Abstract
Purpose.
Using a novel automated perimetry technique, we tested the hypothesis that older adults will have increased latency and decreased accuracy of saccades, as well as higher visual thresholds, to peripheral visual stimuli when compared with younger adults.
Methods.
We tested 20 healthy subjects aged 18 to 30 years (“young”) and 21 healthy subjects at least 60 years old (“older”) for detection of briefly flashed peripheral stimuli of differing sizes in eight locations along the horizontal meridian (±4°, ±12°, ±20°, and ±28°). With the left eye occluded, subjects were instructed to look quickly toward any seen stimuli. Right eye movements were recorded with an EyeLink 1000 infrared camera system. Limiting our analysis to the four stimulus positions in the nasal hemifield (−4°, −12°, −20°, and −28°), we evaluated for group-level differences in saccadic latency, accuracy, and visual threshold at each stimulus location.
Results.
Saccadic latency increased as stimulus size decreased in both groups. Older subjects had significantly increased saccadic latencies (at all locations; P < 0.05), decreased accuracies (at all locations; P < 0.05), and higher visual thresholds (at the −12°, −20°, and −28° locations; P < 0.05). Additionally, there were significant relationships between visual threshold and latency, visual threshold and accuracy, and latency and accuracy (P < 0.0001).
Conclusions.
Older adults have increased latency and decreased accuracy of saccades, as well as higher visual thresholds, to peripheral visual stimuli when compared with younger adults. Saccadic latency and accuracy are related to visual threshold, suggesting that saccadic latency and accuracy could be useful as perimetric outcome measures.
Keywords: saccades, visual threshold, automated perimetry
Using a novel automated perimetry technique, we evaluated the latency and accuracy of saccades to peripheral visual stimuli in younger compared with older adults. We found that older adults had increased latencies, decreased accuracies, and higher visual thresholds compared with young adults.
Introduction
Static automated perimetry permits rapid estimation of visual thresholds based on manual responses to peripheral, near-threshold stimuli, but ignores eye movement responses that could provide useful insight into visual function. Because eye movements could be used to evaluate for visual field loss,1–4 we sought to develop a saccade-based perimetry technique that estimated visual threshold and also used the latency and amplitude of saccades to assess for visual field loss. This novel approach allowed us to examine variation in visual threshold, saccadic latency, and saccadic accuracy across the visual field in healthy young and older subjects, and to evaluate the relationship between these dependent variables.
In healthy humans, saccadic latency and accuracy vary depending on stimulus characteristics. Saccadic latency increases with increasing stimulus eccentricity,5,6 decreasing luminance,5,7,8 decreasing contrast,9 and increasing spatial frequency.9 Likewise, saccadic accuracy decreases with increasing stimulus eccentricity,6,10,11 whereas saccadic amplitude variability increases with increasing stimulus eccentricity12 and decreasing luminance.8 Variations in stimulus size have a modest effect on saccadic latency and accuracy,10,13,14 but stimulus sizes near visual threshold have not been evaluated in young or older populations.
Many studies have evaluated the effects of senescence on saccades. Although saccadic velocity tends to be preserved, older adults' saccadic latencies are increased6,11,15–22 and more variable17,18,20 compared with those of younger adults. Furthermore, older adults' saccadic amplitudes are often smaller than those of younger adults, and this effect is exaggerated with increasing stimulus eccentricity.6,11,19,22 In this study, we aimed to determine, using a gap stimulus paradigm, the latency and accuracy of saccades to peripheral visual stimuli with sizes close to threshold in healthy young adults (18–30 years old) compared with healthy older adults (60 or more years old), and to determine if the latency and accuracy of these saccades were related to threshold at each stimulus location. We hypothesized that older adults would have increased saccadic latencies, decreased accuracies, and increased visual thresholds compared with the young adults.
Methods
Subjects
We invited adults aged 18 to 30 years and 60 or more years to participate. We recruited subjects with no history of neurologic or ophthalmic disease, a normal ophthalmic examination with corrected visual acuities of at least 20/30, and normal 30-1 Humphrey Matrix perimetry (Welch-Allyn, Skaneateles, NY). We excluded subjects with refractive error greater than 6.0 diopters (D) of sphere when corrected with spectacles or 8.0 D of sphere when corrected with contact lenses, astigmatism greater than 3.0 D of cylinder, eye movement or pupil abnormalities, amblyopia, poorly controlled diabetes or hypertension, or a prior history of pilocarpine or hydroxychloroquine treatment. The Institutional Review Board (IRB) of the University of Iowa approved the experimental protocols. All subjects gave written informed consent in accordance with the Declaration of Helsinki and the University of Iowa IRB.
Subjects were recruited by advertisements in the hospital newspaper and were paid for participating. We recruited 20 subjects into the 18- to 30-year-old (“young”) group (9 females/11 males; age: 24 ± 3 years [mean ± SD]), and 21 subjects into the 60-year old and older (“older”) group (13 females/8 males; age: 66 ± 5 years).
Experiment Setup
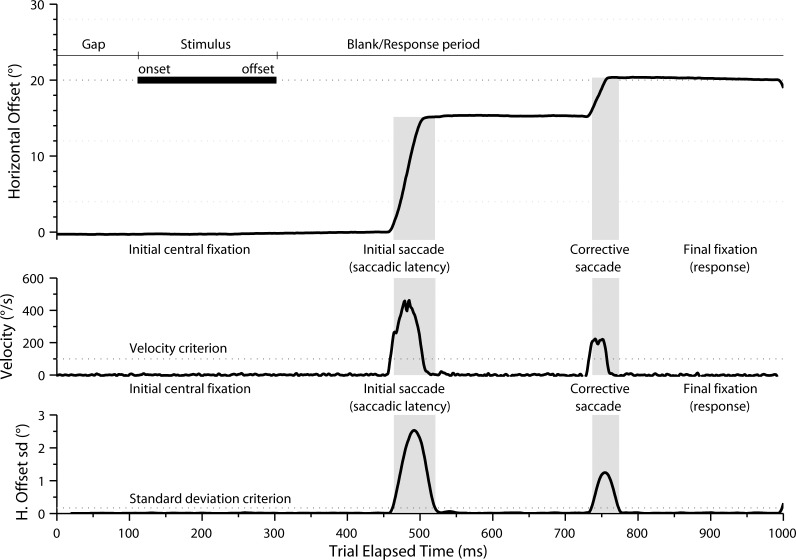
Right eye position was recorded with a temporal resolution of 1000 Hz using an EyeLink 1000 infrared camera system (SR Research, Ltd., Kanata, Ontario, Canada). Visual stimuli were presented on a 598 × 337-mm liquid crystal display monitor (model W2753VC; LG Electronics, Slough, Berkshire, UK) with a vertical refresh rate of 60 Hz and a resolution of 1920 × 1080 pixels. Visual stimuli were generated using MatLab (R2007b; The MathWorks, Inc., Natick, MA) with the Psychophysics Toolbox extension.23,24 The peripheral visual stimuli were light gray (250 cd/m2) filled circles of varying size, presented at eight horizontal locations on a darker gray (150 cd/m2) background: 4° right (4°), 4° left (−4°), 12° right (12°), 12° left (−12°), 20° right (20°), 20° left (−20°), 28° right (28°), and 28° left (−28°). Stimulus size was varied from 0 to 5 dB, with increases in size as close as possible to 100.1; stimulus visual angles were 0.05° (0 dB), 0.11° (1 dB), 0.22° (2 dB), 0.30° (3 dB), 0.37° (4 dB), and 0.48° (5 dB). Before each test, the EyeLink system was calibrated: subjects fixated a visual stimulus at five locations on the screen (center, 15.9° up, 15.9° down, 29.5° left, and 29.5° right). Calibration accuracy was immediately verified by having subjects fixate stimuli at the same five locations. If the calibrated eye position was not less than or equal to 0.5° from the stimulus position at each location, the calibration was repeated. For each trial, eye movements were recorded for 1050 ms (Fig. 1).
Figure 1.
Time series plot of a representative eye movement response (position, in degrees, and velocity, in degrees per second) to a peripheral visual stimulus from a subject in the older group. Following a gap of 100 ms, the peripheral visual stimulus is displayed for 200 ms. The subject's initial saccade begins approximately 350 ms following the onset of the peripheral stimulus. A second corrective saccade is made following the initial saccade; note that, in this example, the eye position following the corrective saccade (indicated as “final fixation”) was used to determine response accuracy.
Experiment Protocols
Subjects sat with their head supported and their eyes 41 cm from the display monitor, and were instructed to keep their head still. If subjects normally wore glasses, trial lenses were used based on an over-refraction within 0.5 D. Otherwise, subjects wore their contact lenses. Subjects with refractive error of 6 to 8 D were required to wear contact lenses. The left eye was occluded with an opaque patch. Following calibration, subjects were instructed to fixate on a central visual stimulus. For a trial to begin, eye position had to be maintained steady within 2° of the center of the fixation stimulus. Following a 100-ms gap, during which no stimulus was displayed, a stimulus was presented randomly at one of the eight peripheral locations for approximately 200 ms (191 ± 3 ms, mean ± SD; see Fig. 1 for an annotated trial sequence). Subjects were instructed to look quickly, without blinking or moving their head, toward any peripheral visual stimulus that was seen. At each location, stimulus size was varied from 0 to 4 dB (or 1–5 dB at the ±12°, ±20°, and ±28° locations for the older subject group). To permit calculation of frequency of seeing curves, each stimulus size was presented at each location 9 to 10 times, in a random order, giving a total of 300 to 400 trials per testing session. Each session lasted for approximately 20 minutes. Subjects were allowed to rest after every 100 trials; the EyeLink system was recalibrated after each break.
Data and Statistical Analysis
Data were analyzed in MatLab and R (version 2.15.1; http://www.r-project.org) using custom software (written by DEW). Eye position data were scaled according to calibration data. Trials showing blinks or head movement were excluded from analysis, as were trials containing saccades less than 100 ms after stimulus onset. Horizontal eye velocity was calculated by differentiating the horizontal eye position data and was smoothed.25
Eye movement latency was defined as the duration of time between stimulus onset and the first saccadic eye movement in the direction of the stimulus, calculated as the time when horizontal eye velocity exceeded 100° per second (to prevent inclusion of microsaccades26; Fig. 1). Eye movement accuracy was defined as the horizontal eye position closest to the stimulus position for 50 ms or more in the interval between the onset of the stimulus and 100 ms from the end of the recording period. Since the initial saccade was often hypometric (Fig. 1), especially in older subjects, we considered the stimulus as seen if the response amplitude was ±2° of the stimulus for the ±4° locations, ±5° of the stimulus for the ±12° locations, or ±10° of the stimulus for the ±20° and ±28° locations, during the analysis period. Thus, correct responses were in the hemifield and locale of the stimulus.
We calculated localization error as the absolute magnitude of the difference (in degrees) between horizontal response amplitude and stimulus location.27 We also calculated relative localization error (RLE) as the error (in degrees) in the horizontal response amplitude relative to stimulus location,28 such that hypometric and hypermetric responses could be distinguished.
Frequency of seeing curves was generated for each stimulus location in each subject by plotting the proportion of stimuli seen versus stimulus size (in dB). We used a 4-parameter probit model (Psignifit for MatLab) to fit the curve of maximum likelihood.29,30 The four parameters fitted for each subject and stimulus location were visual threshold (stimulus size corresponding to 50% of stimuli seen), slope (steepness of curve), false-positive rate (correct responses below threshold), and false-negative rate (incorrect responses above threshold). Threshold estimates outside of the 0- to 5-dB range were considered poor fits and were excluded from analysis. In the nasal hemifield, 4 subjects had an estimated threshold beyond the 0- to 5-dB range (all at the −28° location), whereas 16 subjects had estimated thresholds beyond the 0- to 5-dB range in the temporal hemifield (at the 12° [n = 9], 20° [n = 4], and 28° [n = 3] locations).
Statistical analysis was performed in R. Saccadic latencies, accuracies, and visual thresholds for the two groups were compared using repeated-measures ANOVA on ranks (nparLD library31), complemented by planned comparisons between groups and stimulus positions (Wilcoxon signed-rank tests). We corrected for multiple comparisons using Bonferroni's method (α = 0.05). Because the visual stimulus at 12° corresponded with the physiologic blind spot, only data for stimuli presented in the nasal hemifield were included in our statistical analysis.
Results
Visual Threshold
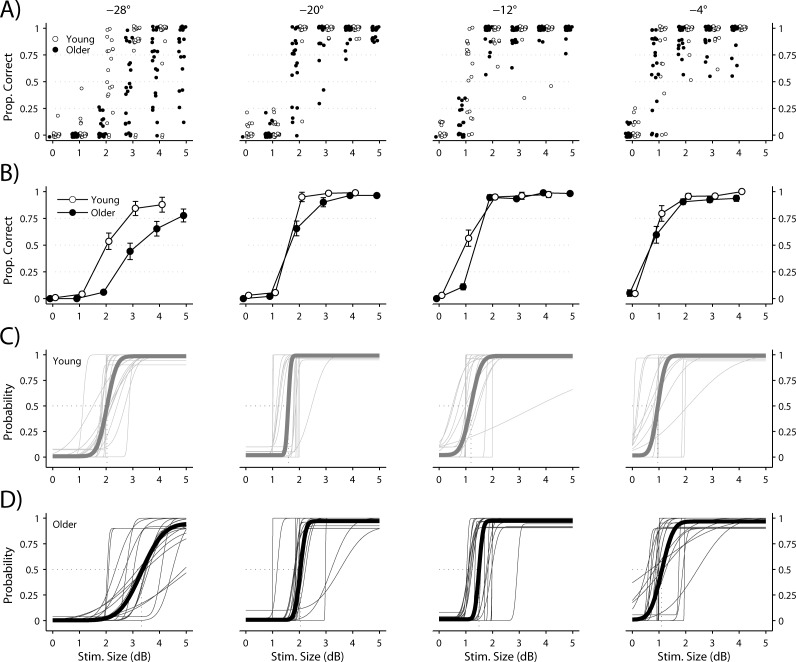
The proportion of visual stimuli considered “seen” by each subject is plotted for each stimulus size at each location in Figure 2A, with the mean ± SEM for the young and older groups plotted in Figure 2B. The frequency of seeing curve for each subject in the young and older groups is plotted for each stimulus location in Figures 2C and 2D, with the mean curves in bold. The mean ± SEM for visual threshold at each location is indicated for each group in Table 1. Note that visual threshold increased with increasing stimulus eccentricity (P < 0.05) and was significantly higher in the older compared with the young group at the −12°, −20°, and −28° locations (test for group factor: P < 0.005; Fig. 2, Table 1). The false-positive response rates were 1.5% ± 3.2% (mean ± SD) and 0.8% ± 2.0% in the young and older groups, respectively. False-negative response rates were 1.0% ± 2.3% and 3.5% ± 4.1% in the young and older groups, respectively. The false-negative response rates differed by group (test for group factor: P < 0.005), because the older group missed more suprathreshold targets than the younger group, whereas the false-positive rates did not differ between groups (test for group factor: P = 0.66).
Figure 2.
Proportions of visual stimuli seen at each location are plotted relative to stimulus size (in dB). (A) Responses from each subject. (B) Mean ± SEM for young and older subjects. (C) Frequency of seeing curves for all young subjects; mean curve is indicated by the bold line. (D) Frequency of seeing curves for all older subjects; mean curve is indicated by the bold line.
Table 1. .
Mean ± SD for Visual Threshold (in dB)
|
Stimulus Location |
Visual Threshold, dB |
||
|
Young Subjects |
Older Subjects |
Significance,
P |
|
| −4° | 0.96 ± 0.53 | 1.10 ± 0.49 | >0.05 |
| −12° | 1.19 ± 0.61 | 1.49 ± 0.47 | <0.01 |
| −20° | 1.59 ± 0.41 | 2.04 ± 0.58 | <0.05 |
| −28° | 2.03 ± 0.39 | 3.33 ± 0.80 | <0.0001 |
Saccadic Latency
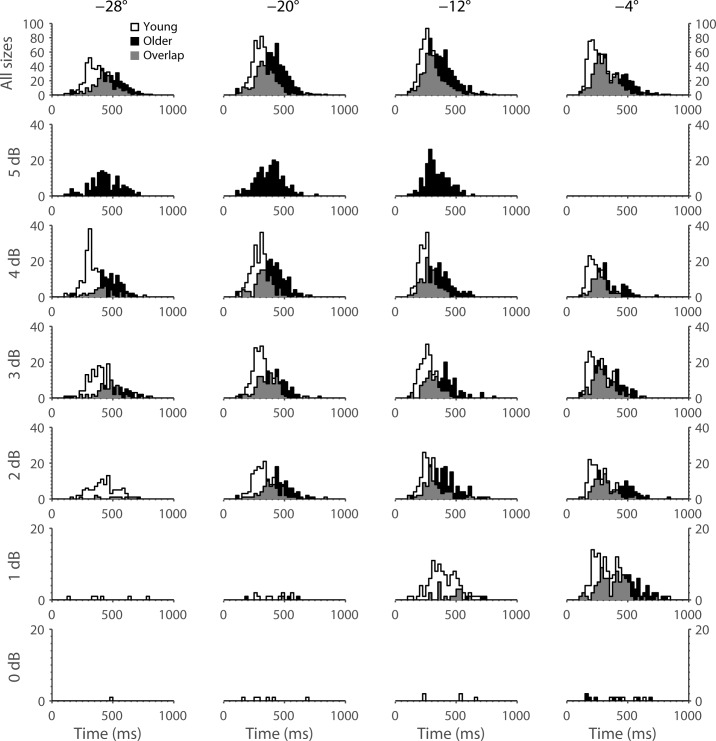
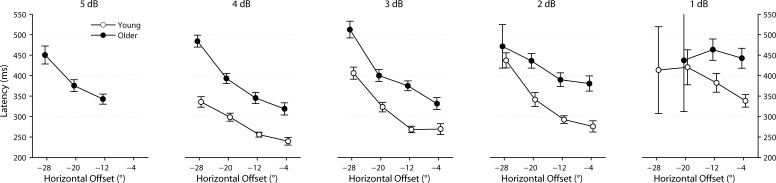
Saccadic latencies of all subjects are plotted for each stimulus size at each location in Figure 3. The mean ± SEM of latency for each location is indicated for the two groups in Table 2 and plotted in Figure 4. Latency increased with increasing stimulus eccentricity and decreasing stimulus size in both groups. Latency was significantly increased in the older compared with the young group at all locations (test for group factor: P < 0.0001). “Express” saccades (latency <150 ms) were rarely observed in either subject group.
Figure 3.
Frequency of response latencies (in ms) for each stimulus size at each location. Data from young subjects are plotted in white, whereas those from older subjects are plotted in black. Areas of data overlap are indicated by gray shading.
Table 2. .
Mean ± SEM for Response Latency (in ms)
|
Stimulus Location |
Latency, ms |
||
|
Young Subjects |
Older Subjects |
Significance,
P |
|
| −4° | 282 ± 11 | 361 ± 14 | <0.0005 |
| −12° | 292 ± 7 | 365 ± 11 | <0.0005 |
| −20° | 321 ± 11 | 400 ± 11 | <0.0001 |
| −28° | 388 ± 13 | 479 ± 16 | <0.005 |
Figure 4.
Mean ± SEM of response latency (in ms) for each stimulus size at each location. Data from young subjects are plotted in white, whereas those from older subjects are in black.
Saccadic Error (Accuracy)
RLE for all subjects in the two groups is plotted for each stimulus size at each location in Figure 5. The means ± SEMs of localization error and RLE for each stimulus location are indicated for the two groups in Table 3 and, for RLE, plotted in Figure 6. Localization error was increased in the older compared with the young group at all locations (test for group factor: P < 0.0001). Localization error increased with increasing stimulus eccentricity, significantly so in the older group. Pairwise comparisons between adjacent stimulus positions were all significant for the older group (P < 0.0001), whereas only −28° vs. −20° and −12° vs. −4° were significant in the younger group (P < 0.05); the difference was reflected in a significant effect of group by eccentricity (test for group-by-position interaction: P < 0.01).
Figure 5.
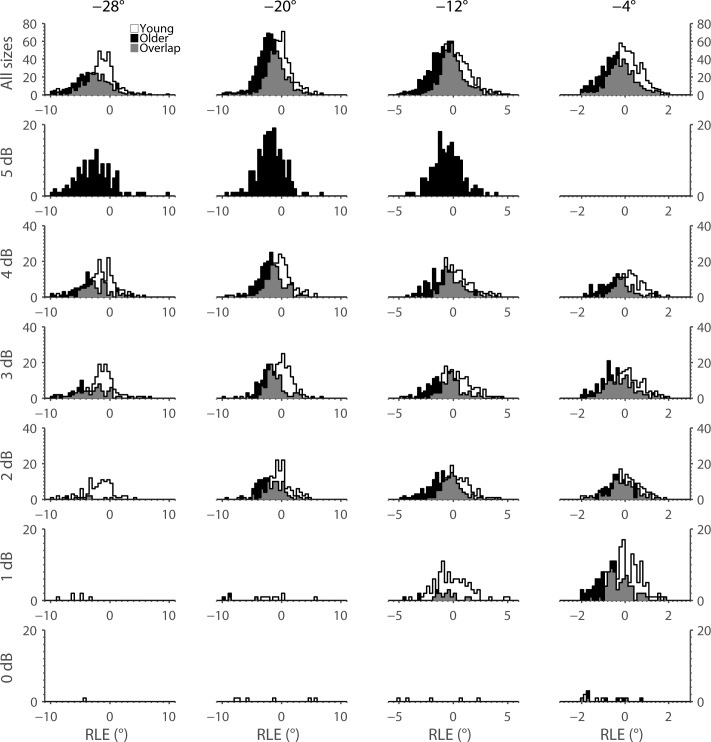
Frequency of RLE (in degrees) for each stimulus size at each location. Data from young subjects are plotted in white, whereas those from older subjects are plotted in black. Areas of data overlap are indicated by gray shading. Note that a negative RLE indicates a hypometric response, whereas a positive RLE indicates a hypermetric response.
Table 3. .
Mean ± SEM for Localization Error and RLE (in Degrees)
|
Stimulus Location |
Localization Error, ° |
Relative Localization Error, ° |
||||
|
Young |
Older |
Significance,
P |
Young |
Older |
Significance,
P |
|
| −4° | 0.55 ± 0.03 | 0.68 ± 0.03 | 0.066 | 0.05 ± 0.07 | −0.38 ± 0.07 | <0.005 |
| −12° | 1.11 ± 0.11 | 1.30 ± 0.09 | 0.122 | 0.32 ± 0.20 | −0.72 ± 0.13 | <0.001 |
| −20° | 1.59 ± 0.16 | 2.47 ± 0.18 | <0.005 | −0.40 ± 0.29 | −1.98 ± 0.29 | <0.005 |
| −28° | 2.21 ± 0.19 | 3.94 ± 0.34 | <0.001 | −1.66 ± 0.30 | −3.38 ± 0.45 | <0.05 |
Figure 6.
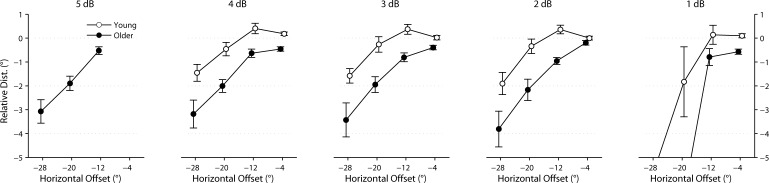
Mean ± SEM of RLE (in degrees) for each stimulus size at each location. Data from young subjects are plotted in white, whereas those from older subjects are in black. Note that a negative RLE indicates a hypometric response, whereas a positive RLE indicates a hypermetric response.
The mean RLE at the −4° and −12° stimulus locations was positive in the young group, indicating slightly hypermetric responses (overshoots). In contrast, the mean RLE at the same locations was negative in the older group, indicating hypometric responses (undershoots). Both groups showed hypometric responses at the −20° and −28° stimulus locations, and this effect was exaggerated in the older group. Pairwise comparisons between adjacent stimulus positions were all significant for the older group (P < 0.0001), whereas only −28° vs. −20° and −20° vs. −12° were significant in the young group (P < 0.05). Pairwise comparisons were not significant for −12° vs. −4° (P = 0.703) or −20° vs. −4° (P = 0.222) in the young group. There was a significant effect of group by eccentricity (test for group-by-position interaction: P < 0.05).
Relationships Among Variables
To examine the relationship among our dependent variables of visual threshold, saccadic accuracy (i.e., RLE), and saccadic latency, we implemented a series of linear mixed-effects (LME) regression models. Each model included one dependent variable as continuous outcome measure, another as a linear predictor (fixed effect), stimulus location as a four-level categorical predictor (fixed effect), and a subject-level term (random effect). Significance of fixed effects was evaluated using an ANOVA applied to the fitted parameter values of the LME model. We implemented three such models: first, visual threshold predicting latency; second, visual threshold predicting RLE; third, RLE predicting latency. Additionally, we fitted a variant of each model that included a predictor-by-stimulus location interaction to test whether the relationship between the predictor and predicted dependent variables differed by stimulus location. Each pair of models (i.e., those with and without the interaction term) was compared using a likelihood ratio test.
Saccadic Latency Versus Visual Threshold.
There was a significant relationship between latency and visual threshold (P < 0.0001) that varied by stimulus location (likelihood ratio = 15.776, P = 0.001). In the three most peripheral stimulus locations (i.e., −28°, −20°, and −12°), the relationship was positive (i.e., higher visual thresholds predicted longer latencies), whereas the relationship was negative at the −4° location.
Relative Localization Error Versus Visual Threshold.
There was a significant relationship between RLE and visual threshold (P < 0.0001) that did not vary by stimulus location (likelihood ratio = 3.549, P = 0.314). Overall, increased visual thresholds were related to more hypometric localization errors (i.e., increasingly negative RLEs).
Saccadic Latency Versus Relative Localization Error.
There was a significant relationship between latency and RLE (P < 0.0001) that did not vary by stimulus location (likelihood ratio = 2.962, P = 0.398). Overall, more hypometric (i.e., increasingly negative) RLEs were related to increased latency.
Discussion
We used a novel eye-movement perimetry technique to determine the latency and accuracy of saccades to peripheral visual stimuli of differing sizes approaching visual threshold in healthy young adults compared with healthy older adults. We found that older adults had increased saccadic latencies, decreased accuracies, and increased visual thresholds. Also, we found that latency, accuracy, and visual threshold were significantly related.
Saccadic Latency
Saccadic latency was influenced by stimulus eccentricity and size in both subject groups, increasing with stimulus eccentricity (Table 2, Fig. 4) in agreement with prior studies.5,6 The modulation of saccadic latency with stimulus eccentricity is thought to relate to the degree of cone and rod stimulation,8,32 which varies depending on stimulus eccentricity. In nonhuman primates, rods determine the response latency for dim stimuli, whereas cones determine the response latency when stimulus intensity exceeds their activation threshold.33 Recordings from optic tract axons in cats during stimulation of receptive fields within 15° of area centralis have demonstrated increased and more variable response latencies with perithreshold stimuli.34
Although the relationships between stimulus intensity (luminance) and both saccadic and manual response latencies have been extensively studied, there are few studies evaluating the relationship with stimulus size. Suprathreshold variation in stimulus size reportedly produces modest effects on saccadic latency,10,13,14 but stimulus sizes close to visual threshold have not been evaluated previously. We evaluated stimulus sizes spanning visual threshold and found that saccadic latencies increased with decreasing stimulus size (Figs. 3, 4). Prior studies have found that the latencies of manual responses and visual-evoked potentials in humans increase as the area of retinal stimulation decreases35; likewise, the latencies of optic nerve discharges in vertebrates increase as the area of retinal stimulation decreases.36,37 Together, these findings suggest that saccadic latency to eccentric visual stimuli is influenced by the convergence (spatial summation) of photoreceptor responses on retinal ganglion cells.
Although saccadic latencies partly depend on physiologic processes in the retina, we found that they were increased in older compared with young adults at all locations, consistent with prior studies.6,11,15–22 The mechanisms underlying these age-related increases in saccadic latencies remain unclear, but age-related cortical changes may contribute. Imaging studies have demonstrated significant age-related decreases in the volume of the gray matter of most cortical regions,38 with the frontal lobes being most severely affected.39,40 Indeed, prior studies have found that saccadic tasks requiring frontal lobe integrity, such as the antisaccade task, show a more prominent latency increase compared with visually guided saccadic tasks in healthy older subjects.20 Visually guided saccadic tasks like ours are more dependent on parietal than frontal lobe integrity,41 which may explain why the latency increase is less prominent than for voluntary saccadic tasks (e.g., antisaccade task).20
Saccadic latency is significantly decreased when there is a gap rather than overlap between the offset of the fixation stimulus and the onset of the peripheral stimulus.42,43 Prior studies using a gap paradigm have observed that a number of saccades have very short latencies (<150 ms). It has previously been documented that older subjects make fewer of these so-called “express” saccades than young subjects.19,44 In our study, express saccades occurred rarely in both subject groups, despite a gap paradigm. Factors that might have limited express saccades in our study include randomization of stimulus location45,46 and use of low-contrast stimuli.47 The low frequency of express saccades produced a more normal distribution of latencies (Fig. 3) rather than the bimodal distribution seen with paradigms evoking larger numbers of express saccades.
Response Accuracy
In the current study, young adults showed hypermetric responses to visual stimuli close to fixation and hypometric responses to eccentric stimuli, whereas older adults showed hypometric responses to all stimuli (Figs. 5, 6, Table 3). Hypermetric responses for stimuli close to visual fixation have been reported in younger subjects.5,11 One study found that hypermetric responses occurred for stimuli that had intensities only close to foveal threshold; suprathreshold stimuli produced responses that were accurate or hypometric.5
A robust finding in our study was that response amplitudes became more hypometric as stimulus eccentricity increased. Indeed, it is well established that saccadic gain decreases with increasing stimulus eccentricity.6,11 The superior colliculus plays an important role in the generation of visually guided saccades. The collicular map is arranged in two-dimensional retinotopic coordinates; saccadic direction and amplitude vary depending on where the colliculus is stimulated.48–50 However, this map is nonhomogeneous, with saccades to locations close to fixation having disproportionally greater representation than saccades to more eccentric locations.48,51 Furthermore, the map is anisotropic, being more expanded along the direction than along the amplitude representation.52 The properties of the collicular map likely explain the less accurate and more variable saccades to eccentric visual stimuli.
Older subjects showed more hypometric responses than younger subjects in our study, as in previous work.6,11,19,22 Although the properties of the collicular map might explain the decreased accuracy of saccades to more eccentric stimuli, cerebellar dysfunction may also contribute; loss of cerebellar Purkinje cells has been documented in healthy older humans.53,54
Visual Threshold
We found that older adults had increased visual thresholds when compared with young adults (Fig. 2, Table 1). It is established that visual field sensitivity decreases with increasing age in healthy adults.55,56 Possible explanations include changes in the optic media and pupil size (which could reduce retinal image quality), loss of neurons in the afferent visual pathway, and possible increase in subclinical pathology in older subjects.55
An important finding of our study was that saccadic latency, accuracy, and visual threshold were related. Although several studies have suggested that eye movements could be used to evaluate visual field loss,1–4 these have not evaluated the latency and amplitude of saccades to peripheral visual stimuli as a means for determining visual threshold. Saccadic latency and accuracy may be important measures that change not only with aging, but with damage to visual sensory structures. For example, an increased latency of saccades to stimuli presented in areas of visual field loss has been demonstrated in patients with resolved optic neuritis,57 optic chiasm compression,57 amblyopia,58 and glaucoma.59 Furthermore, increased error in manually localizing peripheral visual stimuli has been reported in patients with glaucoma,60 even at test locations with normal visual sensitivity on the basis of standard automated perimetry. Because there are highly developed neural networks in the retina and cortex, visual testing aimed at evaluating functions that change as these networks fail (e.g., speed and accuracy) could have promise as perimetric outcome measures.
In conclusion, our study demonstrates that speed (saccadic latency) and accuracy (saccadic error) of response can be assessed while determining visual threshold, without added overhead to testing time, by monitoring subjects' eye movements during perimetric testing. We hope these measures will find utility not only for detecting early visual field loss, but also for confirming visual threshold, thereby allowing faster and more accurate clinical testing strategies.
Acknowledgments
Supported by a Veterans Affairs Rehabilitation Research and Development Merit Review grant. David E. Warren was supported by NIMH R01 MH062500.
Disclosure: D.E. Warren, None; M.J. Thurtell, None; J.N. Carroll, None; M. Wall, None
References
- 1. Jernigan ME. Visual field plotting using eye movement response. IEEE Trans Biomed Eng. 1979; 26: 601–606. [DOI] [PubMed] [Google Scholar]
- 2. Jernigan ME. Structural analysis of eye movement response to visual field stimuli. Comput Biol Med. 1980; 10: 11–22. [DOI] [PubMed] [Google Scholar]
- 3. Murray IC, Fleck BW, Brash HM, Macrae ME, Tan LL, Minns RA. Feasibility of saccadic vector optokinetic perimetry: a method of automated static perimetry for children using eye tracking. Ophthalmology. 2009; 116: 2017–2026. [DOI] [PubMed] [Google Scholar]
- 4. Trope GE, Eizenman M, Coyle E. Eye movement perimetry in glaucoma. Can J Ophthalmol. 1989; 24: 197–199. [PubMed] [Google Scholar]
- 5. Kalesnykas RP, Hallett PE. Retinal eccentricity and the latency of eye saccades. Vision Res. 1994; 34: 517–531. [DOI] [PubMed] [Google Scholar]
- 6. Sharpe JA, Zackon DH. Senescent saccades. Effects of aging on their accuracy, latency and velocity. Acta Otolaryngol. 1987; 104: 422–428. [DOI] [PubMed] [Google Scholar]
- 7. Wheeless LL Jr, Boynton RM, Cohen GH. Luminance as a parameter of the eye-movement control system. J Opt Soc Am. 1967; 57: 394–400. [Google Scholar]
- 8. Doma H, Hallett PE. Dependence of saccadic eye-movements on stimulus luminance, and an effect of task. Vision Res. 1988; 28: 915–924. [DOI] [PubMed] [Google Scholar]
- 9. Ludwig CJ, Gilchrist ID, McSorley E. The influence of spatial frequency and contrast on saccade latencies. Vision Res. 2004; 44: 2597–2604. [DOI] [PubMed] [Google Scholar]
- 10. Fischer B, Weber H. Effects of stimulus conditions on the performance of antisaccades in man. Exp Brain Res. 1997; 116: 191–200. [DOI] [PubMed] [Google Scholar]
- 11. Irving EL, Steinbach MJ, Lillakas L, Babu RJ, Hutchings N. Horizontal saccade dynamics across the human life span. Invest Ophthalmol Vis Sci. 2006; 47: 2478–2484. [DOI] [PubMed] [Google Scholar]
- 12. Van Opstal AJ, Van Gisbergen JA. Scatter in the metrics of saccades and properties of the collicular motor map. Vision Res. 1989; 29: 1183–1196. [DOI] [PubMed] [Google Scholar]
- 13. Dick S, Ostendorf F, Kraft A, Ploner CJ. Saccades to spatially extended targets: the role of eccentricity. Neuroreport. 2004; 15: 453–456. [DOI] [PubMed] [Google Scholar]
- 14. Ploner CJ, Ostendorf F, Dick S. Target size modulates saccadic eye movements in humans. Behav Neurosci. 2004; 118: 237–242. [DOI] [PubMed] [Google Scholar]
- 15. Abel LA, Troost BT, Dell'Osso LF. The effects of age on normal saccadic characteristics and their variability. Vision Res. 1983; 23: 33–37. [DOI] [PubMed] [Google Scholar]
- 16. Carter JE, Obler L, Woodward S, Albert ML. The effect of increasing age on the latency for saccadic eye movements. J Gerontol. 1983; 38: 318–320. [DOI] [PubMed] [Google Scholar]
- 17. Kaneko R, Kuba Y, Sakata Y, Kuchinomachi Y. Aging and shifts of visual attention in saccadic eye movements. Exp Aging Res. 2004; 30: 149–162. [DOI] [PubMed] [Google Scholar]
- 18. Klein C, Foerster F, Hartnegg K, Fischer B. Lifespan development of pro- and anti-saccades: multiple regression models for point estimates. Brain Res Dev Brain Res. 2005; 160: 113–123. [DOI] [PubMed] [Google Scholar]
- 19. Munoz DP, Broughton JR, Goldring JE, Armstrong IT. Age-related performance of human subjects on saccadic eye movement tasks. Exp Brain Res. 1998; 121: 391–400. [DOI] [PubMed] [Google Scholar]
- 20. Peltsch A, Hemraj A, Garcia A, Munoz DP. Age-related trends in saccade characteristics among the elderly. Neurobiol Aging. 2011; 32: 669–679. [DOI] [PubMed] [Google Scholar]
- 21. Schik G, Mohr S, Hofferberth B. Effect of aging on saccadic eye movements to visual and auditory targets. Int Tinnitus J. 2000; 6: 154–159. [PubMed] [Google Scholar]
- 22. Tedeschi G, Di Costanzo A, Allocca S, et al. Age-dependent changes in visually guided saccadic eye movements. Funct Neurol. 1989; 4: 363–367. [PubMed] [Google Scholar]
- 23. Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997; 10: 433–436. [PubMed] [Google Scholar]
- 24. Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997; 10: 437–442. [PubMed] [Google Scholar]
- 25. Engbert R, Kliegl R. Microsaccades uncover the orientation of covert attention. Vision Res. 2003; 43: 1035–1045. [DOI] [PubMed] [Google Scholar]
- 26. Otero-Millan J, Troncoso XG, Macknik SL, Serrano-Pedraza I, Martinez-Conde S. Saccades and microsaccades during visual fixation, exploration, and search: foundations for a common saccadic generator. J Vis. 2008; 8: 21.1–21.18. [DOI] [PubMed] [Google Scholar]
- 27. Wall M, Montgomery EB. Using motion perimetry to detect visual field defects in patients with idiopathic intracranial hypertension: a comparison with conventional automated perimetry. Neurology. 1995; 45: 1169–1175. [DOI] [PubMed] [Google Scholar]
- 28. Wall M, Woodward KR, Brito CF. The effect of attention on conventional automated perimetry and luminance size threshold perimetry. Invest Ophthalmol Vis Sci. 2004; 45: 342–350. [DOI] [PubMed] [Google Scholar]
- 29. Treutwein B, Strasburger H. Fitting the psychometric function. Percept Psychophys. 1999; 61: 87–106. [DOI] [PubMed] [Google Scholar]
- 30. Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys. 2001; 63: 1293–1313. [DOI] [PubMed] [Google Scholar]
- 31. Noguchi K, Gel YR, Brunner E, Konietschke F. nparLD: an R software package for the nonparametric analysis of longitudinal data in factorial experiments. J Stat Softw. 2012; 50: 1–23. 25317082 [Google Scholar]
- 32. Doma H, Hallett PE. Rod-cone dependence of saccadic eye-movement latency in a foveating task. Vision Res. 1988; 28: 899–913. [DOI] [PubMed] [Google Scholar]
- 33. Gouras P, Rod Link K. and cone interaction in dark-adapted monkey ganglion cells. J Physiol. 1966; 184: 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lennie P. The physiological basis of variations in visual latency. Vision Res. 1981; 21: 815–824. [DOI] [PubMed] [Google Scholar]
- 35. Vaughan HG Jr, Costa LD, Gilden L. The functional relation of visual evoked response and reaction time to stimulus intensity. Vision Res. 1966; 6: 645–656. [DOI] [PubMed] [Google Scholar]
- 36. Adrian ED, Matthews R. The action of light on the eye. Part I. The discharge of impulses in the optic nerve and its relation to the electric changes in the retina. J Physiol. 1927; 63: 378–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hartline HK. The effects of spatial summation in the retina on the excitation of the fibers of the optic nerve. Am J Physiol. 1940; 130: 700–711. [Google Scholar]
- 38. Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR. Cerebral structure on MRI, part 1: localization of age-related changes. Biol Psychiatry. 1991; 29: 55–67. [DOI] [PubMed] [Google Scholar]
- 39. Jernigan TL, Archibald SL, Fennema-Notestine C, et al. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001; 22: 581–594. [DOI] [PubMed] [Google Scholar]
- 40. Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003; 23: 3295–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pierrot-Deseilligny C, Rivaud S, Gaymard B, Agid Y. Cortical control of reflexive visually-guided saccades. Brain. 1991; 114: 1473–1485. [DOI] [PubMed] [Google Scholar]
- 42. Fischer B, Ramsperger E. Human express saccades: extremely short reaction times of goal directed eye movements. Exp Brain Res. 1984; 57: 191–195. [DOI] [PubMed] [Google Scholar]
- 43. Weber H, Fischer B. Gap duration and location of attention focus modulate the occurrence of left/right asymmetries in the saccadic reaction times of human subjects. Vision Res. 1995; 35: 987–998. [DOI] [PubMed] [Google Scholar]
- 44. Klein C, Fischer B, Hartnegg K, Heiss WH, Optomotor Roth M. and neuropsychological performance in old age. Exp Brain Res. 2000; 135: 141–154. [DOI] [PubMed] [Google Scholar]
- 45. Fischer B, Ramsperger E. Human express saccades: effects of randomization and daily practice. Exp Brain Res. 1986; 64: 569–578. [DOI] [PubMed] [Google Scholar]
- 46. Dickov LA, Morrison JD. Effects of uncertainty and target displacement on the latency of express saccades in man. Vision Res. 2006; 46: 2505–2512. [DOI] [PubMed] [Google Scholar]
- 47. Nothdurft HC, Parlitz D. Absence of express saccades to texture or motion defined targets. Vision Res. 1993; 33: 1367–1383. [DOI] [PubMed] [Google Scholar]
- 48. Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res. 1972; 12: 1795–1808. [DOI] [PubMed] [Google Scholar]
- 49. Van Opstal AJ, Hepp K, Hess BJ, Straumann D, Henn V. Two- rather than three-dimensional representation of saccades in monkey superior colliculus. Science. 1991; 252: 1313–1315. [DOI] [PubMed] [Google Scholar]
- 50. Hepp K, Van Opstal AJ, Straumann D, Hess BJ, Henn V. Monkey superior colliculus represents rapid eye movements in a two-dimensional motor map. J Neurophysiol. 1993; 69: 965–979. [DOI] [PubMed] [Google Scholar]
- 51. Sparks DL. Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus. Physiol Rev. 1986; 66: 118–171. [DOI] [PubMed] [Google Scholar]
- 52. Ottes FP, Van Gisbergen JA, Eggermont JJ. Visuomotor fields of the superior colliculus: a quantitative model. Vision Res. 1986; 26: 857–873. [DOI] [PubMed] [Google Scholar]
- 53. Hall TC, Miller AK, Corsellus JA. Variations in the human Purkinje cell population according to age and sex. Neuropathol Appl Neurobiol. 1975; 1: 267–292. [Google Scholar]
- 54. Andersen BB, Gundersen HJ, Pakkenberg B. Aging of the human cerebellum: a stereological study. J Comp Neurol. 2003; 466: 356–365. [DOI] [PubMed] [Google Scholar]
- 55. Spry PG, Johnson CA. Senescent changes of the normal visual field: an age-old problem. Optom Vis Sci. 2001; 78: 436–441. [DOI] [PubMed] [Google Scholar]
- 56. Gardiner SK, Johnson CA, Spry PG. Normal age-related sensitivity loss for a variety of visual functions throughout the visual field. Optom Vis Sci. 2006; 83: 438–443. [DOI] [PubMed] [Google Scholar]
- 57. Brigell MG, Goodwin JA, Lorance R. Saccadic latency as a measure of afferent visual conduction. Invest Ophthalmol Vis Sci. 1988; 29: 1331–1338. [PubMed] [Google Scholar]
- 58. Ciuffreda KJ, Kenyon RV, Stark L. Increased saccadic latencies in amblyopic eyes. Invest Ophthalmol Vis Sci. 1978; 17: 697–702. [PubMed] [Google Scholar]
- 59. Lamirel C, Milea D, Cochereau I, Duong MH, Lorenceau J. Impaired saccadic eye movement in primary open-angle glaucoma [published online ahead of print June 14, 2012] J Glaucoma. doi:10.1097/IJG.0b013e31825c10dc. [DOI] [PubMed] [Google Scholar]
- 60. Wall M, Jennisch CS. Random dot motion stimuli are more sensitive than light stimuli for detection of visual field loss in ocular hypertension patients. Optom Vis Sci. 1999; 76: 550–557. [DOI] [PubMed] [Google Scholar]