Abstract
Visual symptoms are relatively common in Parkinson’s disease (PD) and optical coherence tomography has indicated possible retinal thinning. Accumulation of aggregated α-synuclein is thought to be a central pathogenic event in the PD brain but there have not as yet been reports of retinal synucleinopathy. Retinal wholemounts were prepared from subjects with a primary clinicopathological diagnosis of PD (N = 9), dementia with Lewy bodies (DLB; N = 3), Alzheimer’s disease (N = 3), progressive supranuclear palsy (N = 2) as well as elderly normal control subjects (N = 4). These were immunohistochemically stained with an antibody against α-synuclein phosphorylated at serine 129, which is a specific molecular marker of synucleinopathy. Phosphorylated α-synuclein-immunoreactive (p-syn IR) nerve fibers were present in 7/9 PD subjects and in 1/3 DLB subjects; these were sparsely distributed and superficially located near or at the inner retinal surface. The fibers were either long and straight or branching, often with multiple en-passant varicosities along their length. The straight fibers most often had an orientation that was radial with respect to the optic disk. Together, these features are suggestive of either retinopetal/centrifugal fibers or of ganglion cell axons. In one PD subject there were sparse p-syn IR neuronal cell bodies with dendritic morphology suggestive of G19 retinal ganglion cells or intrinsically photosensitive ganglion cells. There were no stained nerve fibers or other specific staining in any of the non-PD or non-DLB subjects. It is possible that at least some of the observed visual function impairments in PD subjects might be due to α-synucleinopathy.
Keywords: Lewy body, Pathology, Autopsy, Diagnosis, Melanopsin, Ganglion cell
1. Introduction
Visual symptoms are relatively common in Parkinson’s disease (PD), including reading difficulties and perceptual distortions. Detailed examinations suggest that visual acuity, contrast sensitivity and color vision are impaired [1–4]. A number of ocular structural and functional abnormalities have been reported in PD subjects and much attention has been paid to the possibility of an ophthalmological biomarker or diagnostic test. Perhaps the most activity has come from the field of optical coherence tomography (OCT), which has enabled high resolution structural imaging of the retina. Several groups have used OCT to measure the thickness of retinal layers and generally concur that the retina is thinner in PD subjects as compared to healthy controls [5]. It is unclear, however, whether the differences are sufficient and consistent enough to serve as a diagnostic marker and whether they are specific to PD or are a change held in common with other neurodegenerative conditions. Retinal thinning has also been reported, for example, in Alzheimer’s disease [6], which often co-exists with PD [7].
The cellular basis of retinal thinning has been ascribed by some to loss of dopaminergic amacrine neurons and/or retinal ganglion cells. In some animal models of Parkinson’s disease there is both loss of dopaminergic retinal cells and development of parkinsonian motor disabilities [8,9]. In monkeys treated with MPTP there is a decrease in the number of retinal dopaminergic cell bodies, dendritic processes and branching complexity; amacrine cells located postsynaptically are also affected [8]. However, the status of intrinsic retinal neurons in human subjects with PD is still unknown.
The protein that accumulates to form the characteristic Lewy bodies of PD, α-synuclein, is normally present in several types of retinal cells in a wide range of vertebrate species including humans [10] and therefore there is a possibility that this may accumulate in PD retinas as it does in the brain. One group has reported that perikaryal α-synuclein cytoplasmic staining increases with age in neurologically normal elderly humans but PD cases were not studied [11]. There is a case report of unusual retinal inclusions in a subject with DLB but these were not immunoreactive for α-synuclein and their composition is still unknown [12]. A single previous study reported no pathological α-synuclein, tau or Aβ deposits in the retinas of 6 PD subjects, 11 AD subjects and 6 age-similar control subjects [13]; we sought to extend the search for retinal synucleinopathy with alternative methodologies, using retinal wholemounts and an immunohistochemical method for phosphorylated α-synuclein, reasoning that these changes might offer greater sensitivity.
2. Materials and methods
2.1. Source of human subjects
This study was conducted as part of the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND), by the Arizona Parkinson Disease Consortium and the Banner Sun Health Research Institute Brain and Body Donation Program (BBDP; www.brainandbodydonationprogram.org). Brain necropsies and neuropathological examinations were performed on elderly subjects who had volunteered for the BBDP [14]. The BBDP has been approved by the BSHRI Institutional Review Board and research conducted on BBDP subjects is carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
2.2. Clinical and neuropathological characterization of human subjects
Subjects are clinically characterized with annual standardized test batteries that include general neurological, cognitive, and movement disorder components [14]. Additionally, private medical records are requisitioned, reviewed and abstracted for each subject. Deceased subjects received standardized neuropathological examinations as described previously [14]. Specific neuropathological diagnostic criteria were used for PD [15], dementia with Lewy bodies (DLB) [16], Alzheimer’s disease (AD) [17] and progressive supranuclear palsy (PSP) [18,19]. Alzheimer’s disease cases with synucleinopathy but not meeting clinicopathological diagnostic criteria for PD or DLB were designated as AD with Lewy bodies (ADLB), versus AD without Lewy bodies (ADNLB).
2.3. Tissue processing
Eyes were fixed in neutral-buffered 10% formalin and retinal wholemounts prepared [20]. To increase antibody penetration, retinal wholemounts were cryoprotected with 15% sucrose in 0.1 M phosphate buffer and subjected to three freeze–thaw cycles by placing and removing from a slab of dry ice [21]. Wedge-shaped pieces of retina were cut with the optic disk at the apex; these were immunohistochemically stained as free-floating preparations with an antibody against α-synuclein phosphorylated at serine 129 [22–25]. Positive and negative control sections were processed with each batch of stained sections. The stained wholemounts were dehydrated in an ethanol series and then cleared in two changes of dibenzyl ether (Sigma–Aldrich, St. Louis, MO).
3. Results
3.1. Human subject characteristics
There were nine subjects with a primary neuropathological diagnosis of PD, three with DLB (two also met neuropathological diagnostic criteria for AD), three with AD (excluding the two cases that also had DLB), of which one was ADLB and two were ADNLB), two with PSP (one also met neuropathological diagnostic criteria for PD) and four normal elderly control subjects. One additional subject had mild cognitive impairment and parkinsonism with non-specific neuropathology. Subject age ranges and means were: PD 63–90, 82.3; DLB 78–82, 80.1; AD 74–87, 82.6; PSP (both subjects were 83); control 86–92, 89.5. For PD subjects, symptom duration ranged from 2 to 13 years with a mean of 9.7 years; final motor scores on the Unified Parkinson’s Disease Rating Scale (UPDRS; except one case with no scores available) ranging from 15 to 73, with a mean score of 42.4.
Nine subjects had a medical history of either cataract (five subjects), glaucoma (five subjects) or macular degeneration (two subjects), with three subjects having more than one eye condition. Four of these nine had a non-PD, non-DLB diagnosis while five had PD or DLB.
3.2. Microscopic observations
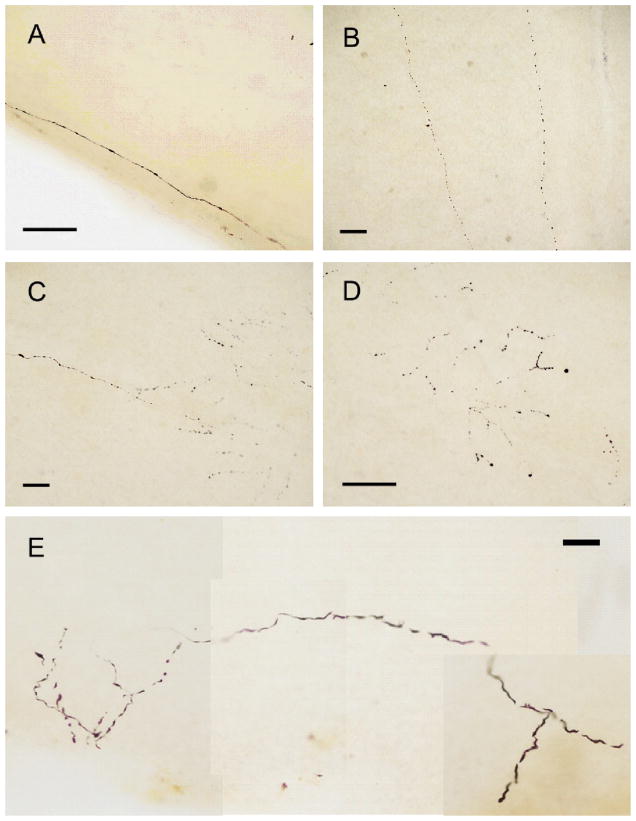
Phosphorylated α-synuclein-immunoreactive (p-syn IR) nerve fibers were present in 7/9 PD subjects and in 1/3 DLB subjects. There were no stained nerve fibers or other specific staining in any of the other subjects. Stained nerve fibers (Fig. 1) were sparsely distributed and superficially located on the inner retinal surface (the surface facing the vitreous). Focusing on the fibers, as well as the top and bottom surfaces of the retinal wholemount, with a 100× oil-immersion objective indicated that they were either right at the inner retinal surface or were located within the inner 25% of the retina, with an average depth equal to 14% of the entire retinal thickness. This is consistent with locations within the nerve fiber layer, the ganglion cell layer and the inner strata of the inner plexiform layer. The fibers were either long and straight (Fig. 1A and B) or branching (Fig. 1C–E); both types often had multiple en-passant varicosities along their length. The straight fibers most often had an orientation that was radial with respect to the optic disk and thus parallel to the radially-oriented dissection margin (Fig. 1A). Neuronal inclusions were not present and neuronal perikarya, glia or other features were not stained.
Fig. 1.
Photomicrographs of p-syn IR nerve fibers, seen in the retinas of 7/9 PD subjects. Stained fibers were either long and straight, without branches (a, b) or had multiple branches (c–e). Both types of fibers often had multiple varicosities. All calibration bars equal 100 μm.
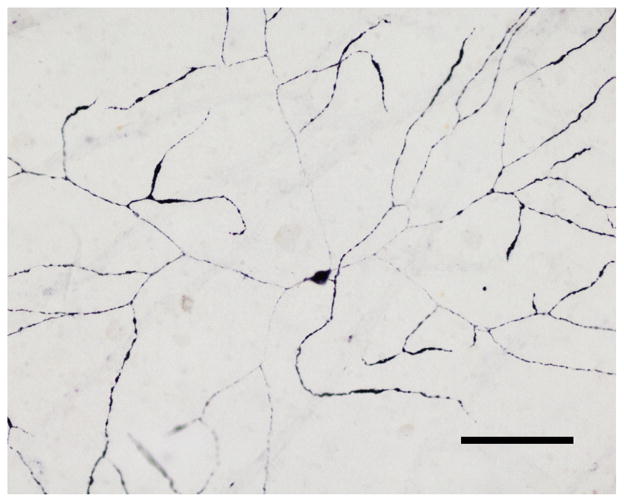
In a single PD subject, a very few p-syn IR neuronal perikarya, complete with extensive, planar, beaded dendritic trees, were strongly stained (Fig. 2). These had large cell bodies (22 × 25 μm diameter) and their dendritic trees were the largest of any ganglion cell types at more than 1 mm dendritic spread (Fig. 2). Surface focusing indicated their cell bodies are likely to be located in the ganglion cell layer and their dendritic trees in the lower inner plexiform layer (IPL). By morphology and location, these cells are very likely a type of retinal ganglion cell.
Fig. 2.
Photomicrograph of a neuronal type found in the retina of a single PD subject. These neurons were located near the inner retinal surface. Their dendritic trees were large, planar and beaded. Their size and morphology are consistent with a type of retinal ganglion cell. Calibration bar = 500 μm.
4. Discussion
As α-synuclein phosphorylated at serine 129 is only immunohistochemically detectable in the nervous systems of human subjects with α-synucleinopathies [7,25–27], its presence in the retina in these seven PD subjects and one DLB subject is consistent with retinal synucleinopathy. This is in contrast to a recent report [13] that did not find pathological α-synuclein, tau or Aβ deposits in the retinas of six PD subjects, 11 AD subjects and six age-similar control subjects. As the p-syn IR nerve fibers detected in the present report were sparsely distributed, it is possible that the much greater sample volume obtained with retinal whole-mounts (versus paraffin-embedded retinal cross-sections in the previous study) allowed greater sensitivity. Other methodological differences were the usage of an antibody against unmodified α-synuclein in the earlier study while the present study employed an antibody against phosphorylated α-synuclein. The presence of phosphorylated α-synuclein is restricted to the brains of subjects with synucleinopathy while unmodified α-synuclein is extremely abundant in normal brains and therefore precautions must be used to ensure differential staining of normal and pathological α-synuclein. Two prior publications have noted the presence of immunohistochemical staining for unmodified α-synuclein within retinal neuronal perikarya [10,13].
From the great length and radial orientation of the straight fibers, and the probable location of fibers within the optic nerve fiber layer, ganglion cell layer and inner plexiform layer, together with the absence of stained retinal neuronal perikarya in all subjects except one, it is possible that these are centrifugal/retinopetal fibers originating from the brain [28]. Such fibers are generally sparsely distributed in the retina, and most arise from either histaminergic neurons in the hypothalamus or dorsal raphe serotonergic neurons, although tyrosine hydroxylase-immunoreactive fibers arising from the hypothalamus have also been reported in the mouse [29]. Neurons in all of these brain locations are selectively vulnerable to the development of synucleinopathy in PD [30]. Alternatively, it is possible that these represent centripetal/retinofugal fibers arising from retinal ganglion cells, although retinal ganglion cell bodies were not stained in 6/7 of these subjects. The superficial inner retinal location and the dendritic morphology of the p-syn IR neurons seen in one PD subject are suggestive of both a G19 retinal ganglion cell, as described by Kolb and co-workers [31] and a melanopsin-positive intrinsically photosensitive retinal ganglion cell (ipRGC), as described by Schmidt and co-workers [32]. Co-localization studies will be needed to conclusively identify the observed fibers and neuronal cell bodies.
There were p-syn IR fibers in the retina of 1/3 subjects with DLB. These were similar to those seen in the PD subjects. As with PD, retinal thinning has been reported with OCT in DLB [33]. To our knowledge, there has been only a single prior report of retinal histology in DLB subjects; cytological inclusions were reported but these were not immunoreactive for α-synuclein and did not resemble Lewy bodies ultrastructurally [12].
The numbers of subjects included in this study is small and therefore while we have shown that pathological α-synuclein deposits are present in some Lewy body disease subjects, it is not yet possible to conclude that these are restricted to such subjects. Additionally, the association is not universal as only one of three DLB subjects, and seven of nine PD subjects, had retinal p-syn IR. Previous investigations [26] have noted that synucleinopathy does not extend quite as widely outside of the brain in DLB as in PD. It may be that extension of synucleinopathy to the retina is not an early event, as for both of the p-syn IR-negative PD subjects, the symptom durations were less than the median for the group (3 and 7 years versus 9.7 years). Only one p-syn IR-negative PD subject had a UPDRS motor score available, and, at 15, it was well below the mean PD group score of 42. Again, however, with the small subject numbers it is difficult to conclude much with certainty from this.
Histaminergic and serotonergic retinal inputs modulate the maintained activity and light responses of retinal ganglion cells [28] while ipRGCs mediate aspects of circadian rhythm, pupillary light reflex and sleep, as well as contributing to image formation [32]. At least some of the reported visual function impairments in PD subjects might be due to α-synucleinopathy. It is possible that the psyn-IR fibers might allow an ophthalmological diagnostic test for PD should appropriate technology be developed for this.
HIGHLIGHTS.
Structural and functional retinal changes are present in Parkinson’s disease (PD).
Phosphorylated α-synuclein (psyn) is a specific biomarker of PD.
Psyn-immunoreactive nerve fibers were present in the retina in 7/9 subjects with PD.
Psyn-immunoreactive neuronal perikarya were present in 1 PD subject.
This pathology may contribute to structural and functional retinal changes in PD.
Acknowledgments
We would like to acknowledge the assistance of other members of the Arizona Parkinson’s Disease Consortium and Brain and Body Donation Program, including John Caviness, Erika Driver-Dunckley, Sandra Jacobson, Christine Belden and Kathryn Davis. We thank Helga Kolb of the University of Utah for generously giving her advice and encouragement. This project was funded by the Michael J. Fox Foundation for Parkinson’s Research. Funding for the recruitment and clinical and neuropathological characterization of human subjects, as well as other operating costs of the Brain and Body Donation Program, have been partially supplied by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research. These sponsors had no role in the study design, collection, analysis and interpretation of data, the writing of the report or the decision to submit the article for publication.
References
- 1.Lin T, Adler CH, Hentz JG, Adler J, Balcer L, Galetta S, Devick S, Cronin R Arizona Parkinson’s Disease Consortium. Abnormal variable contrast acuity in Parkinson’s disease. Neurology. 2013;80:P04.143. [Google Scholar]
- 2.Muller T, Kuhn W, Buttner T, Przuntek H. Distorted colour discrimination in Parkinson’s disease is related to severity of the disease. Acta Neurol Scand. 1997;96:293–296. doi: 10.1111/j.1600-0404.1997.tb00286.x. [DOI] [PubMed] [Google Scholar]
- 3.Buttner T, Kuhn W, Klotz P, Steinberg R, Voss L, Bulgaru D, Przuntek H. Disturbance of colour perception in Parkinson’s disease. J Neural Transm Park Dis Dement Sect. 1993;6:11–15. doi: 10.1007/BF02252618. [DOI] [PubMed] [Google Scholar]
- 4.Archibald NK, Clarke MP, Mosimann UP, Burn DJ. Visual symptoms in Parkinson’s disease and Parkinson’s disease dementia. Mov Disord. 2011;26:2387–2395. doi: 10.1002/mds.23891. [DOI] [PubMed] [Google Scholar]
- 5.Bodis-Wollner I, Miri S, Glazman S. Venturing into the noman’s land of the retina in Parkinson’s disease. Mov Disord. 2014;29:15–22. doi: 10.1002/mds.25741. [DOI] [PubMed] [Google Scholar]
- 6.Simao LM. The contribution of optical coherence tomography in neurodegenerative diseases. Curr Opin Ophthalmol. 2013;24:521–527. doi: 10.1097/ICU.0000000000000000. [DOI] [PubMed] [Google Scholar]
- 7.Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, Eschbacher J, White CL, III, Akiyama H, Caviness J, Shill HA, Connor DJ, Sabbagh MN, Walker DG. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–634. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuenca N, Herrero MT, Angulo A, de JE, Martinez-Navarrete GC, Lopez S, Barcia C, Martin-Nieto J. Morphological impairments in retinal neurons of the scotopic visual pathway in a monkey model of Parkinson’s disease. J Comp Neurol. 2005;493:261–273. doi: 10.1002/cne.20761. [DOI] [PubMed] [Google Scholar]
- 9.Esteve-Rudd J, Fernandez-Sanchez L, Lax P, de JE, Martin-Nieto J, Cuenca N. Rotenone induces degeneration of photoreceptors and impairs the dopaminergic system in the rat retina. Neurobiol Dis. 2011;44:102–115. doi: 10.1016/j.nbd.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Navarrete GC, Martin-Nieto J, Esteve-Rudd J, Angulo A, Cuenca N. Alpha synuclein gene expression profile in the retina of vertebrates. Mol Vis. 2007;13:949–961. [PMC free article] [PubMed] [Google Scholar]
- 11.Leger F, Fernagut PO, Canron MH, Leoni S, Vital C, Tison F, Bezard E, Vital A. Protein aggregation in the aging retina. J Neuropathol Exp Neurol. 2011;70:63–68. doi: 10.1097/NEN.0b013e31820376cc. [DOI] [PubMed] [Google Scholar]
- 12.Maurage CA, Ruchoux MM, de VR, Surguchov A, Destee A. Retinal involvement in dementia with Lewy bodies: a clue to hallucinations? Ann Neurol. 2003;54:542–547. doi: 10.1002/ana.10730. [DOI] [PubMed] [Google Scholar]
- 13.Ho CY, Troncoso JC, Knox D, Stark W, Eberhart CG. Beta-amyloid, phospho-tau and alpha-synuclein deposits similar to those in the brain are not identified in the eyes of Alzheimer’s and Parkinson’s disease patients. Brain Pathol. 2014;24:25–32. doi: 10.1111/bpa.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beach TG, Sue LI, Walker DG, Roher AE, Lue L, Vedders L, Connor DJ, Sabbagh MN, Rogers J. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2008;9:229–245. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 16.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del ST, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 17.Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 18.Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I. Preliminary NINDS neuropathologic criteria for Steele–Richardson–Olszewski syndrome (progressive supranuclear palsy) Neurology. 1994;44:2015–2019. doi: 10.1212/wnl.44.11.2015. [DOI] [PubMed] [Google Scholar]
- 19.Dickson DW, Rademakers R, Hutton ML. Progressive supranuclear palsy: pathology and genetics. Brain Pathol. 2007;17:74–82. doi: 10.1111/j.1750-3639.2007.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ullmann JF, Moore BA, Temple SE, Fernandez-Juricic E, Collin SP. The retinal wholemount technique: a window to understanding the brain and behaviour. Brain Behav Evol. 2012;79:26–44. doi: 10.1159/000332802. [DOI] [PubMed] [Google Scholar]
- 21.Wassle H, Grunert U, Rohrenbeck J. Immunocytochemical staining of AII-amacrine cells in the rat retina with antibodies against parvalbumin. J Comp Neurol. 1993;332:407–420. doi: 10.1002/cne.903320403. [DOI] [PubMed] [Google Scholar]
- 22.Obi K, Akiyama H, Kondo H, Shimomura Y, Hasegawa M, Iwatsubo T, Mizuno Y, Mochizuki H. Relationship of phosphorylated alpha-synuclein and tau accumulation to Abeta deposition in the cerebral cortex of dementia with Lewy bodies. Exp Neurol. 2008;210:409–420. doi: 10.1016/j.expneurol.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Beach TG, White CL, Hamilton RL, Duda JE, Iwatsubo T, Dickson DW, Roncaroli F, Buttini M, Hladik CL, Sue LI, Noorigian JV, Adler CH. Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol. 2008;116:277–288. doi: 10.1007/s00401-008-0409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker DG, Lue LF, Adler CH, Shill HA, Caviness JN, Sabbagh MN, Akiyama H, Serrano GE, Sue LI, Beach TG. Changes in properties of serine 129 phosphorylated alpha-synuclein with progression of Lewy-type histopathology in human brains. Exp Neurol. 2013;240:190–204. doi: 10.1016/j.expneurol.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. Alpha-synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 26.Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White CL, III, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, Walker DG. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beach TG, Adler CH, Dugger BN, Serrano G, Hidalgo J, Henry-Watson J, Shill HA, Sue LI, Sabbagh MN, Akiyama H. Submandibular gland biopsy for the diagnosis of Parkinson disease. J Neuropathol Exp Neurol. 2013;72:130–136. doi: 10.1097/NEN.0b013e3182805c72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gastinger MJ, Tian N, Horvath T, Marshak DW. Retinopetal axons in mammals: emphasis on histamine and serotonin. Curr Eye Res. 2006;31:655–667. doi: 10.1080/02713680600776119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon A, Martin-Martinelli E, Savy C, Verney C, Raisman-Vozari R, Nguyen-Legros J. Confirmation of the retinopetal/centrifugal nature of the tyrosine hydroxylase-immunoreactive fibers of the retina and optic nerve in the weaver mouse. Brain Res Dev Brain Res. 2001;127:87–93. doi: 10.1016/s0165-3806(01)00103-1. [DOI] [PubMed] [Google Scholar]
- 30.Braak H, Braak E. Pathoanatomy of Parkinson’s disease. J Neurol. 2000;247(Suppl 2):II3–II10. doi: 10.1007/PL00007758. [DOI] [PubMed] [Google Scholar]
- 31.Kolb H, Linberg KA, Fisher SK. Neurons of the human retina: a Golgi study. J Comp Neurol. 1992;318:147–187. doi: 10.1002/cne.903180204. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt TM, Do MT, Dacey D, Lucas R, Hattar S, Matynia A. Melanopsin-positive intrinsically photosensitive retinal ganglion cells: from form to function. J Neurosci. 2011;31:16094–16101. doi: 10.1523/JNEUROSCI.4132-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno-Ramos T, Ito-Leon J, Villarejo A, Bermejo-Pareja F. Retinal nerve fiber layer thinning in dementia associated with Parkinson’s disease, dementia with Lewy bodies, and Alzheimer’s disease. J Alzheimers Dis. 2013;34:659–664. doi: 10.3233/JAD-121975. [DOI] [PubMed] [Google Scholar]