Abstract
Background and purpose of the study
Renal injury and dysfunction are serious complications after major surgery, which may lead to increased morbidity and mortality. The objective of our study was to identify the possible risk factors for renal dysfunction after total hip joint replacement surgery.
Methods
A retrospective study was conducted among 599 consecutive primary hip joint replacements performed between January 2011 and December 2013. According to the RIFLE criteria, increased postoperative serum creatinine was considered indicative of postoperative renal injury. The Welch two-sample test, chi-square test, and Fisher exact test were used for statistical analysis.
Results
Eighty-one patients (13.8 %) had significant moderate or severe postoperative renal dysfunction in which 10 patients (1.7 %) acquired severe and permanent renal impairment.
Conclusion
We identified advanced age, hypertension, general anesthesia, high ASA scores, low intra-operative systolic BP, and prophylactic dicloxacillin as significant risk factors. Low baseline systolic BP, low baseline diastolic blood pressure, and hip fracture diagnosis were independent risk factors for postoperative increase in serum creatinine. Smoking, diabetes mellitus, high BMI, gender, and duration of surgery were not identified as significant risk factors.
Introduction
Total hip joint replacement is indicated mainly for hip osteoarthritis, for complications after osteosynthesis of hip fractures, and for the treatment of femoral neck fractures in relatively young patients. Possible complications are deep venous thrombosis [1–3], infection [4–6], dislocation of the hip prosthesis [7, 8] and increased creatinine levels, and impaired renal function [9–11]; the latter may in turn increase mortality and morbidity among patients who are already affected by diseases such as diabetes mellitus, hypertension, heart disease, and obesity [12–16]. The aim of this study was to identify patients with renal injury after total hip joint replacement and to detect possible risk factors and their clinical relevance in our retrospective material of 599 consecutive total hip joint replacements. In recent years, a few studies identified renal impairment as a complication to be considered after major surgery [17–21].
Materials and methods
A retrospective study was performed which included a consecutive cohort of patients who underwent primary total hip joint replacement using cementless CORAIL®stem with either Pinnacle or Avantage cup, between January 2011 and December 2013. Indications for surgery were primary osteoarthritis (n = 551), femoral neck fractures, and complications after osteosynthesis of hip fractures (n = 48). A total of 599 patients with a total of 599 hip joint replacements were included. Data was obtained from our computerized database and hospital charts. Charts were reviewed for at least 9 months after surgery. Out of the 599 total hip joint replacements, 588 had complete data sets matching our investigation criteria. The following variables were selected [17, 18]: age, sex, body mass index (BMI), hypertension, diabetes mellitus, smoking, American Society of Anesthesiologists (ASA) physical status, prophylactic antibiotics according to our protocol (one dose immediately preoperatively and three doses in the first postoperative day), duration of surgery, type of anesthesia, baseline systolic blood pressure (BP), baseline diastolic BP, intra-operative systolic BP, and intra-operative diastolic BP (lowest measured blood pressure intra-operatively).
Furthermore, 11 patients were not included due to the missing intra-operative BP data. Two patients were excluded due to pre-existing severe renal dysfunction (in hemodialysis) because any new renal injury could not have been detected. Five hundred eighty-six patients, with complete data set and inclusion criteria, were available for analysis.
In our department, the protocol for elective total hip joint replacement surgery includes measuring serum creatinine; once preoperatively and three consecutive days postoperatively. Increased postoperative serum creatinine was monitored and controlled daily until it decreased or the patient was referred to the nephrology department. During the first postoperative week, the highest serum creatinine was chosen as a sign for maximum renal injury. Dicloxacillin was the antibiotic of choice for prophylaxis and cefuroxime used as the alternative in cases of allergies to penicillin.
Patients were identified as renally impaired using the relative increase in serum creatinine and the RIFLE classification proposed by the Acute Dialysis Quality Initiative Group to identify patients with renal impairment [19–21]. The patients were accordingly divided into two groups, those with RIFLE < 1.5 times increase in serum creatinine where renal impairment is absent or mild and those with RIFLE ≥ 1.5 times increase in serum creatinine indicating moderate or severe renal impairment (Table 1).
Table 1.
The RIFLE classification
| GFR criteria | Urine output criteria | |
|---|---|---|
| Risk | SCr increased 1.5 times | 0.5 ml(kg h) for 6 h |
| Injury | SCr increased 2.0 times | 0.5 ml(kg h) for 12 h |
| Failure | SCr increased 3.0 times or creatinine = 355 μmol/l when there was an acute rise of >44 μmol/l |
0.3 ml(kg h) for 24 h or anuria for 12 h |
| Loss | Persistent ARF; complete loss of kidney function for >4 weeks | |
| End-stage renal disease | End-stage renal disease for >3 months |
For statistical analysis and graphics, the free R software was used (www.r-project.org). Multivariate regression analysis was performed using the command generalized linear model. Model reduction was performed objectively using an automated procedure (step) maximizing Akaike’s information criterion (AIC). For the multivariate regression model, the relative increase in serum creatinine was used as a continuous variable. Univariate comparisons were made using the Welch two-sample test, chi-square test, and Fishers exact test. A P value of 0.05 or less was considered significant.
The study has been approved by the Danish Data Management Board, and it has been conducted in accordance with the ethical and legal requirements of the Institutional Review Board of Sjaelland region.
Results
During the study, 81 out of 586 patients had significant moderate or severe renal impairment (RIFLE ≥ 1.5) resulting in an overall incidence of 13.8 % (Table 2). Forty-six patients (7.8 %) had RIFLE 1.5–2, 19 patients (3.2 %) had RIFLE 2–3, and 16 patients (2.7 %) had RIFLE ≥ 3. Out of these 81 patients, 71 improved but 10 patients acquired severe and permanent renal impairment (i.e., in dialysis) with an incidence of 1.7 %. Seven patients had postoperative serum creatinine above the defined failure limit (355 μmol/l). This was not correlated with a higher preoperative serum creatinine (Fig. 1a). The two patients with high preoperative serum creatinine were already above 200 μmol/l. They had only a smaller relative increase in serum creatinine (Fig. 1a, b). The renal status of the 81 patients was observed through electronic charts for at least 9 months after surgery.
Table 2.
The variables advanced age, general anesthesia, hypertensive disease, and high ASA scores revealed significant postoperative renal dysfunction. Patients not receiving dicloxacillin preoperatively were given cefuroxime
| Variables | RIFLE < 1.5 | RIFLE ≥ 1.5 | P value | Test |
|---|---|---|---|---|
| n = 505 | n = 81 | |||
| Mean age | 69 (range 37–93) | 73 (range 49–91) | 0.002* | T |
| Mean BMI | 27.4 (range 15–46) | 27.5 (range 18–42) | 0.77 | T |
| Duration of Surgery (minutes) | 64 (range 30–223) | 65 (range 30–161) | 0.67 | T |
| Baseline systolic BP | 147 (range 90–206) | 154 (range 115–231) | 0.011 | T |
| Baseline diastolic BP | 83 (range 40–121) | 80 (range 50–114) | 0.05 | T |
| Intra-operative systolic BP | 90 (range 60–145) | 89 (range 60–170) | 0.53 | T |
| Intra-operative diastolic BP | 52 (range 30–90) | 50 (range 35–80) | 0.24 | T |
| General anesthesia | 265 yes/240 no | 53 yes/28 no | 0.04* | C |
| Gender | 229 M/276 F | 31 M/50 F | 0.28 | C |
| Smoking | 386 no/119 yes | 65 no/16 yes | 0.53 | C |
| Hypertensive patients | 264 | 56 | 0.006* | C |
| Normotensive patients | 241 | 25 | ||
| Diabetes mellitus | 456 no/49 yes | 73 no/8 yes | 1 | C |
| ASA score 1 | 91 | 5 | 0.006* | C |
| ASA score 2 | 319 | 52 | ||
| ASA score 3 | 95 | 24 | ||
| Dicloxacillin | 53 no/452 yes | 3 no/78 yes | 0. 084 | F |
T Welch two sample test, C chi-square test, F Fisher exact test
Fig. 1.
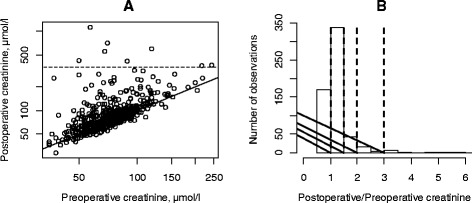
a XY plot of preoperative versus postoperative serum creatinine. The patients had a mean increase in postoperative serum creatinine of 8 μmol/l (0.0–15.4, 95 % confidence interval on the difference, P = 0.05 paired t-test). The diagonal line depicts no change. The broken line is set at the limit of 355 μmol/l (see Table 2). The normal range for women is 50–90 μmol/l and for men 60–105 μmol/l. b Histogram of relative change in serum creatinine. The mean relative change was 1.2. The vertical broken lines depict 1 = no change, 1.5, 2, and 3 according to the RIFLE classification
Table 2 reveals advanced age, hypertension, general anesthesia, high ASA scores, low intra-operative BP, and using prophylactic dicloxacillin as being significant risk factors for renal impairment, after total hip joint replacement on univariate analysis.
Generalized multivariate modeling was performed using the relative change in serum creatinine as a dependent variable. It confirmed that advanced age, hypertension, general anesthesia, prophylactic dicloxacillin, low baseline systolic and diastolic BP, and having a hip fracture diagnosis were significant independent risk factors for a rise in serum creatinine (Table 3).
Table 3.
Model output after stepwise reduction. The dependent variable was the relative change in serum creatinine defined as postoperative creatinine/preoperative creatinine
| Estimate | Std. error | P value | |
|---|---|---|---|
| Age | 0.003509 | 0.001502 | 0.0198* |
| BMI | 0.004841 | 0.002954 | 0.1018 |
| Diabetes mellitus | −0.076695 | 0.046419 | 0.0990 |
| Hypertension | 0.043715 | 0.02879 | 0.1285 |
| General anesthesia | 0.072565 | 0.027415 | 0.0083* |
| Dicloxacillin | 0.157739 | 0.046075 | 0.0007* |
| Baseline systolic BP | −0.003150 | 0.000755 | 0.0001* |
| Baseline diastolic BP | 0.004641 | 0.001362 | 0.0007* |
| Diagnosis fracture | 0.136877 | 0.051407 | 0.0079* |
BMI, duration of surgery, gender, diabetes mellitus, and smoking were not considered significant risk factors.
Discussion
Increased hospital stay, morbidity, mortality, and increased cost may all be consequences of acute postoperative renal dysfunction [22, 23]. To date, preventative strategies are the only effective measures to reduce morbidity in cases of postoperative renal dysfunction. Therefore, in order to influence our guidelines, it is imperative to identify the risk factors of renal dysfunction after total hip joint replacement surgery.
In spite of the retrospective design, data was complete for most patients; only 11 patients were excluded from the study due to missing data. However, an important limitation was the missing information on fluid input and output which would have potential influence on renal function. Unfortunately, these charts were unreliable and had frequent missing records of blood loss during surgery. Therefore, data regarding perioperative blood loss was not collected. None of our patients had received blood transfusions perioperatively, and very few patients received blood transfusion postoperatively (<1 %) indicating minimal blood loss during surgery. Excessive blood loss during surgery may lead to decreased intra-operative BP and renal blood flow predisposing the patients to pre-renal failure. Our study shows that a higher preoperative serum creatinine is not a predictor for either a higher postoperative serum creatinine above the limit of 355 μmol/l or a higher relative change (Fig. 1a).
In accordance with Mantilla et al. [1], Parvizi et al. [3], Aveline et al. [9], Nergelius et al. [10], Abelha et al. [11], and Jämsen et al. [23], we found increased age as an independent risk factor for renal dysfunction after major surgery. However, Sharrock et al. [13] was not able to confirm the age factor in this regard. This may have been due to the relatively small number of patients included.
Our patients received either general anesthesia (n = 318) or spinal anesthesia (n = 268). General anesthesia was an independent risk factor for the development of postoperative renal dysfunction [24]. The type of anesthesia was chosen by the attending anesthesiologist only after an individual clinical assessment of each patient was performed. Thus, this observation may have been influenced by preferences of the anesthesiologist. Jafari et al. [17] did not report this finding—perhaps due to inadequate data regarding the number of patients who received general anesthesia or other forms of anesthesia.
Our patients received prophylactic antibiotics in the form of either dicloxacillin (n = 530) or cefuroxime (n = 56). Those receiving the former had a significant increased risk of increased postoperative serum creatinine. Baily et al. [25], Solgaard et al. [26], and Isacson and Collert [27] developed the same conclusion in their respective studies. Dicloxacillin has been the local recommendation for many years due to the narrow bacterial spectrum relevant to prevent infections with Staphylococcus aureus. In addition, dicloxacillin compared to cefuroxime is known to have a lower risk of complications concerning gastrointestinal problems and induction of bacterial resistance [28, 29].
The ASA score was an independent significant risk factor for the development of renal impairment, thus corresponding with the findings of Parvizi et al. [3], Abelha et al. [11], Belmont et al. [16], and Jafari et al. [17].
In our study, hypertensive disease (under treatment) had a significant increase in the risk for renal impairment as supported by Nergelius et al. [10], Naik et al. [21], and Weingarten et al. [24]. In addition, patients with low baseline systolic and diastolic BP, before anesthesia induction, also had an increased risk for renal impairment. This may be due to a reduced capacity to tolerate an additional drop in BP during anesthesia induction.
Several authors [3, 15–17, 24, 30] have indicated that high BMI was an independent risk factor after joint replacement surgery. Although our BMI range was 15 to 46, we could not confirm this finding.
Weingarten et al. [24] found that diabetes mellitus was independently associated with a high risk of developing acute kidney injury after total joint replacement, which was not the case in our study. However, Weingarten et al. [24] did not mention the actual diabetic disease control whereby our patients were meticulously controlled preoperatively.
Our study revealed a relatively high incidence of renal impairment (2.7 %) after primary total hip replacement compared to other studies [3, 17, 24]. The retrospective study conducted by Jafari et al. [17] showed an incidence of 0.55 % of acute renal failure or injury after joint arthroplasties (98 out of 17,938 joint arthroplasties including revision arthroplasties). Parvizi et al. [3] had an incidence of 0.85 % of acute renal failure in their prospective study of 1636 primary hip and knee joint replacements. The incidence was higher (1.82 %) in the retrospective study conducted by Weingarten et al. [24] which included a cohort of 9171 patients in which 167 patients showed acute kidney injury postoperatively. Nykoebing Falster Hospital serves an area of Denmark with a relatively older population and relatively low social status which would explain the higher risk of renal impairment.
Therefore, it is recommended that further studies be conducted and include controlled randomization to elucidate causal factors concerning postoperative renal impairment, after major surgery.
Conclusion
Our study, in accordance with other studies, confirms the increased risk of renal injury after total hip joint replacement surgery. These findings may warrant a change in the protocol for informed consent as well as preoperative preparation protocols. Patients intended for total hip joint replacement may have to be informed preoperatively of any increased risk of renal impairment. High-risk patients (advanced age, hypertensive disease, and high ASA scores) should be indentified early for further optimization pre- and intra-operatively.
Acknowledgements
The authors would like to thank the department secretary Stine Kruse for her contribution in obtaining the medical charts.
Footnotes
Competing interest
The authors declare that they have no competing interests.
Authors’ contributions
BKH: data collection and manuscript writing. AS: manuscript writing. RBCD: statistical analysis. All authors read and approved the final manuscript.
Contributor Information
Basim Kamil Hassan, Phone: +45 51281986, Email: basimkhassan@hotmail.com.
Arne Sahlström, Phone: +45 56516510, Email: svas@regionsjaelland.dk.
Ram Benny Christian Dessau, Phone: +45 28699280, Email: ramd@regionsjaelland.dk.
References
- 1.Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology. 2002;97(2):531. doi: 10.1097/00000542-200208000-00053. [DOI] [PubMed] [Google Scholar]
- 2.Bruce W, Van der Wall H, Peters M, Liaw Y, Morgan L, Storey G. Occurrence of pulmonary thromboembolism immediately after arthroplasty. Nucl Med Commun. 2001;22:1237–42. doi: 10.1097/00006231-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27–32. doi: 10.2106/JBJS.E.01443. [DOI] [PubMed] [Google Scholar]
- 4.Lindeque B, Hartman Z, Noshchenko A, Cruse M. Infection after primary total hip arthroplasty. Orthopedics. 2014;37(4):257–65. doi: 10.3928/01477447-20140401-08. [DOI] [PubMed] [Google Scholar]
- 5.Kane P, Chen C, Post Z, Radcliff K, Orozco F, Ong A. Seasonality of infection rates after total joint arthroplasty. Orthopedics. 2014;37(2):e182–6. doi: 10.3928/01477447-20140124-23. [DOI] [PubMed] [Google Scholar]
- 6.Delaunay C, Hamadouche M, Girard J, Duhamel A, SoFCOT Group What are the causes for failures of primary hip arthroplasties in France? Clin Ortho Relat Res. 2013;471(12):3863–9. doi: 10.1007/s11999-013-2935-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heithoff BE, Callaghan JJ, Goetz DD, Sullivan PM, Pedersen DR, Johnston RC. Dislocation after total hip arthroplasty: a single surgeon’s experience. Ortho Clin North Am. 2001;32(4):587–91. doi: 10.1016/S0030-5898(05)70229-7. [DOI] [PubMed] [Google Scholar]
- 8.Jørgensen CC, Kjaersgaard-Andersen P, Solgaard S, Kehlet H, Lundbeck Foundation Centre for Fast-track Hip and Knee Replacement Collaborative Group Hip dislocations after 2,734 elective unilateral fast-track total hip arthroplasties: incidence, circumstances and predisposing factors. Arch Orthop Trauma Surg. 2014;134(11):1615–22. doi: 10.1007/s00402-014-2051-3. [DOI] [PubMed] [Google Scholar]
- 9.Aveline C, Leroux A, Vautier P, Cognet F, Le Hetet H, Bonnet F. Risk factors for renal dysfunction after total hip arthroplasty. Ann Fr Anesth Reanim. 2009;28(9):728–34. doi: 10.1016/j.annfar.2009.07.077. [DOI] [PubMed] [Google Scholar]
- 10.Nergelius G, Vinge E, Grubb A, Lidgren L. Renal impairment after hip or knee arthroplasty. Urinary excretion of protein markers studied in 59 patients. Acta Orthop Scand. 1997;68(4):410–1. doi: 10.3109/17453679708996191. [DOI] [PubMed] [Google Scholar]
- 11.Abelha FJ, Botelho M, Fernandes A, Barros H. Determinants of postoperative acute kidney injury. Crit Care. 2009;13(3):R79. doi: 10.1186/cc7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pulido L, Parvizi J, Macgibeny M, Sharkey PF, Purtill JJ, Rothman RH, et al. In hospital complications after total knee joint arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):139–45. doi: 10.1016/j.arth.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Sharrock NE, Cazan MG, Hargett MJ, Williams-Russo P, Wilson PD., Jr Changes in mortality after total hip and knee arthroplasty over a ten-year period. Anesth Analg. 1995;80(2):242–8. doi: 10.1097/00000539-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Lalmohamed A, Vestergaard P, de Boer A, Leufkens HG, van Staa TP, de Vries F. Changes in mortality patterns following total hip or knee arthroplasty over the past two decades: a nationwide cohort study. Arthritis Rheumatol. 2014;66(2):311–8. doi: 10.1002/art.38232. [DOI] [PubMed] [Google Scholar]
- 15.Suleiman LI, Ortega G, Ong’uti SK, Gonzalez DO, Tran DD, Onyike A, et al. Does BMI affect perioperative complications following total knee and hip arthroplasty. J Surg Res. 2012;174(1):7–11. doi: 10.1016/j.jss.2011.05.057. [DOI] [PubMed] [Google Scholar]
- 16.Belmont PJ, Jr, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am. 2014;96(1):20–6. doi: 10.2106/JBJS.M.00018. [DOI] [PubMed] [Google Scholar]
- 17.Jafari SM, Huang R, Joshi A, Parvizi J, Hozack WJ. Renal impairment following total joint arthroplasty: who is at risk? J Arthroplasty. 2010;25(6 Suppl):49–53. doi: 10.1016/j.arth.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Novis BK, Roizen MF, Aronson S. Association of preoperative risk factors with postoperative acute renal failure. Anesth Analg. 1994;78:143. doi: 10.1213/00000539-199401000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Bellomo R, Ronco C, Kellum JA. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kheterpal S, Tremper KK, Englesbe MJ. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology. 2007;107:892. doi: 10.1097/01.anes.0000290588.29668.38. [DOI] [PubMed] [Google Scholar]
- 21.Naik BI, Colquhoun DA, McKinney WE, Smith AB, Titus B, McMurry TL, et al. Incidence and risk factors for acute kidney injury after spine surgery using the RIFLE classification. J Neurosurg Spine. 2014;20(5):505–11. doi: 10.3171/2014.2.SPINE13596. [DOI] [PubMed] [Google Scholar]
- 22.Doddakula K, Al-Sarraf N, Gately K. Predictors of acute renal failure requiring renal replacement therapy post cardiac surgery in patients with preoperatively normal renal function. Interact Cardiovasc Thorac Surg. 2007;6:314. doi: 10.1510/icvts.2006.148874. [DOI] [PubMed] [Google Scholar]
- 23.Jämsen E, Puolakka T, Eskelinen A, Jäntti P, Kalliovalkama J, Nieminen J, et al. Predictors of mortality following primary hip and knee replacement in the aged. Acta Orthop. 2013;84(1):44–53. doi: 10.3109/17453674.2012.752691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weingarten TN, Gurrieri C, Jarret PD, Brown DR, Berntson NJ, Calaro RD, Jr, et al. Acute kidney injury following total joint arthroplasty: retrospective analysis. Can J Anaesth. 2012;59(12):1111–8. doi: 10.1007/s12630-012-9797-2. [DOI] [PubMed] [Google Scholar]
- 25.Baily O, Torkington MS, Anthony I, Wells J, Blyth M, Jones B. Antibiotic-related acute kidney injury in patients undergoing elective joint replacement. Bone Joint J. 2014;96-B(3):395–8. doi: 10.1302/0301-620X.96B3.32745. [DOI] [PubMed] [Google Scholar]
- 26.Solgaard L, Tuxoe JI, Mafi M, Due Olsen S, Toftgaard JT. Nephrotoxicity by dicloxacillin and gentamycin in 163 patients with intertrochanteric hip fractures. Int Orthop. 2000;24(3):155–7. doi: 10.1007/s002640000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isacson J, Collert S. Renal impairment after high doses of dicloxacillin-prophylaxis in joint replacement surgery. Acta Orthop Scand. 1984;55(4):407–10. doi: 10.3109/17453678408992384. [DOI] [PubMed] [Google Scholar]
- 28.Tängdén T, Eriksson BM, Melhus A, Svennblad B, Cars O. Radical reduction of cephalosporin use at a tertiary hospital after educational antibiotic intervention during an outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. J Antimicrob Chemother. 2011;66:1161–1167. doi: 10.1093/jac/dkr053. [DOI] [PubMed] [Google Scholar]
- 29.Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis. 2011;53(1):42–8. doi: 10.1093/cid/cir301. [DOI] [PubMed] [Google Scholar]
- 30.Kelz RR, Reinke CE, Zubizarreta JR, Wang M, Saynisch P, Even-Shoshan O, et al. Acute kidney injury, renal function, and the elderly obese surgical patient: a matched case-control study. Ann Surg. 2013;258(2):359–63. doi: 10.1097/SLA.0b013e31829654f3. [DOI] [PMC free article] [PubMed] [Google Scholar]


