful, perineural involvement may be associated with pain and paresthesia. These aggressive SCCs are characterized by rapid growth and may track back through the foramen into bone if left untreated. In advanced cases, the larger cranial nerves may become involved.
Patient education is an important element in care of the SCC. It is crucial that patients make the mental connection between their “skin problem,” on the one hand, and cancer on the other.
Organ transplant recipients. There are more than 20,000 organ transplants performed each year in the United States with approximately 140,000 organ transplant recipients (OTRs) alive today. The immunosuppressive pharmacological regimens taken by OTRs increase their rates of malignancy, in particular skin cancers.133 The relative risk increase for skin cancer in OTRs is a 3.4-fold increase for melanoma, a tenfold increase for BCC, and a 65-fold increase for SCC. Further, the metastatic rate from SCC in OTRs is seven percent and has been associated with a poor prognosis. Among OTRs, risks are elevated for older patients (with more cumulative UV exposure), longer duration of immunosuppression, intensity of immunosuppression, Fitzpatrick type I to III, significant history of UV exposure, and the type of organ transplant. The risk is greater for heart transplant patients, which reflects differences in immune suppression.
Treating OTRs with cutaneous cancers requires a special level of expertise and highly individualized care. Transplant patients must have a way to rapidly access the clinical team, for example, with emergency phone numbers.
Metastatic SCC. High-risk SCC accounts for up to 2,500 deaths/year. When clinically detectable nodal metastases occur, five-year survival is under 30 percent. The treatment for metastatic SCC may involve “watchful waiting,” adjuvant radiation, elective lymph node dissection, and sentinel node biopsy. Patients must be educated about the seriousness of this condition. For example, if an initial procedure gives them early positive results, patients should be counseled regarding the risk of recurrence, what “metastases” means, and the importance of surveillance.
Treatment options for BCC and SCC. There are a variety of treatment options for patients with BCC or SCC, which can be broadly categorized as surgical versus nonsurgical and those that involve or do not involve margin control. Among the nonsurgical options, one may include radiation, PDT, 5-fluorouracil, and imiquimod. Surgical options include curettage, cryosurgery, excision, laser, and Mohs surgery. Cryosurgery may be particularly useful for superficial lesions, small lesions, or nodular BCC, and should be considered in patients who may not otherwise be good surgical candidates. Among the potential drawbacks to cryosurgical approaches are slow healing, hypopigmentation, and no margin control.
Curettage is an important procedure with several clear advantages for the busy dermatology clinic; it can be done quickly, the learning curve is short, only minimal equipment (investment) is required, and it has a cure rate of more than 90 percent. The main drawbacks to curettage are that occasionally there is unpredictable cosmesis, it may be associated with slower healing rates, there is no margin control, and it is not suitable for high-risk tumors. Curettage is indicated for superficial or nodular, noninfiltrative, well-defined, primary, smaller, low-risk BCC as well as for low-risk SCC, such as Bowen’s superficial SCC. Curettage should not be used when dealing with recurrent tumors or tumors with full thickness into the subcutis. Curettage may be effectively combined with electrodessication, cryosurgery, or imiquimod. When curettage was combined with electrodessication, the overall five-year recurrence rates for treated BCC were 13 percent, with rates varying by size (8.5% for 0-5mm, 15% for 6-9mm and 10-14mm, 20% for 15-19mm, and 26% for >20mm).134
The excision of BCC offers good margin control, improved cosmesis, and more rapid healing with suture closure. The potential disadvantages of BCC excision are that the “breadloaf sectioning” may miss areas of the tumor, there is a longer learning curve, and considerable technical expertise is required for consistently outstanding results. Excision can be a time-consuming approach compared to other treatment options. For excisions, 4mm or smaller BCC and 4 to 6mm SCC are considered low-risk interventions, while interventions involving BCC or SCC 6 to 10mm are high risk.
Incompletely excised BCC present clinicians with the question whether to re-excise, in that tumor persistence was shown in a retrospective study to be about 28 percent.135 Recurrence after excision with a positive margin is about 35 percent; therefore, these are typically either re-excised or treated with Mohs surgery. For a select subset of patients, a watch-and-wait attitude may be appropriate in that the immune response may clear most residual cells, and the inflammatory process is stopped when the lesion is closed.135 Low-risk parameters include superficial or nodular subtypes, small size (<1cm), and location not on the nose or ear. Thus, most—but not all—patients should undergo re-excision or Mohs surgery because of the high rate of tumor persistence. Re-excision should also be considered for high-risk tumors and for positive deep margins.
Mohs surgery remains the “gold standard” treatment for tumor extirpation and is indicated for high-risk BCC. High-risk parameters include recurrent nature, larger tumors, aggressive histology, location, and incomplete excision.136 The Mohs surgical procedure is unique in that the surgeon serves as his or her own pathologist, the lab is onsite, meticulous mapping and horizontal sectioning are required, and the procedure looks at 100 percent of the tumor. The main advantages of Mohs surgery is its high cure rate, its tissue-sparing and structure-sparing benefits, and the fact that immediate reconstruction can be performed. The drawbacks to Mohs surgery are the fact that experienced technical personnel are required to support the procedures, which require additional equipment. Mohs surgery can be expensive and may take a comparatively long time. Recurrence rates for primary BCC can be one percent for Mohs surgery, compared to rates of 10 percent for excision and rates around 7 or 8 percent for electrodessication and curettage, radiation, and cryosurgery.137
Disruptive Technologies for Skin Cancer: Brachytherapy and Superficial Radiation
Electronic brachytherapy (EBX) and superficial radiation will be important therapeutic options in the future because of their impressive and durable cure rates (87-100% at 2-5 years). Many cancers, including prostate and cervical cancer, have been effectively treated with EBX. In some instances EBX may be combined with 30 to 40Gy high-dose radiation (HDR) to optimize cure rates and possibly shorten the course of therapy. EBX is recommended for use in nonmelanoma skin cancers. In a study of 520 mostly BCC and SCC patients over the course of 10 years, investigators achieved a 92-percent cure rate. The local control rate for HDR brachytherapy is between 93.7 and 97.5 percent.
HDR brachytherapy relies on iridium-192 and can be performed, but EBX does not use any radioisotope. This means that EBX still uses the high-dose fractionation, but is potentially safer with less collateral damage and may even be more effective, in that the beam is flatter and has less penumbra (less scatter). HDR brachytherapy can be performed in a dermatology clinic or physician’s office.
The workflow of HDR brachytherapy involves a radiation oncologist, a radiation therapist, and a physicist. A portable lead shield can be employed to protect the patient rather than lead walls. The patient enters the room and the machine is calibrated based on the patient’s individual dosing parameters. A typical treatment may take about 15 minutes. The course of therapy is individualized, but often includes twice-weekly sessions over a course of 8 to 12 treatments.
In a study of 187 patients (275 nonmelanoma skin cancers), the surface applicator was 10, 20, 35, or 50mm with a 2mm margin and a depth of 3mm (determined by computed tomography for thick lesions).138 Patients were treated twice weekly; dose fractionation was 5Gy for eight fractions, resulting in a total of 40Gy. The study population was 63 percent men, 97 percent Caucasian, with a mean age of 73 years. Types of cancer treated were predominantly BCC (n=159) and SCC (n=109). With a mean follow-up of 10 months (range 1-28), no recurrences and no adverse events > grade 3 have been observed. Mild grade 1 dermatitis occurred in 84 percent of patients treated and 25 percent experienced grade 1 pruritus. The most common adverse event reported was hypopigmentation, which occurred in 10.9 percent of 46 lesions at one year (all grade 1). Cosmesis at one year was evaluated in 42 lesions, with 92.9 percent rated “excellent.”138
Another treatment model is superficial radiation, in which low-energy photons (rather than charged particles) are delivered in a highly focused beam with the ability to penetrate 0 to 1cm. Treatment duration is about 90 seconds. The course of treatment (5 to 22 fractions) depends on the size, site, and type of tumor being treated. A key advantage of superficial radiation is that it can be performed in a doctor’s office or clinic, providing a radiation therapist carries out the treatment. In a retrospective, single-center study (n=1,715 histologically confirmed primary cutaneous BCC or SCC), most patients had multiple tumors and could select either Mohs surgery or superficial radiation treatment (5 to 7 fractions for a total of 35Gy).139 The recurrence rate for all tumors was 1.9 percent at two and five percent at five years; recurrence specifically for SCC was 0.8 percent at two years and 8.7 percent at five years. Recurrence was most likely to occur in men and tumors >2cm. Superficial radiation may be appropriate for older, frail patients; those with larger tumors in cosmetically sensitive areas, such as around the eyelids or in areas that do not heal well, such as the shins; or in patients taking blood thinners.
Reimbursement for these therapies reflects the high cost of the equipment and energy required and currently relies on a temporary code that will sunset in 2017.
Superficial radiation treatments cost substantially more than other treatments, raising a potential ethical issue. However, it must be recognized that reimbursement is typically more generous when a new technology emerges in medicine, with the rationale that it incentivizes practitioners to adopt new treatment modalities plus it allows Medicare the opportunity to gather data. As the technology becomes more entrenched and established, reimbursement decreases. Reimbursement differentials exist in other areas of medicine and even with Mohs surgery codes where Mohs procedures may cost many times more than traditional extirpation methods but can still be ethically selected in certain cases. The use of these emerging technologies may provide the patient with a better informed consent, in that patients can compare the proposed procedure to established treatments with demonstrated cure rates. While our healthcare system is moving toward cost-containment strategies, there is simultaneously a drive toward more patient-centered care paradigms. Skin cancer patients, such as cosmetic dermatology patients, prefer less invasive treatments and want to avoid needles, bandages, blood, and long treatment courses as much as possible. That is likely why EBX and superficial radiation are being offered at more and more clinics today.
Mohs surgery and dermatology procedures have already achieved high surgical cure rates. The evolution of field treatment of nonmelanoma skin cancers include outpatient and office-based procedures under local anesthesia, aesthetic reconstruction, and scar revision with laser technology. All of healthcare is evolving toward personalized medicine through molecular targeting, and in dermatology, minimally invasive radiation treatments appears to be an interim step in that march toward personalized medicine.
To advance these new treatment modalities, dermatologists and Mohs surgeons should participate in multidisciplinary brachytherapy societies.
Dermatologists should participate in multicenter clinical trials and launch dermatologist-led specialty societies for brachytherapy. These novel approaches should be incorporated where appropriate into our skin cancer treatment protocols. It would be useful to create a paradigm for the area-under-the-dose-response curve (AUC) for radiation therapies. There may be value in developing a menu-type system of treatment hierarchies and options with stronger “brands” and awareness of the particular benefits and risks of various treatment modalities. Within the next few years, dermatologists will have five-year data for these treatments, reimbursements will have decreased for EBX, and private insurance may start to cover EBX, particularly if there is patient-led demand for these procedures.
Treating Basal Cells with Drugs
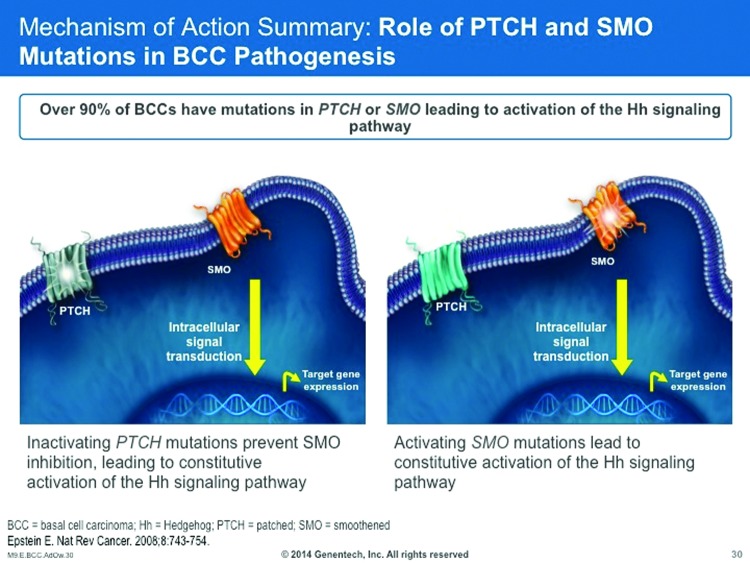
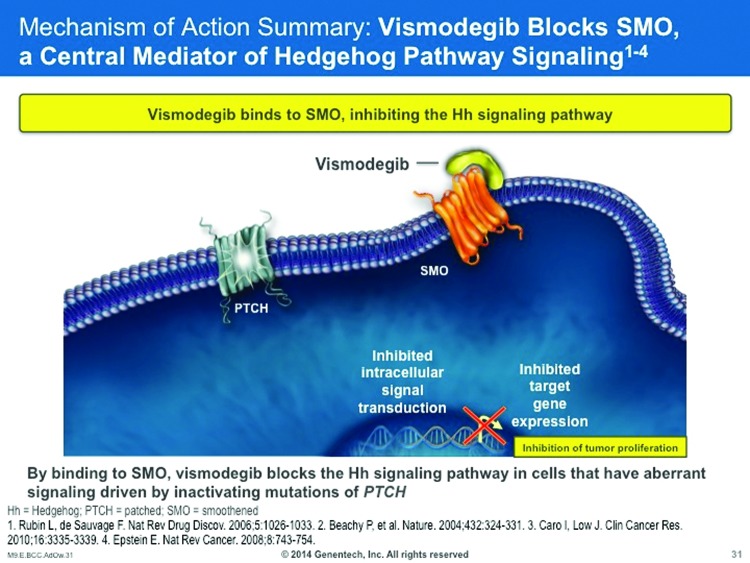
The Hedgehog (Hh) signaling pathway is important in human embryogenesis, with reduced or altogether absent activity in adults. Aberrant Hh pathway signaling can result in developmental abnormalities of the brain, face, or midline.140 Hh pathways may be a factor in the development of certain malignancies and, for that reason, Hh inhibition may be an important treatment strategy for cancer care.141 About 90 percent of sporadic BCCs have a PTCH1 loss of heterozygosity and/or other mutations in the PTCH1 genes, resulting in PTCH1 inactivation and activation of the Hh pathway. About 10 percent of sporadic BCCs have activating mutations in the SMO gene, resulting in constitutively active SMO and Hh pathway activation (Figure 6).142
Figure 6.
The HH pathway is active during normal embryonic development, but becomes inactive in adulthood. The PTCH and SMO mutations in BCC pathogenesis activate the Hh pathway in certain adults.140
Thus, aberrant Hh pathway activation has been identified in both hereditary and sporadic BCCs. For patients with hereditary BCCs, patients with basal cell nevus syndrome (BCNS) carry germ-line hetereozygous mutations in the PTCH1 gene that results in loss of PTCH1 function; such individuals may be highly predisposed to developing multiple BCCs. 142 On the other hand, about 90 percent of sporadic BCCs have PTCH1 loss of heterozygosity, mutations in the PTCH1 gene, or both, resulting in PTCH1 inactivation and Hh pathways activation. About 10 percent of sporadic BCCs will exhibit activating mutations in the SMO gene that result in constitutively active SMO and Hh pathway activation.140
Four agents are currently being evaluated for their use in mediating Hh pathway signaling for the treatment of BCC—vismodegib, sondigib/erismodegib (LDE225), CUR61414, and saridegib (IPI-926).
Vismodegib. Vismodegib was cleared for market release in the United States in early 2012 by the FDA and can be considered a first-in-class drug. It is indicated for locally advanced BCC not amenable to surgery or radiation and metastatic BCC (formerly treated with cisplatin-based chemotherapeutic regimens).143 It is contraindicated for use during pregnancy. The commonly reported adverse events are muscle spasms, alopecia, dysgeusia, weight loss, fatigue, anorexia, asymptomatic hyponatremia, hyperglycemia, presyncope, pyelo¬nephritis, and amenorrhea.143 The recommended vismodegib dose is 150mg orally once a day.
The mechanism of action for vismodegib has been described as SMO blockade, that is, it binds to SMO and in that way blocks the Hh signaling pathway in cells with either aberrant signaling driven by inactivating mutations of PTCH or activating mutations of SMO (Figure 7).
Figure 7.
Vismodegib binds to SMO and thus inhibits the HH signaling pathway.140,141
Vismodegib fits well into the dermatology clinic in that it has similarities to biologics, methotrexate, cyclosporine, and other drugs that require close clinical supervision. Side effects are predictable, although some, such as alopecia in women, may be treatment limiting. Serious adverse events have occurred. Vismodegib is a teratogen, and contraception is required for both men and women taking this agent. It may be used preoperatively to shrink a tumor or postoperatively. An important treatment consideration is duration and whether or not treatment might be interrupted (and possibly biopsies scheduled periodically). The author recommends treatment for 6 to 9 months with breaks for most patients, although some may benefit from treatment five days a week. In an international, multicenter, two-cohort, nonrandomized phase 2 vismodegib study in 96 advanced BCC patients, patients were treated with 150mg of oral vismodegib daily. Among patients with metastastic BCC (n=33), the response rate was 30 percent (95% confidence interval, range 16-48, p=0.001), while among patients with locally advanced BCC (n=63), the independently assessed response rate was 43 percent (95% confidence interval, range 31-56, p<0.001) with 13 patients achieving complete response.143 Results endured 7.6 months for both cohorts. About 30 percent of patients experienced adverse events, including serious adverse events (25%) and seven deaths attributed to adverse events. In a subsequent study of the same patients offering 12 additional months of follow-up, the objective response rate increased from 30 to 33 percent for metastatic BCC patients and from 43 to 48 percent for locally advanced BCC patients; the median duration of response extended from 7.6 months to 9.5 months. There were no new adverse events reported.144
Sonidegib/erismodegib. Clinical trials are underway for dose-ranging and safety for patients with locally advanced or metastatic BCC being treated with sonidegrib (also known as erismodegib and LDE225). Sonidegrib is available in oral and topical formulations (0.75%). In a study of eight patients with nevoid BCC (27 lesions, total) treated with 0.75% topical sonidegib or vehicle twice daily, among the 13 lesions treated with the active agent, three had a complete response and nine partial responses after four weeks.145 A quality-of-life study for patients taking 200 to 800mg of sonidegib found both doses improved quality of life and had similar tolerability.
CUR61414. This new agent has been formulated as a topical product. In a murine preclinical evaluation, there was an in vivo down-regulation of Gli-1 expression, but a phase 1 study did not show efficacy in human superficial BCC or nodular BCC.146
Saridegib (IPI-926). Saridegib (also known as IPI-926) has entered phase 1 and 2 testing. It was originally developed to treat chondrosarcoma, primary myelofibrosis, pancreatic cancer, and various head and neck cancers.
Other SMO inhibitors. Smoothened (SMO) inhibitors are currently being developed by several pharmaceutical manufacturers. These agents include BMS-833923 (Bristol-Myers-Squibb, currently in phase 1 and 2 testing); LEQ506 (Novartis, currently in phase 1 testing); PF-04449913 (Pfizer, currently in phase 1 testing); TAK-441 (Takeda Pharmaceuticals, currently in phase 1 testing).
Itraconazole, now in phase 2 clinical studies, binds SMO at a distinct site from cyclopamine and inhibits the growth of Hh-dependent BCCs and medullablastomas in mice. Studies of an oral formulation itraconazole with BCCs >4mm in size found it decreased cell proliferation, reduced Hh pathway activity, and shrank tumor size.147,148 A topical formulation is also being evaluated.
Large Congenital Nevi and Proliferative Nodules: What’s New?
New recommendations have been published with respect to the categorization of cutaneous features of congenital melanocytic nevi (CMN), in particular, CMN of the head (enlargement by factor 1.7), of trunk and arms (enlargement factor 2.8), and legs (3.3).149 These revisions may help better stratify risk and improve dermatologic and interdisciplinary communication. The phenotype observed is actually a reflection of the patient’s underlying mutation, allowing for more precise prediction of clinical behavior and possible therapeutic response.
A number of activating mutations in RAS genes have been seen in large and giant nevi. Speckled lentiginous nevi are often due to somatic-activating HRAS mutations. Both large bathing trunk melanocytic nevi and nevus spilus type LCMN are often caused by activating mutations in the NRAS150 Recently, some cases of LCMN have been reported to be cause by BRAF mutations, specifically BRAF V600E.151 However, NRAS Q61 mutations predominate as a cause of L/GCMN with or without NCM.152
Proliferating nodule (PN) in GCMN. It can be challenging to differentiate proliferative nodules (PNs) from malignant melanomas (MMs) that occur in the setting of GCMN.153 PNs are more likely to develop in the first five years of life. Although typically benign (not melanoma), clinicians should consider deep, firm, or ulcerated nodules to be more concerning than soft, superficial nodules. Interpretation of biopsies of proliferative nodules and those in the newborn period can be challenging and these specimens should ideally be evaluated by dermatopathologists with experience in understanding the findings. Ancillary tests, such as genomic testing, are emerging, but remain of uncertain value in terms of disease management. PNs often manifest with a blended appearance of expansive nodules of epitheloid cells. There may be considerable atypia and sometimes mitotic activity (malignant melanoma in GCMN have increased mitoses relative to PN and ulceration of the epidermis).154
Some report that incidence of PNs to occur in 3 to 19 percent of CGMN. The estimated lifetime risk for melanoma developing in the setting of GCMN is approximately 5 to 10 percent. Overall patterns comparing PN and MM include the following:154
PNs are more common in GCMN than MMs
MMs exhibit more mitoses than PNs (1.67 vs. 12.5 mitoses per square mm)
MMs exhibit more ulceration than PNs
MMs show more partial chromosome gains (6p25).
Phadke et al155 proposed a classification system based on histopathological interpretation in PNs, but recognized that their atypical features can make them difficult to distinguish from melanoma. Atypical PNs exhibited sharp demarcation, expansile growth, epidermal effacement, and increased mistosis compared to benign PNs. While molecular analysis of these mutations has resulted in the detection of numerous mutations, the practical diagnostic value of molecular analysis remains unclear.
Conclusion
The Maui Derm conference convenes every year to present the latest findings in dermatology, highlights of new drug and device developments, and new care paradigms for dermatologic patients in our changing healthcare system. The opinions stated herein reflect those of the authors speaking freely to their peers and colleagues.
Biographies












Contributor Information
Seemal R Desai, University of Texas Southwestern Medical Center Dallas, TX.
Ilona J Frieden, University of California, San Francisco San Francisco, CA.
Joel M Gelfand, University of Pennsylvania Philadelphia, PA.
Whitney High, University of Colorado Denver, CO.
Arthur Kavanaugh, University of California, San Diego San Diego, CA.
Ashfaq A Marghoob, Memorial Sloan Kettering Cancer Center Hauppauge, NY.
David M Ozog, Henry Ford Hospital and Wayne State University Detroit, MI.
Ted Rosen, Baylor College of Medicine Houston, TX.
Linda Stein Gold, Henry Ford Hospital Detroit, MI.
Bruce Strober, University of Connecticut Health Center Farmington, CT.
Neil Swanson, Oregon Health & Science University Portland, OR.
George Martin, Dermatology Laser Center Maui Kihei, Hawaii.
REFERENCES
- 1.Kerdel FA, Strober BE. Tumor necrosis factor inhibitors in psoriasis: an update. Sem Cutan Med Surg. 2014;33(2) Suppl 2:S31–36. doi: 10.12788/j.sder.0066. [DOI] [PubMed] [Google Scholar]
- 2.De Souza A, Strober BE, Merola JF, et al. Apremilast for discoid lupus erythematosus: results of a phase 2, open-label, single-arm, pilot study. J Drugs Dermatol. 2012;11(10):1224–1226. [PubMed] [Google Scholar]
- 3.Papp, K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1) J Am Acad Dermatol. 2015;73(1):37–49. doi: 10.1016/j.jaad.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 4.Chimenti MS, Gramiccia T, Saraceno R, et al. Apremilast for the treatment of psoriasis. Expert Opin Pharmacother. doi: 10.1517/14656566.2015.1076794. 2015 August 4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Fraser K. 72nd annual meeting of the American Academy of Dermatology. Am J Clin Derm. 2014;15(2):143–145. doi: 10.1007/s40257-014-0073-9. [DOI] [PubMed] [Google Scholar]
- 6. Highlights of Prescribing Information: Otezla (apremilast) tablets for oral use. 2014. http://www.otezla.com/otezla-prescribing-information.pdf. Accessed on May 24, 2015. [Google Scholar]
- 7.van den Reek JM, Zweegers J, Kievit W, et al. ’Happy’ drug survival of adalimumab, etanercept and ustekinumab in psoriasis in daily practice care: results from the BioCAPTURE network. Brit J Dermatol. 2014;171(5):1189–1196. doi: 10.1111/bjd.13087. [DOI] [PubMed] [Google Scholar]
- 8.Gottlieb AB, Kalb RE, Langley RG, et al. Safety observations in 12,095 patients with psoriasis enrolled in an international registry (PSOLAR): experience with infliximab and other systemic and biologic therapies. J Drugs Dermatol. 2014;13(12):1441–1448. [PubMed] [Google Scholar]
- 9.Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) JAMA Dermatol. doi: 10.1001/jamadermatol.2015.0718. 13 May 2015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Carrascosa JM, Garcia-Doval I, Perez-Zafrilla B, et al. Use of off-label doses is frequent in biologic therapy for moderate to severe psoriasis: a cross-sectional study in clinical practice. J Dermatolog Treat. 2015:1–5. doi: 10.3109/09546634.2015.1034070. Apr 17. [DOI] [PubMed] [Google Scholar]
- 11.Stein Gold LF. Optimizing the use of topical agents in psoriasis. Sem Cutan Med Surg. 2014;33(2) Suppl 2:S24–26. doi: 10.12788/j.sder.0066. [DOI] [PubMed] [Google Scholar]
- 12.Katz HI, Hien NT, Prawer SE, et al. Betamethasone dipropionate in optimized vehicle. Intermittent pulse dosing for extended maintenance treatment of psoriasis. Arch Dermatol. 1987;123(10):1308–1311. doi: 10.1001/archderm.123.10.1308. [DOI] [PubMed] [Google Scholar]
- 13.Svartholm H, Larsson L, Frederiksen B. Intermittent topical treatment of psoriasis with clobetasol propionate (“Dermovate”) Curr Med Res Opin. 1982;8(3):154–157. doi: 10.1185/03007998209112377. [DOI] [PubMed] [Google Scholar]
- 14.Saraceno R, Camplone G, D’Agostino M, et al. Efficacy and maintenance strategies of two-compound formulation calcipotriol and betamethasone dipropionate gel (Xamiol(R) gel) in the treatment of scalp psoriasis: results from a study in 885 patients. J Dermatolog Treat. 2014;25(1):30–33. doi: 10.3109/09546634.2013.800182. [DOI] [PubMed] [Google Scholar]
- 15.Kircik L, Lebwohl MG, Del Rosso JQ, et al. Clinical study results of desoximetasone spray, 0.25% in moderate to severe plaque psoriasis. J Drugs Dermatol. 2013;12(12):1404–1410. [PubMed] [Google Scholar]
- 16.Rivera AM, Hsu S. Topical halobetasol propionate in the treatment of plaque psoriasis: a review. Am J Clin Dermatol. 2005;6(5):311–316. doi: 10.2165/00128071-200506050-00004. [DOI] [PubMed] [Google Scholar]
- 17.Hecker D, Worsley J, Yueh G, Lebwohl M. In vitro compatibility of tazarotene with other topical treatments of psoriasis. J Am Acad Dermatol. 2000;42(6):1008–1011. [PubMed] [Google Scholar]
- 18.Oostveen AM, de Jong EM, Donders AR, et al. Treatment of paediatric scalp psoriasis with calcipotriene/betamethasone dipropionate scalp formulation: effectiveness, safety and influence on children’s quality of life in daily practice. JEADV. doi: 10.1111/jdv.12789. Oct 13 2014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Jemec GB, Ganslandt C, Ortonne JP, et al. A new scalp formulation of calcipotriene plus betamethasone compared with its active ingredients and the vehicle in the treatment of scalp psoriasis: a randomized, double-blind, controlled trial. J Am Acad Dermatol. 2008;59(3):455–463. doi: 10.1016/j.jaad.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 20.Kragballe K, Hoffmann V, Ortonne JP, et al. Efficacy and safety of calcipotriol plus betamethasone dipropionate scalp formulation compared with calcipotriol scalp solution in the treatment of scalp psoriasis: a randomized controlled trial. Brit J Dermatol. 2009;161(1):159–166. doi: 10.1111/j.1365-2133.2009.09116.x. [DOI] [PubMed] [Google Scholar]
- 21.Lebwohl M, Siskin SB, Epinette W, et al. A multicenter trial of calcipotriene ointment and halobetasol ointment compared with either agent alone for the treatment of psoriasis. J Am Acad Dermatol. 1996;35(2 Pt 1):268–269. doi: 10.1016/s0190-9622(96)90349-7. [DOI] [PubMed] [Google Scholar]
- 22.Koo J, Blum RR, Lebwohl M. A randomized, multicenter study of calcipotriene ointment and clobetasol propionate foam in the sequential treatment of localized plaque-type psoriasis: short- and long-term outcomes. J Am Acad Dermatol. 2006;55(4):637–641. doi: 10.1016/j.jaad.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 23.Feldman SR, Matheson R, Bruce S, et al. Efficacy and safety of calcipotriene 0.005% foam for the treatment of plaque-type psoriasis: results of two multicenter, randomized, double-blind, vehicle-controlled, phase III clinical trials. Am J Clin Dermatol. 2012;13(4):261–271. doi: 10.2165/11630710-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Feldman SR, Mills M, Brundage T, Eastman WJ. Amulticenter, randomized, double-blind study of the efficacy and safety of calcipotriene foam, 0.005%, vs. vehicle foam in the treatment of plaque-type psoriasis of the scalp. J Drugs Dermatol. 2013;12(3):300–306. [PubMed] [Google Scholar]
- 25.Wojciechowski D, Vincenti F. Targeting JAK3 in kidney transplantation: current status and future options. Curr Opin Organ Transplant. 2011;16(6):614–619. doi: 10.1097/MOT.0b013e32834c23ce. [DOI] [PubMed] [Google Scholar]
- 26.Seavey MM, Dobrzanski P. The many faces of Janus kinase. Biochem Pharmacol. 2012;83(9):1136–1145. doi: 10.1016/j.bcp.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 27.Trelinski J, Robak T. JAK inhibitors: pharmacology and clinical activity in chronic myeloprolipherative neoplasms. Curr Med Chem. 2013;20(9):1147–1161. doi: 10.2174/0929867311320090004. [DOI] [PubMed] [Google Scholar]
- 28.O’Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28(4):477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vafadari R, Weimar W, Baan CC. Phosphospecific flow cytometry for pharmacodynamic drug monitoring: analysis of the JAK-STAT signaling pathway. Clin Chim Acta. 2012;413(17-18):1398–1405. doi: 10.1016/j.cca.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 30.Aittomaki S, Pesu M. Therapeutic targeting of the Jak/STAT pathway. Basic Clin Pharmacol Toxicol. 2014;114(1):18–23. doi: 10.1111/bcpt.12164. [DOI] [PubMed] [Google Scholar]
- 31.Mavers M, Ruderman EM, Perlman H. Intracellular signal pathways: potential for therapies. Curr Rheumatol Rep. 2009;11(5):378–385. doi: 10.1007/s11926-009-0054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiricozzi A, Faleri S, Saraceno R, et al. Tofacitinib for the treatment of moderate-to-severe psoriasis. Expert Rev Clin Immunol. 2015;11(4):443–455. doi: 10.1586/1744666X.2015.1013534. [DOI] [PubMed] [Google Scholar]
- 33.West K. CP-690550, a JAK3 inhibitor as animmunosuppressant for the treatment of rheumatoid arthritis, transplant rejection, psoriasis and other immune¬mediated disorders. Curr Opin Investig Drugs. 2009;10(5):491–504. [PubMed] [Google Scholar]
- 34.Papp K, Pariser D, Catlin M, et al. Phase 2a randomised, double-blind, placebo-controlled, sequential dose-escalation study to evaluate the efficacy and safety of ASP015K, a novel Janus kinase (JAK) inhibitor, in patients with moderate to severe psoriasis. Brit J Dermatol. doi: 10.1111/bjd.13745. 21 Feb 2015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Mesa RA. Ruxolitinib, a selective JAK1 and JAK2 inhibitor for the treatment of myeloproliferative neoplasms and psoriasis. IDrugs. 2010;13(6):394–403. [PubMed] [Google Scholar]
- 36.Hansen RB, Kavanaugh A. Novel treatments with small molecules in psoriatic arthritis. Curr Rheumatol Rep. 2014;16(9) doi: 10.1007/s11926-014-0443-6. 443. [DOI] [PubMed] [Google Scholar]
- 37.Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10(11):1236–1242. [PubMed] [Google Scholar]
- 38.Nickoloff BJ, Qin JZ, Nestle FO. Immunopathogenesis of psoriasis. Clin Rev Allergy Immunol. 2007;33(1-2):45–56. doi: 10.1007/s12016-007-0039-2. [DOI] [PubMed] [Google Scholar]
- 39.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445(7130):866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 40.Clark R, Chong B, Michandani N, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol Methods. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 41.Curry J, Qin J, Robinson J, Nickoloff B. Reactivity of resident immunocytes in normal and prepsoriatic skin using an ex vivo skin-explant model system. Arch Path Lab Med. 2003;127:289–296. doi: 10.5858/2003-127-0289-RORIIN. [DOI] [PubMed] [Google Scholar]
- 42.Kauffman C, Aria N, Toichi E, et al. A phase 1 study evaluating the safety, pharmacokinetics, and clinical response of a human IL-12 p40 antibody in subjects with plaque psoriasis. J Invest Dermatol. 2004;123(6):1037–1044. doi: 10.1111/j.0022-202X.2004.23448.x. [DOI] [PubMed] [Google Scholar]
- 43.Nestle F, Conrad C. The IL-12 family member p40 chain as a master switch and novel therapeutic target in psoriasis. J Invest Dermatol. 2004;123:xiv–xxv. doi: 10.1111/j.0022-202X.2004.23488.x. [DOI] [PubMed] [Google Scholar]
- 44.Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. New Engl J Med. 2014;371(4):326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 45.Oussova T. Cosentyx (Secukinumab) for injection, for subcutaneous use for the treatment of moderate to severe plaque psoriasis. Dermatologic and Ophthalmic Drugs Advisory Committee Meeting 2014; http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DermatologicandOphthalmicDrugsAdvisoryCommittee/UCM420461.pdf. Accessed 25 May 2015.
- 46.Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. New Engl J Med. 2012;366(13):1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- 47.Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. New Engl J Med. 2012;366(13):1181–1189. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 48.Citrome L, Ketter TA. When does a difference make a difference? Interpretation of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Int J Clin Pract. 2013;67(5):407–411. doi: 10.1111/ijcp.12142. [DOI] [PubMed] [Google Scholar]
- 49.Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013;69(5):729–735. doi: 10.1016/j.jaad.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 50.Gladman DD, Antoni C, Mease P, et al. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2:ii):14–17. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mease P, Goffe BS. Diagnosis and treatment of psoriatic arthritis. J Am Acad Dermatol. 2005;52(1):1–19. doi: 10.1016/j.jaad.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 52.Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs. 2014;74(4):423–441. doi: 10.1007/s40265-014-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lebwohl M, Swensen AR, Nyirady J, et al. The Psoriasis Symptom Diary: development and content validity of a novel patient-reported outcome instrument. Int J Dermatol. Jun 2014;53(6):714–722. doi: 10.1111/j.1365-4632.2012.05798.x. [DOI] [PubMed] [Google Scholar]
- 54.Haroon M, Gallagher P, Fitzgerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. doi: 10.1136/annrheumdis-2013-204858. Feb 27 2014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 55.Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis and Rheumatism. 2006;54(8):2665–2673. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 56.Kavanaugh AF, Ritchlin CT, Committee GTG. Systematic review of treatments for psoriatic arthritis: an evidence based approach and basis for treatment guidelines. J Rheumatol. 2006;33(7):1417–1421. [PubMed] [Google Scholar]
- 57.Strober BE, Armour K, Romiti R, et al. Biopharmaceuticals and biosimilars in psoriasis: what the dermatologist needs to know. J Am Acad Dermatol. 2012;66(2):317–322. doi: 10.1016/j.jaad.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 58.Beck A, Reichert JM. Approval of the first biosimilar antibodies in Europe: a major landmark for the biopharmaceutical industry. MAbs. 2013;5(5):621–623. doi: 10.4161/mabs.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar R, Singh J. Biosimilar drugs: current status. Int J App Basic Med Res. 2014;4(2):63–66. doi: 10.4103/2229-516X.136774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vogelzang EH, Kneepkens EL, Nurmohamed MT, et al. Anti-adalimumab antibodies and adalimumab concentrations in psoriatic arthritis; an association with disease activity at 28 and 52 weeks of follow-up. Ann Rheum Dis. 2014;73(12):2178–2182. doi: 10.1136/annrheumdis-2014-205554. [DOI] [PubMed] [Google Scholar]
- 61.Cantini F, Niccoli L, Cassara E, et al. Sustained maintenance of clinical remission after adalimumab dose reduction in patients with early psoriatic arthritis: a long-term follow-up study. Biologics. 2012;6:201–206. doi: 10.2147/BTT.S31145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Araujo EG, Finzel S, Englbrecht M, et al. High incidence of disease recurrence after discontinuation of disease-modifying anti-rheumatic drug treatment in patients with psoriatic arthritis in remission. Ann Rheum Dis. 2015;74(4):655–660. doi: 10.1136/annrheumdis-2013-204229. [DOI] [PubMed] [Google Scholar]
- 63.Yoshida K, Sung YK, Kavanaugh A, et al. Biologic discontinuation studies: a systematic review of methods. Ann Rheum Dis. 2014;73(3):595–599. doi: 10.1136/annrheumdis-2013-203302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kavanaugh A, Smolen JS. The when and how of biologic agent withdrawal in rheumatoid arthritis: learning from large randomised controlled trials. Clin Exp Rheumatol. 2013;31(4) Suppl 78:S19–S21. [PubMed] [Google Scholar]
- 65.Mease P. Secukinumab in PsA: FUTURE1 Study. Boston: ACR Annual Meeting; 2014 [Google Scholar]
- 66.Kavanaugh A, Ritchlin C, Rahman P, et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double¬blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann Rheum Dis. doi: 10.1136/annrheumdis-2013-204741. 19 Feb 2014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73(6):1020–1026. doi: 10.1136/annrheumdis-2013-205056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mease P. PALACE 1-3 Study. Paris, France: European League against Rheumatism; 2014 [Google Scholar]
- 69.Azfar RS, Gelfand JM. Psoriasis and metabolic disease: epidemiology and pathophysiology. Curr Opin Rheumatol. 2008;20(4):416–422. doi: 10.1097/BOR.0b013e3283031c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gelfand JM1, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 71.Gelfand JM, Shin DB, Neimann AL, et al. The risk of lymphoma in patients with psoriasis. J Invest Dermatol. 2006;126(10):2194–2201. doi: 10.1038/sj.jid.5700410. Epub 2006 Jun 1. [DOI] [PubMed] [Google Scholar]
- 72.Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3 Pt 1):556–562. doi: 10.1038/jid.2011.365. Epub 2011 Nov 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. JHypertens. 2013;31(3):433–442. doi: 10.1097/HJH.0b013e32835bcce1. discussion 442¬443. [DOI] [PubMed] [Google Scholar]
- 74.Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31(8):1000–1006. doi: 10.1093/eurheartj/ehp567. Epub 2009 Dec 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abuabara K, Azfar RS, Shin DB, et al. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U.K. Brit J Dermatol. 2010;163(3):586–592. doi: 10.1111/j.1365-2133.2010.09941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31(8):1000–1006. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dubreuil M, Rho YH, Man A, et al. Diabetes incidence in psoriatic arthritis, psoriasis and rheumatoid arthritis: a UK population-based cohort study. Rheumatology (Oxford) 2014;53(2):346–352. doi: 10.1093/rheumatology/ket343. Epub 2013 Oct 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Au SC, Goldminz AM, Loo DS, et al. Association between pediatric psoriasis and the metabolic syndrome. J Am Acad Dermatol. 2012;66(6):1012–1013. doi: 10.1016/j.jaad.2011.11.935. [DOI] [PubMed] [Google Scholar]
- 79.Ogdie A, Haynes K, Troxel AB, et al. Risk of mortality in patients with psoriatic arthritis, rheumatoid arthritis and psoriasis: a longitudinal cohort study. Ann Rheum Dis. 2014;73(1):149–53. doi: 10.1136/annrheumdis-2012-202424. Epub 2012 Dec 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129(10):2411–2418. doi: 10.1038/jid.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Azfar RS, Seminara NM, Shin DB, et al. Increased risk of diabetes mellitus and likelihood of receiving diabetes mellitus treatment in patients with psoriasis. Arch Dermatol. 2012;148(9):995–1000. doi: 10.1001/archdermatol.2012.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 83.Mehta NN, Yu Y, Pinnelas R, et al. Attributable risk estimate of severe psoriasis on major cardiovascular events. Am J Med. 2011;124(8):e771–e776. doi: 10.1016/j.amjmed.2011.03.028. 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y, Gao H, Loyd CM, et al. Chronic skin-specific inflammation promotes vascular inflammation and thrombosis. J Invest Dermatol. 2012;132(8):2067–2075. doi: 10.1038/jid.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suarez-Farinas M, Li K, Fuentes-Duculan J, et al. Expanding the psoriasis disease profile: interrogation of the skin and serum of patients with moderate-to-severe psoriasis. J Invest Dermatol. 2012;132(11):2552–2564. doi: 10.1038/jid.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alamdari HS, Gustafson CJ, Davis SA, et al. Psoriasis and cardiovascular screening rates in the United States. J Drugs Dermatol. 2013;12(1):e14–e19. [PubMed] [Google Scholar]
- 87.Ahlehoff O, Skov L, Gislason G, et al. Pharmacological undertreatment of coronary risk factors in patients with psoriasis: observational study of the Danish nationwide registries. PloS One. 2012;7(4) doi: 10.1371/journal.pone.0036342. e36342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Prodanovich S, Ma F, Taylor JR, et al. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52(2):262–267. doi: 10.1016/j.jaad.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 89.Wu JJ, Shen A, Fisher A, et al. The effect of tumor necrosis factor-alpha inhibitors on the risk of myocardial infarction in patients with psoriasis. Poster P400, presented at the American Academy of Dermatology Annual Meeting; February 4-8, 2011; New Orleans [Google Scholar]
- 90.Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273(2):197–204. doi: 10.1111/j.1365-2796.2012.02593.x. [DOI] [PubMed] [Google Scholar]
- 91.Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108(9):1362–1370. doi: 10.1016/j.amjcard.2011.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149(10):1173–1179. doi: 10.1001/jamadermatol.2013.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Callis Duffin K, Wong B, Horn EJ, Krueger GG. Psoriatic arthritis is a strong predictor of sleep interference in patients with psoriasis. J Am Acad Dermatol. 2009;60(4):604–608. doi: 10.1016/j.jaad.2008.10.059. [DOI] [PubMed] [Google Scholar]
- 94.Wakkee M, de Vries E. van den Haak P, Nijsten T. Increased risk of infectious disease requiring hospitalization among patients with psoriasis: a population-based cohort. J Am Acad Dermatol. 2011;65(6):1135–1144. doi: 10.1016/j.jaad.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 95.van der Voort EA, Koehler EM, Dowlatshahi EA, et al. Psoriasis is independently associated with nonalcoholic fatty liver disease in patients 55 years old or older: results from a population-based study. J Am Acad Dermatol. 2014;70(3):517–524. doi: 10.1016/j.jaad.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 96.Yang YW, Keller JJ, Lin HC. Medical comorbidity associated with psoriasis in adults: a population-based study. British J Dermatol. 2011;165(5):1037–1043. doi: 10.1111/j.1365-2133.2011.10494.x. [DOI] [PubMed] [Google Scholar]
- 97.Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347 doi: 10.1136/bmj.f5961. f5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Balta I, Karadag AS, Selek S, et al. General psychiatric symptoms, quality of sleep, and coping strategies in patients with psoriasis vulgaris. Int J Dermatol. doi: 10.1111/ijd.12649. May 6 2015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 99.Schell C, Schleich R, Walker F, et al. Restless legs syndrome in psoriasis: an unexpected comorbidity. Eur J Dermatol. doi: 10.1684/ejd.2015.2525. Mar 17 2015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 100.Egeberg A, Khalid U, Gislason GH, et al. Association between depression and risk of atrial fibrillation and stroke in patients with psoriasis: a Danish nationwide cohort study. British J Dermatol. doi: 10.1111/bjd.13778. Mar 17 2015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 101.Wine-Lee L, Keller SC, Wilck MB, et al. From the Medical Board of the National Psoriasis Foundation: Vaccination in adult patients on systemic therapy for psoriasis. J Am Acad Dermatol. 2013;69(6):1003–1013. doi: 10.1016/j.jaad.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 102.Chung VQ, Moschella SL, Zembowicz A, Liu V. Clinical and pathologic findings of paraneoplastic dermatoses. J Am Acad Dermatol. 2006;54(5):745–762. doi: 10.1016/j.jaad.2004.06.051. quiz 763-746. [DOI] [PubMed] [Google Scholar]
- 103.Longshore SJ, Taylor JS, Kennedy A, Nurko S. Malignant acanthosis nigricans and endometrioid adenocarcinoma of the parametrium: the search for malignancy. J Am Acad Dermatol. 2003;49(3):541–543. doi: 10.1067/s0190-9622(03)00913-7. [DOI] [PubMed] [Google Scholar]
- 104.Anderson SH, Hudson-Peacock M, Muller AF. Malignant acanthosis nigricans: potential role of chemotherapy. British J Dermatol. 1999;141(4):714–716. doi: 10.1046/j.1365-2133.1999.03116.x. [DOI] [PubMed] [Google Scholar]
- 105.Cohen PR, Grossman ME, Almeida L, Kurzrock R. Tripe palms and malignancy. J Clin Oncol. 1989;7(5):669–678. doi: 10.1200/JCO.1989.7.5.669. [DOI] [PubMed] [Google Scholar]
- 106.Bogner RR, Wetter DA, Dingli D. Scleromyxedema. Intern Med. 2014;53(21):2561–2562. doi: 10.2169/internalmedicine.53.3063. [DOI] [PubMed] [Google Scholar]
- 107.Robinson ND, Hashimoto T, Amagai M, Chan LS. The new pemphigus variants. J Am Acad Dermatol. 1999;40(5 Pt 1):649–671. doi: 10.1016/s0190-9622(99)70145-3. quiz 672-643. [DOI] [PubMed] [Google Scholar]
- 108.Rochet NM, Chavan RN, Cappel MA, et al. Sweet syndrome: clinical presentation, associations, and response to treatment in 77 patients. J Am Acad Dermatol. 2013;69(4):557–564. doi: 10.1016/j.jaad.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 109.Kurzrock R, Cohen PR. Mucocutaneous paraneoplastic manifestations of hematologic malignancies. Am J Med. 1995;99(2):207–216. doi: 10.1016/s0002-9343(99)80141-7. [DOI] [PubMed] [Google Scholar]
- 110.Prat L, Bouaziz JD, Wallach D, et al. Neutrophilic dermatoses as systemic diseases. Clin Derm. 2014;32(3):376–388. doi: 10.1016/j.clindermatol.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 111.Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68(2):e181–121. doi: 10.1016/j.jaad.2012.10.037. 189;quiz 210. [DOI] [PubMed] [Google Scholar]
- 112.Thrash B, Patel M, Shah KR, et al. Cutaneous manifestations of gastrointestinal disease: part II. J Am Acad Dermatol. 2013;68(2) doi: 10.1016/j.jaad.2012.10.037. 211 e211-233; quiz 244-216. [DOI] [PubMed] [Google Scholar]
- 113.Roy DB, Conte ET, Cohen DJ. The treatment of pyoderma gangrenosum using etanercept. J Am Acad Dermatol. 2006;54(3) Suppl 2:S128–134. doi: 10.1016/j.jaad.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 114.Tan MH, Gordon M, Lebwohl O, et al. Improvement of Pyoderma gangrenosum and psoriasis associated with Crohn disease with anti-tumor necrosis factor alpha monoclonal antibody. Arch Dermatol. 2001;137(7):930–933. [PubMed] [Google Scholar]
- 115.Storwick GS, Prihoda MB, Fulton RJ, Wood WS. Pyodermatitis-pyostomatitis vegetans: a specific marker for inflammatory bowel disease. J Am Acad Dermatol. 1994;31:336–341. doi: 10.1016/s0190-9622(94)70167-9. 2 Pt 2. [DOI] [PubMed] [Google Scholar]
- 116.De La Torre-Lugo EM, Sanchez JL. Erythema gyratum repens. J Am Acad Dermatol. 2011;64(5):e89–90. doi: 10.1016/j.jaad.2010.10.006. May. [DOI] [PubMed] [Google Scholar]
- 117.Rongioletti F, Fausti V, Parodi A. Erythema gyratum repens is not an obligate paraneoplastic disease: a systematic review of the literature and personal experience. JEADV. 2014;28(1):112–115. doi: 10.1111/j.1468-3083.2012.04663.x. [DOI] [PubMed] [Google Scholar]
- 118.Wu SL, Bai JG, Xu J, et al. Necrolytic migratory erythema as the first manifestation of pancreatic neuroendocrine tumor. World J Surg Oncol. 2014;12 doi: 10.1186/1477-7819-12-220. 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nashan D, Meiss F, Braun-Falco M, Reichenberger S. Cutaneous metastases from internal malignancies. Dermatol Ther. 2010;23(6):567–580. doi: 10.1111/j.1529-8019.2010.01364.x. [DOI] [PubMed] [Google Scholar]
- 120.Gupta AK, Paquet M, Villanueva E, Brintnell W. Interventions for actinic keratoses. Cochrane Database Syst Rev. 2012;12 doi: 10.1002/14651858.CD004415.pub2. CD004415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goldenberg G, Perl M. Actinic keratosis: update on field therapy. J Clin Aesthet Dermatol. 2014;7(10):28–31. [PMC free article] [PubMed] [Google Scholar]
- 122.Malvehy J. A new vision of actinic keratosis beyond visible clinical lesions. JEADV. 2015;29(Suppl 1):3–8. doi: 10.1111/jdv.12833. [DOI] [PubMed] [Google Scholar]
- 123.Wiegell SR, Wulf HC, Szeimies RM, et al. Daylight photodynamic therapy for actinic keratosis: an international consensus: International Society for Photodynamic Therapy in Dermatology. JEADV. 2012;26(6):673–679. doi: 10.1111/j.1468-3083.2011.04386.x. [DOI] [PubMed] [Google Scholar]
- 124.Vegter S, Tolley K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PloS One. 2014;9(6) doi: 10.1371/journal.pone.0096829. e96829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oseroff AR, Shieh S, Frawley Np, et al. Treatment of diffuse basal cell carcinomas and basaloid follicular hamartomas in nevoid basal cell carcinoma syndrome by wide-area 5-aminolevulinic acid photodynamic therapy. Arch Dermatol. 2005;141(1):60–67. doi: 10.1001/archderm.141.1.60. [DOI] [PubMed] [Google Scholar]
- 126.Kabingu E, Oseroff AR, Wilding GE, Gollnick SO. Enhanced systemic immune reactivity to a basal cell carcinoma associated antigen following photodynamic therapy. Clinical Cancer Research. 2009;15(13):4460–4466. doi: 10.1158/1078-0432.CCR-09-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Swanson N, Abramovits W, Berman B, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo controlled studies of daily application to the face and balding scalp for two 2-week cycles. J Am Acad Dermatol. 2010;62(4):582–590. doi: 10.1016/j.jaad.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 128.Longo C, Neri L, Argenziano G, et al. Management of local skin reactions after the application of ingenol mebutate gel for the treatment of actinic keratosis: four illustrative cases. JEADV. doi: 10.1111/jdv.12714. Sep 3 2014. [DOI] [PubMed] [Google Scholar]
- 129.Berman B, Goldenberg G, Hanke CW, et al. Efficacy and safety of ingenol mebutate 0.015% gel 3 weeks after cryosurgery of actinic keratosis: 11-week results. J Drugs Dermatol. 2014;13(2):154–160. [PubMed] [Google Scholar]
- 130.Berman B, Goldenberg G, Hanke CW, et al. Efficacy and safety of ingenol mebutate 0.015% gel after cryosurgery of actinic keratosis: 12-month results. J Drugs Dermatol. 2014;13(6):741–747. [PubMed] [Google Scholar]
- 131.http://www.cdc.gov/cancer/skin/statistics/ CDC. Skin Cancer Statistics. Skin Cancer 2014; Accessed on June, 9 2015.
- 132.Interventions for non-metastatic squamous cell carcinoma of the skin: a summarised Cochrane review. Clin Exp Dermatol. 2011;36(3):332–333. doi: 10.1111/j.1365-2230.2011.04056.x. [DOI] [PubMed] [Google Scholar]
- 133.Berg D, Otley CC. Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. J Am Acad Dermatol. 2002;47(1):1–17. doi: 10.1067/mjd.2002.125579. quiz 18-20. [DOI] [PubMed] [Google Scholar]
- 134.Silverman MK, Kopf AW, Grin CM, et al. Recurrence rates of treated basal cell carcinomas. Part 2: Curettage-electro-desiccation. J Dermatol Surg Oncol. 1991;17(9):720–726. doi: 10.1111/j.1524-4725.1991.tb03425.x. [DOI] [PubMed] [Google Scholar]
- 135.Berlin J, Katz KH, Helm KF, Maloney ME. The significance of tumor persistence after incomplete excision of basal cell carcinoma. J Am Acad Dermatol. 2002;46(4):549–553. doi: 10.1067/mjd.2002.117733. [DOI] [PubMed] [Google Scholar]
- 136.Ad Hoc Task F, Connolly SM, Baker DR, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531–550. doi: 10.1016/j.jaad.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 137.Rowe DE, Carroll RJ, Day CL. Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15(3):315–328. doi: 10.1111/j.1524-4725.1989.tb03166.x. [DOI] [PubMed] [Google Scholar]
- 138.Bhatnagar A. Nonmelanoma skin cancer treated with electronic brachytherapy: results at 1 year. Brachytherapy. 2013;12(2):134–140. doi: 10.1016/j.brachy.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 139.Cognetta AB, Howard BM, Heaton Hp, et al. Superficial x-ray in the treatment of basal and squamous cell carcinomas: a viable option in select patients. J Am Acad Dermatol. 2012;67(6):1235–1241. doi: 10.1016/j.jaad.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 140.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer. 2008;8(10):743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Caro I, Low JA. The role of the hedgehog signaling pathway in the development of basal cell carcinoma and opportunities for treatment. Clinical Cancer Research. 2010;16(13):3335–3339. doi: 10.1158/1078-0432.CCR-09-2570. [DOI] [PubMed] [Google Scholar]
- 142.Evans DG, Farndon PA. Nevoid basal cell carcinoma syndrome. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. Seattle: University of Washington, Seattle. GeneReviews®; 1993-2015. Epub 2002 Jun 20 [updated 2013 Mar 7] [PubMed] [Google Scholar]
- 143.Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. New Engl J Med. 2012;366(23):2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sekulic A, Migden MR, Lewis K, et al. Pivotal ERIVANCE basal cell carcinoma (BCC) study: 12-month update of efficacy and safety of vismodegib in advanced BCC. J Am Acad Dermatol. 2015;72(6):1021–1026. doi: 10.1016/j.jaad.2015.03.021. e1028. [DOI] [PubMed] [Google Scholar]
- 145.Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131(8):1735–1744. doi: 10.1038/jid.2011.48. [DOI] [PubMed] [Google Scholar]
- 146.Tang T, Tang JY, Li D, et al. Targeting superficial or nodular basal cell carcinoma with topically formulated small molecule inhibitor of smoothened. Clinical Cancer Research. 2011;17(10):3378–3387. doi: 10.1158/1078-0432.CCR-10-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32(8):745–751. doi: 10.1200/JCO.2013.49.9525. [DOI] [PubMed] [Google Scholar]
- 148.Yun JI, Kim HR, Park H, et al. Small molecule inhibitors of the hedgehog signaling pathway for the treatment of cancer. Arch Pharm Res. 2012;35(8):1317–1333. doi: 10.1007/s12272-012-0801-8. [DOI] [PubMed] [Google Scholar]
- 149.Krengel S, Scope A, Dusza SW, et al. New recommendations for the categorization of cutaneous features of congenital melanocytic nevi. J Am Acad Dermatol. 2013;68(3):441–451. doi: 10.1016/j.jaad.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 150.Kinsler VA, Krengel S, Riviere JB, et al. Next-generation sequencing of nevus spilus-type congenital melanocytic nevus: exquisite genotype-phenotype correlation in mosaic RASopathies. J Invest Dermatol. 2014;134(10):2658–2660. doi: 10.1038/jid.2014.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Salgado CM, Basu D, Nikiforova M, et al. BRAF mutations are also associated with neurocutaneous melanocytosis and large/giant congenital melanocytic nevi. Pediatr Dev Pathol. 2015;18(1):1–9. doi: 10.2350/14-10-1566-OA.1. [DOI] [PubMed] [Google Scholar]
- 152.Kusters-Vandevelde HV, Willemsen AE, Groenen PJ, et al. Experimental treatment of NRAS-mutated neurocutaneous melanocytosis with MEK162, a MEK-inhibitor. Acta Neuropathol Commun. 2014;2(41) doi: 10.1186/2051-5960-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Machan S, Molina-Ruiz AM, Fernandez-Acenero MJ, et al. Metastatic melanoma in association with a giant congenital melanocytic nevus in an adult: controversial CGH findings. Am JDermatopathol. 2015;37(6):487–494. doi: 10.1097/DAD.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 154.Yelamos O, Arva NC, Obregon R, et al. A comparative study of proliferative nodules and lethal melanomas in congenital nevi from children. Am J Surg Pathol. 2015;39(3):405–415. doi: 10.1097/PAS.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 155.Phadke PA, Rakheja D, Le LP, et al. Proliferative nodules arising within congenital melanocytic nevi: a histologic, immuno-histochemical, and molecular analyses of 43 cases. Am J Surg Pathol. 2011;35(5):656–669. doi: 10.1097/PAS.0b013e31821375ea. [DOI] [PubMed] [Google Scholar]