Abstract
The European Myeloma Network provides recommendations for the management of the most common complications of multiple myeloma. Whole body low-dose computed tomography is more sensitive than conventional radiography in depicting osteolytic disease and thus we recommend it as the novel standard for the detection of lytic lesions in myeloma (grade 1A). Myeloma patients with adequate renal function and bone disease at diagnosis should be treated with zoledronic acid or pamidronate (grade 1A). Symptomatic patients without lytic lesions on conventional radiography can be treated with zoledronic acid (grade 1B), but its advantage is not clear for patients with no bone involvement on computed tomography or magnetic resonance imaging. In asymptomatic myeloma, bisphosphonates are not recommended (grade 1A). Zoledronic acid should be given continuously, but it is not clear if patients who achieve at least a very good partial response benefit from its continuous use (grade 1B). Treatment with erythropoietic-stimulating agents may be initiated in patients with persistent symptomatic anemia (hemoglobin <10g/dL) in whom other causes of anemia have been excluded (grade 1B). Erythropoietic agents should be stopped after 6–8 weeks if no adequate hemoglobin response is achieved. For renal impairment, bortezomib-based regimens are the current standard of care (grade 1A). For the management of treatment-induced peripheral neuropathy, drug modification is needed (grade 1C). Vaccination against influenza is recommended; vaccination against streptococcus pneumonia and hemophilus influenza is appropriate, but efficacy is not guaranteed due to suboptimal immune response (grade 1C). Prophylactic aciclovir (or valacyclovir) is recommended for patients receiving proteasome inhibitors, autologous or allogeneic transplantation (grade 1A).
Introduction
Multiple myeloma (MM) is characterized by bone destruction, anemia, renal and immunological impairment. These complications may lead to severe impairment of the quality of life of myeloma patients and may deteriorate their life expectancy. Therefore, prophylaxis and supportive treatment for osteolytic disease, pain, anemia, renal insufficiency, infections, pain, thromboembolic events and peripheral neuropathy are essential for the management of myeloma patients. The aim of this paper of the European Myeloma Network (EMN) is to provide useful guidelines for the management of the most common myeloma-related complications.
Methods
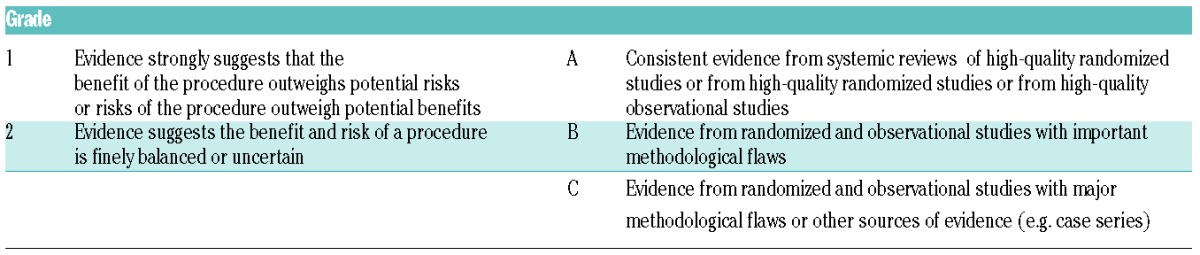
An interdisciplinary panel of myeloma experts on behalf of the EMN reviewed all published randomized clinical studies, guidelines, meta-analyses, systematic reviews, observational studies and case reports on the management of complications in MM. The research was performed in PubMed and ISI until the 28th August 2014. The Grading of Recommendations Assessment Development and Evaluation (GRADE) system was used for the grading of recommendations (Table 1). In cases of lack of sufficient data, an expert consensus was used to develop recommendations. The paper was circulated among the panel members; initial discussion took place at the 6th EMN Trialist meeting (Baveno, Italy, 15th–16th September 2013) and the recommendations were approved by the panel members and the participants at the 7th EMN Trialist meeting (Baveno, 14th–15th September 2014). Subsequently, the manuscript underwent two-round revisions between the panel members.
Table 1.
Grade recommendations for grading levels of evidence.
Bone disease
Osteolytic bone disease is one of the most prominent features of myeloma, and is present in up to 80% of patients at diagnosis.1 Bone destruction leads to skeletal-related events (SREs), i.e. vertebral and other pathological fractures, a need for radiotherapy or surgery to the bone and/or spinal cord compression. It is mainly due to an increased osteoclastic activity which is accompanied by low osteoblastic function.1 Bisphosphonates, radiotherapy, balloon kyphoplasty and surgery are the main therapies used for the management of bone disease in MM.
Imaging for the diagnosis and follow up of myeloma patients
Skeletal survey based on conventional radiography (WBXR) is currently considered to be the standard technique for the detection of lytic lesions in MM patients and is recommended for the detection of bone disease in the CRAB criteria that are used for the definition of myeloma defining events.2,3 However, novel techniques can detect more lytic lesions compared to conventional radiography. Whole-body, multi-detector, low-dose computed tomography (WBLD-CT) is more sensitive for the detection of lytic lesions in myeloma compared to conventional radiography, it is very easy to perform (the examination is performed in 2 min or less), it has a more accurate evaluation of areas with instability or at risk of fracture, and is superior regarding the planning for radiotherapy or surgical interventions.4,5 Similarly, positron emission tomography in combination with CT (PET/CT) is superior to conventional radiography in the detection of lytic disease, while whole-body magnetic resonance imaging (MRI) accurately depicts the marrow involvement in MM patients.6,7 We stress that MRI depicts bone marrow involvement, while CT and skeletal survey reveals lytic lesions. However, there are some issues that need to be clarified when using such sensitive techniques. For example, the issue of the importance of the detection of 2 or 3 small lesions of 3–4 mm of diameter by WBLD-CT or by MRI or PET/CT in a patient with no CRAB criteria has not yet been solved (i.e. whether this patient will develop symptomatic disease earlier than those without such lesions). Furthermore, we do not know the prognostic value of the WBCT, i.e. what the difference is between an MM patient with 4 lytic lesions detected by conventional radiography and another patient with 14 lytic lesions detected by WBLD-CT. Although these questions have not been conclusively answered, data so far support the substitution of conventional radiography by WBLD-CT for the detection of lytic disease in MM. Positive lesions in WBLD-CT are considered those with a diameter of 5 mm or more.
Regarding the definition of myeloma defining events, there are important studies which suggest that asymptomatic patients with more than 1 focal lesions on MRI have a higher risk (more than 70% within 2 years) for progression to symptomatic myeloma.8,9 These patients need to be treated as having symptomatic disease.10 Furthermore, MRI correlates with survival in myeloma patients.11 PET/CT findings have also been correlated with response to therapy and survival.12,13 Prospective comparisons between MRI and PET/CT in patients with asymptomatic disease have not yet been published. In symptomatic patients there is evidence that MRI achieves better results than PET/CT in the staging and disease recurrence, while PET/CT has shown faster changes in imaging findings than MRI in patients who respond to therapy.14 However, in the post-treatment setting, MRI may often be false positive because of persistent non-viable lesions and thus PET/CT might be more suitable for the determination of remission status.15
Recommendation: WBLD-CT is the novel standard procedure for the diagnosis of lytic disease in patients with MM (grade 1A). Conventional radiography can also be used if WBLD-CT is not available. In asymptomatic patients with no lytic disease in WBLD-CT, whole body MRI (or spine and pelvic MRI if WB-MRI is not available) has to be performed and in the presence of more than 1 focal lesion the patients are characterized as having symptomatic disease that needs therapy (grade 1A). PET/CT may be useful for the better definition of complete or stringent complete response (CR or sCR) and for the progression of the disease (grade 2B). Figure 1 presents the imaging algorithm which is proposed by the EMN for use in myeloma-related bone disease.
Figure 1.
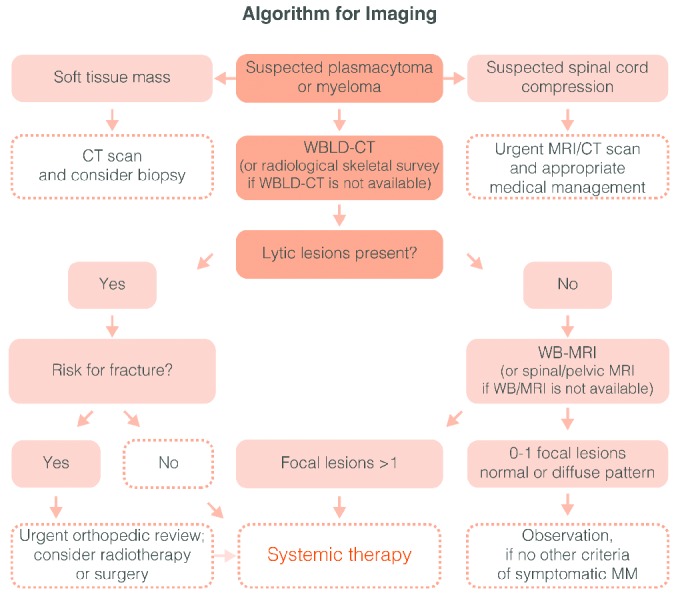
Algorithm for imaging in multiple myeloma (MM). In the case of spinal cord compression an urgent MRI or CT is obligatory in order to assess the better management (radiotherapy or surgery in the cases on the presence of bone fracture segments into the spinal canal). In the suspicion of a plasmacytoma a CT of the area and a needle biopsy is needed. In the case of myeloma the WBLD-CT (or the standard conventional radiographic evaluation of the skeleton if a WBLD-CT is not available) may reveal or not lytic lesions. If lytic lesions are present then the patient fulfils the criteria for symptomatic disease and needs systematic therapy. If not, then a WB-MRI (or a spinal and pelvic MRI if a WB-MRI is not available) has to be performed. In the presence of more than one focal lesion (more than 5 mm of diameter) in MRI the treating physician should treat the patient as having symptomatic myeloma. To date data do not justify the initiation of treatment in asymptomatic patients with diffuse MRI pattern of marrow involvement.
Bisphosphonates
Based on phase III studies, both pamidronate and zoledronic acid (ZA) have been found to reduce SREs compared to placebo.16–18 There are only three randomized studies comparing the effect of two different bisphosphonates (BP) or two different dosages of the same BP. In the first study, ZA was as effective as pamidronate in reducing SREs in the era of conventional chemotherapy.18,19 In the second, two doses of intravenous pamidronate (30 mg vs. 90 mg, monthly) had comparable results regarding time to SRE and SRE-free survival time.20 The major flaw of this study was that it was powered to show differences in quality of life and not in SREs.20 Finally, the third study, which compared intravenous ZA with oral clodronate, found that ZA reduced the SRE risk compared to clodronate in all patients, irrespective of the presence of lytic lesions at diagnosis, but furthermore, improved overall survival (OS) by ten months in MM patients with lytic lesions at diagnosis.21,22 These effects continued in patients who received ZA for more than two years.23 However, there was no sub-analysis according to the response status of the patients, and thus it is not clear if the continuous use of ZA produces similar results in patients who have achieved a CR, sCR, very good partial response (VGPR) or PR. A recent meta-analysis was not able to confirm superiority of ZA over pamidronate, but remarkably revealed a survival advantage of ZA versus placebo.24 This analysis also showed that in order to prevent one SRE, we need to treat 6–15 MM patients with BP.
Recommendations: all MM patients with adequate renal function (creatinine clearance >30 mL/min) and osteolytic disease at diagnosis should be treated with ZA (4 mg, over an at least 15-min infusion, every 3–4 weeks) or pamidronate (90 mg, in a 2–4-h infusion, every 3–4 weeks), intravenously, in addition to specific anti-myeloma therapy (grade 1A). Symptomatic patients, without bone disease assessed by conventional radiography, can be treated with ZA (grade 1B). The advantage is not clear for patients without bone involvement on MRI or PET/CT. In asymptomatic MM, BPs are not recommended (grade 1A); in cases of osteoporosis or vertebral fractures that are not due to myeloma, bisphosphonates should be given in asymptomatic patients with doses as given for osteoporosis (i.e. 5 mg ZA per year). ZA should be given continuously (grade 1B). However, it is currently unknown whether patients who achieve VGPR or better have benefits from the continuous use of ZA. Regarding pamidronate, there are no data to support its continuous use; thus it should be given for two years and then at the physician’s discretion (grade 2C).
Side-effects of bisphosphonates and their management
Side-effects of intravenous BPs include acute phase reactions, inflammatory reactions at the injection site, hypocalcemia, hypophosphatemia, renal impairment (RI) and osteonecrosis of the jaw (ONJ).25–27 For the prevention of hypocalcemia, all patients under BPs should receive calcium and vitamin D3 supplementation (600 mg calcium per day and 400 IU vitamin D3 per day); interestingly, approximately 60% of myeloma patients are vitamin D-deficient or -insufficient.25 The treating physicians are encouraged to perform vitamin D measurements at least once a year and manage their patients accordingly.
RI due to acute tubular damage and deterioration of renal function can be observed with both pamidronate and ZA, but the true incidence of this adverse event remains unknown, as RI is also a common complication of MM.21,25,26 Thus patients with moderate RI need dose reductions of ZA, according to the summary of product characteristics of the drug.25 Regarding pamidronate, its elimination is slower when the CrCl is below 30 mL/min.26
ONJ is an uncommon but sometimes severe complication of BP. Retrospective studies suggest that ONJ is observed more often with ZA, after dental procedures, and is associated with the prolonged administration of the BP.27 It seems that the use of preventive dental measures leads to the reduction of ONJ incidence.28 There are conflicting recommendations regarding precautions before dental extraction in patients who are treated with BP. The most recent American Dental Association (ADA) recommendations do not support the discontinuation of BP in these cases in the absence of any convincing data and because BP remain in the bones for years.29 However the International Myeloma Working Group (IMWG) guidelines suggest the temporary discontinuation of BP for 90 days before and after invasive dental procedures.25
Recommendations: for the prevention of hypocalcemia, calcium and vitamin D3 supplementation should be given in all patients under intravenous BP (grade 1A). The treating physicians are encouraged to perform vitamin D measurements at least once a year and manage their patients accordingly. Renal function should be closely monitored by measuring CrCl, serum electrolytes and urinary albumin in all patients under BP therapy; CrCl should be evaluated before the administration of each intravenous infusion (grade 1A). Patients with CrCl 30–60 mL/min should receive reduced doses of ZA with no change to infusion time (grade 1A), while pamidronate should be given via 4-h infusion (grade 1C). Pamidronate and ZA should not be given in patients with CrCl less than 30 mL/min (grade 1A); alternatively clodronate can be given in patients with a CrCl more than 12 mL/min (grade 2C). Treatment with BP should be discontinued if a patient experiences deterioration of renal function until CrCl returns to within 10% of base-line values (grade 1B). Patients on chronic dialysis without possibility of renal failure reversal should also receive monthly BPs (grade 2C); treating physicians should closely monitor these patients due to high risk for hypocalcemia. For all other patients on dialysis, BPs should be avoided until their independence from dialysis and the reversal of RI to CrCl more than 30 mL/min (grade 2C). Before BP administration, patients should have a thorough dental examination and all major dental problems (i.e. dental extractions or other traumatic dental procedures) should be resolved (grade 2C). In cases of ONJ, BP should be discontinued and can later be re-administered if ONJ has healed, at the physician’s discretion (grade 2C).
Denosumab: denosumab has not yet been licensed for myeloma patients. A large phase III study comparing denosumab with ZA is ongoing. Denosumab can currently be given in myeloma patients only in the rare cases of resistant hypercalcemia to BPs.
Radiotherapy: radiotherapy is mainly used in the cases of solitary plasmacytoma, symptomatic spinal cord compression, extremely painful lytic lesions and for the prevention of pathological fractures. For painful osteolytic lesions, a dose of 3000 cGy in 10–15 fractions is usually adequate. Radiotherapy may cause delays in applying systemic anti-myeloma therapies with radiosensitizing drugs, such as anthracyclinse and proteasome inhibitors.
Balloon kyphoplasty and vertebroplasty: these techniques are mainly used for the management of painful vertebral compression fractures, where almost 80% of patients with pain, non-responsive to pain killers, experience pain relief.30 All recent data, including a phase III study and a large meta-analysis, suggest that balloon kyphoplasty is the treatment of choice for the reduction of pain due to cancer-related vertebral fractures and is associated with reduced rates of cement leakage30,31 (grade 1A).
Surgery: the administration of very effective novel anti-myeloma regimens has reduced the need for surgery during the last decade. Currently, surgery should be used in the following cases: i) to fix pathological fractures of the long bones; ii) to prevent and restore axial skeleton in cases of unstable spinal fractures; and iii) for spinal cord compression with bone fragments within the spinal route (grade 2C).
Anemia
Anemia, which is usually normochromic and normocytic, is another common complication of MM. It is present in approximatley 75% of patients at diagnosis32,33 and in almost all patients with uncontrolled disease. Several factors contribute to the development of anemia in MM patients: the BM infiltration by the myeloma itself leads to reduced numbers of erythroid precursors, erythropoietin deficiency (in patients with RI), decreased responsiveness of the pro-erythroblasts and CFU-E cells to erythropoietin, impaired iron utilization due to increased production of hepcidin because of chronic inflammation, and paraprotein-induced increase of the plasma volume.33 However, the major cause of anemia in myeloma is the induction of apoptosis of erythroblast by myeloma cells.34 Furthermore, anti-myeloma therapy and radiotherapy can either cause anemia or exacerbate pre-existing anemia.35
Red blood cell transfusions are helpful for patients who need rapid improvement of their anemic condition. Moreover, several prospective studies have shown that erythropoiesis-stimulating agents (ESAs), such as erythropoietin (Epo)-α and β as well as darbepoetin are able to increase hemoglobin (Hb) levels by 2 g/dL or more in 60% to 75% of myeloma patients with symptomatic anemia. ESAs mainly reduce transfusion requirements and improve quality of life.36–40 Predictors of response to ESAs include the ratio of observed to expected Hb (<0.9) and the preserved BM function, reflected by the platelet counts (>150×109/L).33,38,41 A systematic review of the use of ESAs in more than 20,000 cancer patients confirmed that their use reduced the relative risk of transfusions due to increase of erythroid responses, but there was evidence that ESAs increased mortality during ESA administration and thus decreased OS.42 Although anemia is common in MM patients, no clear consensus exists as to the use and impact of ESAs on outcome in MM and randomized studies in MM patients are still limited. However, one randomized study (VISTA sub-analysis) showed no evidence of inferior outcome after ESA treatment, albeit patient numbers were very limited and, therefore, statistically under-powered.43 The most recent guidelines from the American Society of Hematology (ASH) and American Society of Clinical Oncology (ASCO) recommend the administration of ESAs at the lowest possible dose to avoid transfusions. In case of iron deficiency, which is indicated by low transferrin saturation (<20%) and/or high numbers of hypochromic red cells (>5%), the iron should be given intravenously.44 Important side-effects of ESAs include thromboembolic complications, hypertension and possibly increased mortality.42,45 Here, it is important to stress that nowadays, with very effective combination therapies that rapidly control the disease, the systematic need of ESAs in myeloma is a subject of debate.
Recommendations: treatment with ESAs may be initiated in patients with persistent symptomatic anemia (usually Hb levels <10 g/dL) in whom other causes of anemia (i.e. iron or B12 deficiency, hemolysis, etc.) have been excluded (grade 1B). The standard dose of Epo-α is 40,000 U/week, of Epo-β 30,000 U/week and of darbepoetin 150 μg/week or 500 μg every three weeks. Hb levels should not increase more than 12 g/dL. ESAs should be stopped after 6–8 weeks if adequate Hb response is not achieved. True or functional iron deficiency during treatment with an ESA should be treated with intravenous iron (grade 1A).
Renal Impairment
Incidence and assessment of renal impairment
Mild renal impairment (RI) (estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2), if carefully monitored, can be observed in at least 25%–50% of patients during the course of their disease.46 The pathophysiology of RI in MM is complex and associated with various underlying processes. The principal renal mechanism is tubulointerstitial lesions, such as cast nephropathy, a direct consequence of the high serum concentration of immunoglobulin free light chains (SFLCs; Figure 2), characterized by tubular atrophy and tubular-interstitial fibrosis and the most frequent (approx. 90%) form of renal damage.47–51 FLCs can also cause functional impairment, resulting in Fanconi’s syndrome, characterized by failure of the reabsortive capacity of the proximal renal tubules resulting in glucosuria, aminoaciduria, hypophosphatemia and hypouricemia.52 Moreover, deposition of monoclonal light chains can occur in several organs (kidney, heart, liver, small intestine), leading to the development of amyloid light chain (AL) amyloidosis or light chain deposition disease (LCDD). A variety of other nephrotoxic processes may also contribute to renal damage, including dehydration, hypercalcemia, infections, amyloidosis, and concomitant exposure to nephrotoxic medications, such as non-steroidal anti-inflammatory drugs.46,53
Figure 2.
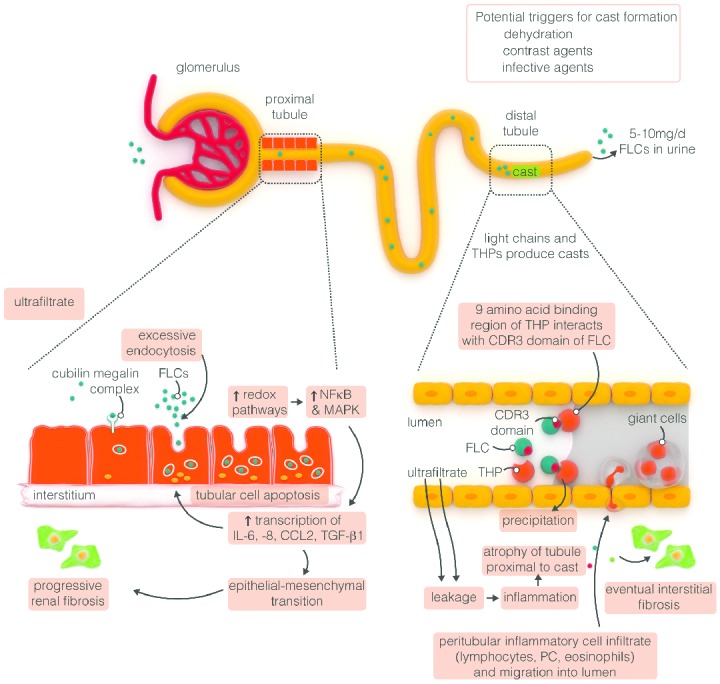
Mechanisms of FLC-induced acute kidney injury. Serum FLCs are primarily cleared by the kidneys through glomerular filtration, endocytosed by the proximal tubule cells and degraded within lysosomes.50 In MM, the Ig light chains are produced in excess and absorption mechanisms in the proximal tubule are overwhelmed. Thus, the excessive light chains reach the distal tubules, where they form tubular casts with Tamm-Horsfall protein (THP), subsequently leading to tubular obstruction.51 Additionally, excess FLCs can cause direct injury to proximal tubular cells through the induction of pro-inflammatory cytokine production and other pathways leading to tubular cell death.52 The very high concentrations of FLCs present in the ultrafiltrate of patients with MM can result in direct injury to PTCs. Activation of redox pathways occurs, with increased expression of NFκB and MAPK, which in turn leads to the transcription of both inflammatory and profibrotic cytokine. In the distal tubules, FLCs can bind to a specific binding domain on THPs and co-precipitate to form casts. These casts result in tubular atrophy proximal to the cast and lead to progressive interstitial inflammation and fibrosis. CCL2: hemokine (C-C motif) ligand 2; CDR: complementarity determining region; FLC: free light chain; IL: interleukin; MAPK: mitogen-activated protein kinase; NFκB: nuclear factor κB; PTC: proximal tubule kidneys; TGF-β1: transforming growth factor β1; THP: Tamm-Horsfall protein (adapted to Hutchison et al.49).
According to Durie & Salmon staging system, serum creatinine-levels of 2 mg/dL or more (sCr) define RI and represent one of the ‘CRAB’ diagnostic criteria for symptomatic MM.54 However, serum creatinine is not a suitable factor for the reflection of GFR.54–55 Therefore predictive of GFR equations based on serum creatinine [Cockcroft-Gault equation and the Modification of Diet in renal Disease (MDRD)] are often used to define the degree of RI.46,55,56 The IMWG has recommended the use of the MDRD equation for the estimation of GFR in MM patients,56 and the Kidney Disease Improving Global Outcomes (KDIGO) classification for the classification of RI in MM patients.57 Recently, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formulas, with or without cystatin C, has been used for the accurate detection of manifest RI,58,59 and especially the CKD-EPI-cystatin C equation seems to provide an independent prognostic value, which needs to be further elucidated and formally compared to currently used equations both in newly diagnosed and relapsed patients.58 The improved prognostic ability and more sensible detection of RI by the CDK-EPI compared to the MDRD estimation therefore enlarges the arsenal of eGFR formulas and should lead to a broader use of CKD-EPI formulas for the estimation of GFR in patients with myeloma.59,60
Supportive care and mechanical approaches
MM patients with RI at presentation should be considered a medical emergency. Management of patients with RI include adequate hydration, urine alkalinization, and treatment off hypercalcemia. High fluid intake alone will at best reduce the urine concentration of the pathogenic light chains and should be combined with prompt anti-myeloma therapy, including agents without nephrotoxic potency. Therapeutic plasma exchange (TPE) has been suggested to impact the outcome of the renal failure by promoting rapid reduction in the levels of free light chain,61 but its role remains controversial.62 The largest prospective randomized trial performed so far found no impact of TPE on the composite end point of death, dialysis dependence, and GFR less than 30 mL/min/1.73 m2 in MM patients with RI.62 A small series of 14 patients with confirmed or presumed cast nephropathy treated with bortezomib, dexamethasone, and TPE reported normalization of serum creatinine in 43% of patients.61 A definitive answer on the role of TPE in MM patients with RI will require larger prospective trials with, for example, proteasome-inhibitor-based uniform pharmacological therapy. The removal of FLCs with dialysis is another method. The issue of whether an extended duration of dialysis with high cut off dialyzers is more effective than plasma exchange at removing FLCs or reversing renal failure is not established but should be solved in the near future and this method can also be combined with bortezomib or other anti-myeloma therapies.63–65
Antimyeloma therapy in multiple myeloma patients with renal impairment
High-dose chemotherapy with autologous stem cell transplantation (ASCT) may be performed in patients with severe RI or under dialysis using melphalan at a reduced dose (140 mg/m2) albeit immediately reconstitution of RI is essential and ASCT may not always be readily applicable; therefore, immediate initiation of effective chemotherapies is recommended.56 Therapy with bortezomib-based regimens plus high-dose dexamethasone [either alone or with the addition of a third agent such as thalidomide (VTD), doxorubicin (PAD) or cyclophosphamide (VCD)] should be used as first choice.56 The prospective, randomized phase III (HOVON-65/GMMG-HD4) trial, including 81 of 827 patients with RI (serum-creatinine ≥2 mg/dL) and investigating PAD versus VAD followed by ASCT and maintenance with thalidomide or bortezomib, showed a substantial improved OS at three years for the patients with serum-creatinine of 2 mg/dL or more of 74% with PAD-ASCT-bortezomib versus 34% with VAD-ASCT-thalidomide (P<0.001).66 In addition, it was also worthy of note that both OS and progression-free (PFS) survival were similar with base-line serum-creatinine of 2 mg/dL or more or less than 2 mg/dL in the PAD-ASCT-bortezomib arm. These results indicated that bortezomib-containing treatment before and after ASCT may overcome the negative prognostic impact of RI.66 In elderly or comorbid patients with RI, the combination with bortezomib with melphalan and prednisone (VMP) may be preferred.67 The second proteasome inhibitor which has been licensed for MM, carfilzomib, has also shown encouraging results in a small phase II study with 38 relapsed/refractory patients with RI (8 on chronic dialysis); responses were similar among patients with different severity of renal dysfunction.68 More studies are needed to reveal the role of carfilzomib in RI.
Immunomodulatory drugs (IMiDs) can also been administered in myeloma patients with RI. Thalidomide and lenalidomide are very effective especially in patients with mild to moderate RI; lenalidomide should be used with the recommended reduced doses based on renal function.56,69 Finally, responses to the combination of pomalidomide and low-dose dexamethasone were similar between patients with relapsed/refractory myeloma, irrespective of renal function.70 Bendamustine in combination with thalidomide or bortezomib and prednisone is also feasible and safe, even in patients with end-stage renal disease.71,72
Analyses of newly diagnosed MM patients with at least moderate renal dysfunction showed that bortezomib-based regimens were the most effective in the reversibility of renal function. Time to major renal response (renalCR or renalPR) for thalidomide, bortezomib and lenalidomide-based regimes was 2, 1.12 and 1.25 months, respectively.73
Recommendations: every myeloma patient with RI needs a thorough workup for the determination of the cause of RI (Figure 3). For the evaluation of RI, the MDRD formula is recommended in patients with stabilized serum creatinine. Patients with renal failure should be classified according to the KDIGO classification (grade 1B). Novel formulas, such as the CKD-EPI, with or without cystatin C, should be further assessed in clinical trials and in large patient cohorts to evaluate their utility and prognostic impact. Available data support the safety and efficacy of bortezomib-based therapies in MM patients with RI and thus bortezomib combined with dexamethasone (with or without thalidomide, doxorubicin or cyclophosphamide) is the recommended treatment (grade 1A). Lenalidomide is a feasible and effective therapy option with mild to moderate RI and is recommended with dose adjustment according to renal function (grade 1B).
Figure 3.
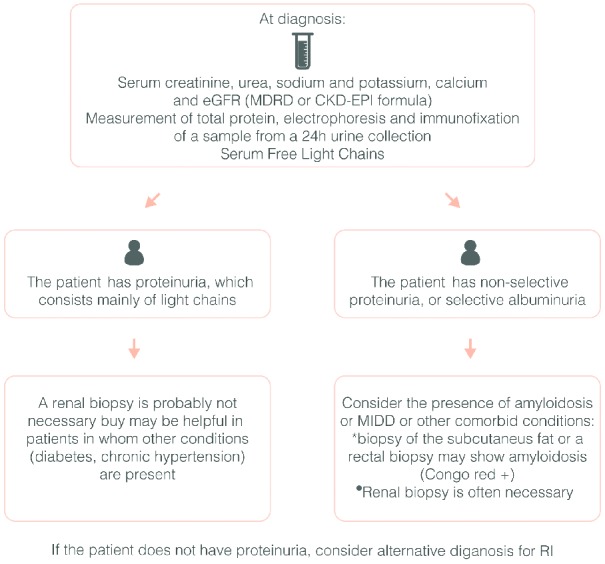
Algorithm for the initial workup of myeloma patients with renal impairment.
Peripheral neuropathy
Peripheral neuropathy (PN) is a significant complication of MM that can be caused by the disease itself or by certain therapies, including thalidomide- and bortezomib-based therapies. Thorough clinical evaluation has shown that up to 20% of MM patients have PN at diagnosis and up to 75% may experience treatment-emergent PN during therapy.74 MM-associated PN is primarily sensory or sensorimotor, and symptoms are predominantly symmetric, including paresthesia, numbness, burning sensation and weakness, often with mild intensity, but rarely with the potency to be inactivating or life-threatening. Treatment-induced PN symptoms are usually symmetric and distal with some differences among therapies.75 PN from thalidomide is cumulative, dose dependent and often permanent, and may also occur after treatment has already stopped.74,75 Bortezomib-induced PN is related to dose, schedule and mode of administration and is mostly reversible. Symptoms of bortezomib-induced PN may start distally and may progress proximally.74,75 A randomized trial of subcutaneous compared to intravenous administration of bortezomib showed a significant decrease in PN of all grades (38% vs. 53%) and grade 3 or 4 (6% vs. 16%) with the former, leading to its universal use.76 Based on these data, the up-dated US prescribing information added that starting subcutaneous bortezomib may be considered for patients with pre-existing PN or at high-risk of PN (Table 3). The incidence of treatment-emergent PN with the newer proteasome inhibitors is relatively low. Experience from 526 relapsed/refractory MM patients in 4 phase II studies with carfilzomib reported an overall incidence of PN of 13.9%, grade 3 PN of 1.3% and no grade 4 or more; moreover all of the grade 3 PN occurred in patients with grade 1 or 2 at baseline.77
Table 3.
Management of hematologic and non-hematologic complication in myeloma patients treated with novel agents
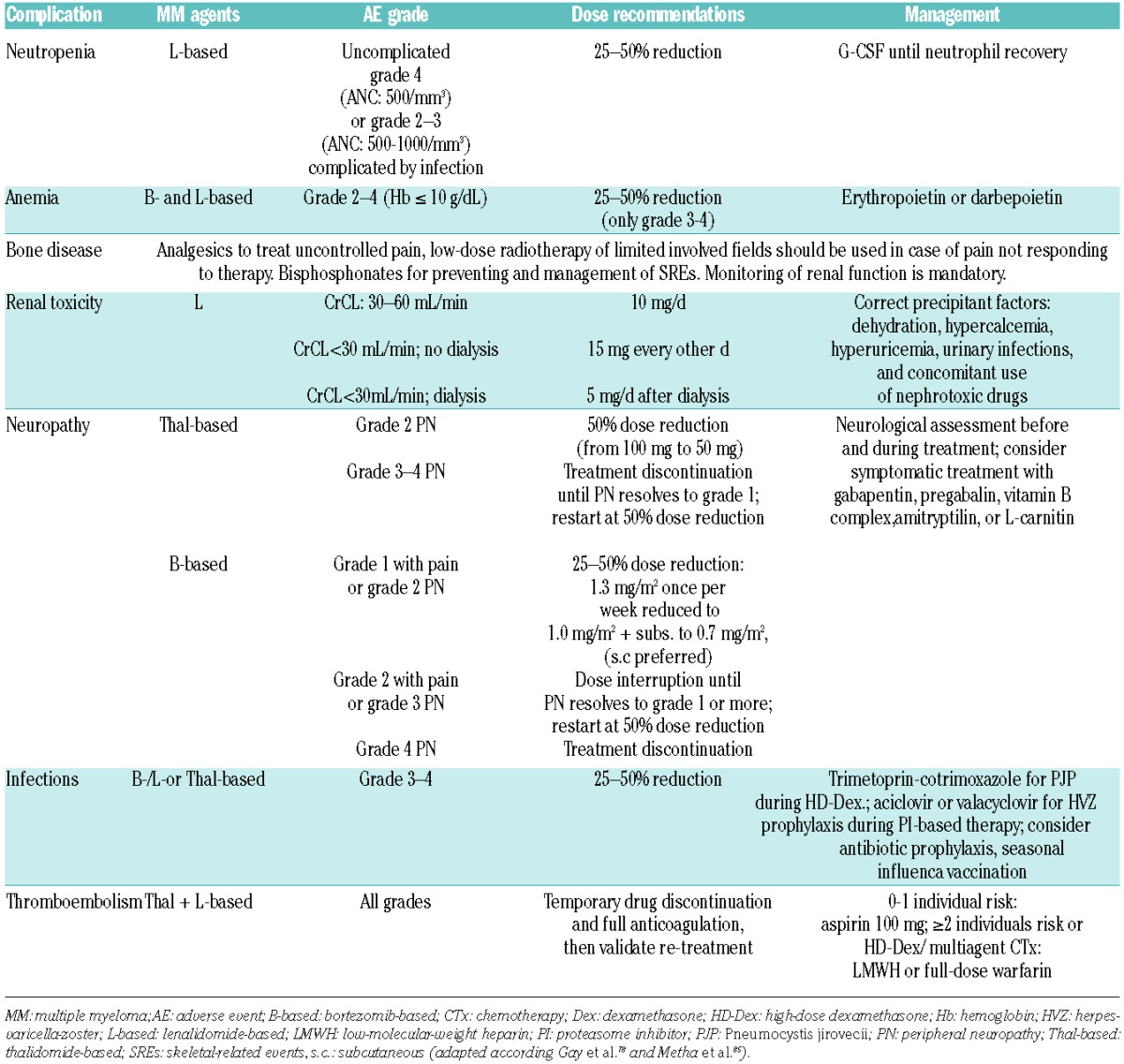
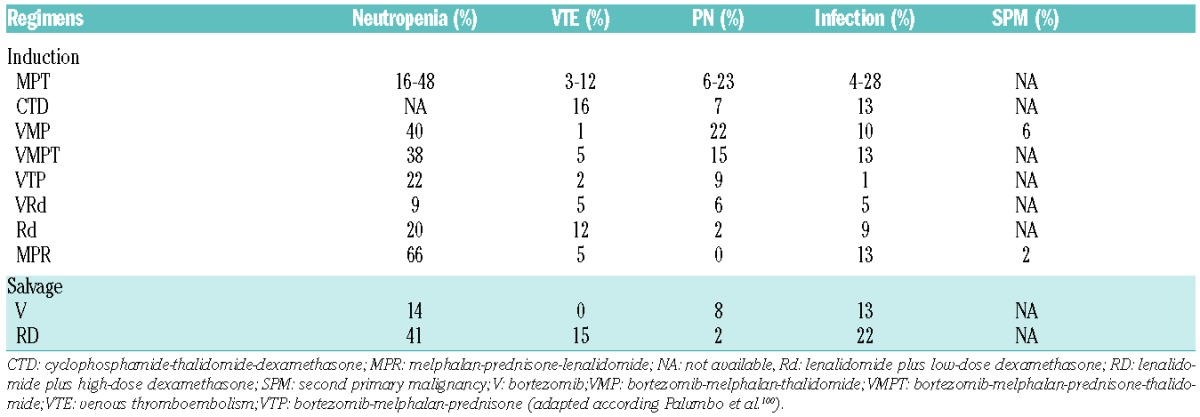
Table 2.
Incidence of adverse events in multiple myeloma patients treated with different therapy regimens.
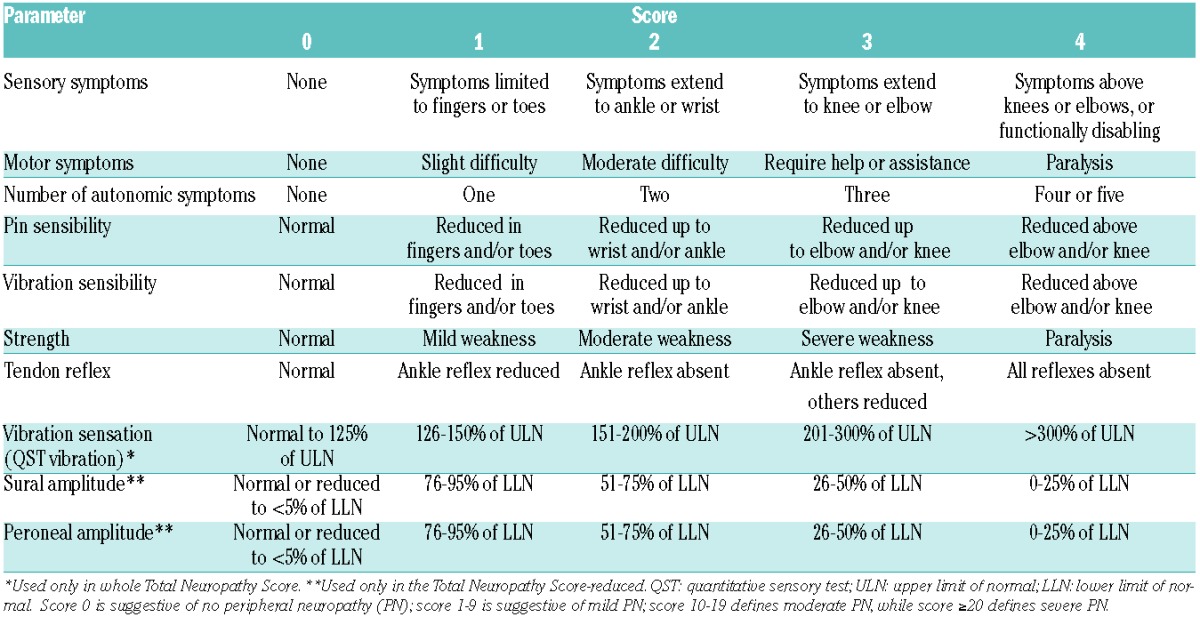
Careful attention to the development of PN is essential, while patients are on therapy and prompt dose reductions are required. Lower doses of bortezomib, weekly administration or different schedules (4- instead of 3-week cycles) may also be used. Regular monitoring for treatment-emergent PN, sensible detection and intervention are relevant to prevent the development of more severe PN (Table 3).74,78 The NCI CTC definition of PN is commonly used in clinical routine, but should be used with neuropathy-specific patient-completed questionnaires, such as the whole or the reduced Total Neuropathy Score (Table 4).79 However, a need remains for more sensitive assessment tools that focus on MM patients with PN.
Table 4.
Total Neuropathy Scores.
Several interventions have been investigated for treatment-induced PN, but prospective analyses are lacking. Acetyl-L-carnitin and alpha lipoic acid has shown activity in the treatment of chemotherapy-induced PN.74 Although the neuropathic pain may often be poorly responsive to standard analgesic treatment, opioids can be effective, which should be combined with other pain modulating drugs.44,74 In addition, calcium channel blocker (e.g. gabapentin and pregabalin), sodium channel blockers, such as oxcarbazepine and serotonin-norepinephrine reuptake inhibitors (e.g. duloxetine) can be very effective, especially in painful PN.44,74 Recent studies in a mouse model with anti-TNF-α showed protection against neuropathy induced by bortezomib, but future studies are essential to elucidate the etiopathogenesis of neuropathy, the role of TNF-α pathway and how bortezomib differentially regulates NF-κB in tumoral and neuronal cells.80
Recommendations: in the treatment for chemotherapy-induced PN, prevention is a key strategy for patients’ quality of life and ongoing treatment options (grade 2C). All MM patients with potential neurotoxic drugs should be routinely and clinically assessed for signs of PN before undergoing treatment; it is advisable that PN is graded with validated tools, such as the Total Neuropathy Score (grade 2C). The use for dose modifications for the management of bortezomib- or thalidomide-induced PN remains the ‘gold standard’ of care (grade 1C). Reduction of PN induced by bortezomib can be achieved by: a) prompt dose modification (1.3→ 1.0→ 0.7 mg/m2); b) once a week instead of twice weekly application; and c) subcutaneous rather than intravenous administration (Table 3).
Infections
Myeloma is associated with increased rate of infections, which is the main cause of death for myeloma patients. A recent population-based study on 9253 myeloma patients showed that the risk of developing a bacterial infection was 7-fold higher and for viral infections 10-fold higher compared to healthy individuals of the same sex and age. At one year of follow up, infection was the underlying cause in 22% of deaths in MM patients.81 Haemophilus influenzae, streptococcus pneumoniae, Gram negative bacilli and viruses (influenza and herpes zoster) are the most frequent causes of infection in myeloma patients.81
The increased susceptibility of patients to infections results from the myeloma itself, therapies and/or age- and disease-related conditions. Myeloma-related innate immunodeficiency involves various parts of the immune system and includes B-cell dysfunction as well as functional abnormalities of dendritic-, T- and natural killer (NK)-cells.82 Myeloma- and treatment-associated organ dysfunction, such as renal and/or pulmonary impairment, alimentary mucosal damage and multiorgan involvement by myeloma-associated deposition disease also increase the risk for infections.83 Finally, MM affects older patients who frequently experience age-related frailty, geriatric conditions and physical dysfunctions making them more susceptible to infections (Figure 3).84
Multiple myeloma patients require thorough infection monitoring and appropriate use of antibiotics (Table 3).85 There are only a few prospective studies evaluating the role of prophylactic antibiotics in MM patients. In a randomized, phase II study in 157 patients who underwent ASCT, the administration of ciprofloxacin and vancomycin reduced the incidence of neutropenic fever, without affecting, however, the total interval of hospitalization, time to engraftment, or all-cause mortality.86 In another study, 212 myeloma patients who received initial chemotherapy were randomized on a 1:1:1 basis to daily ciprofloxacin (500 mg twice daily), trimethoprim-sulfamethoxazole (DS twice daily) or observation. The incidence of severe bacterial infections was similar among the three groups: 12.5%, 6.8% and 5.9%, respectively (P=0.218). Similarly, the incidence of any infection during the first two months of therapy was also comparable (20%, 23% and 22%, respectively; P=0.954).87
Regarding the incidence of infections with different therapies, this has been reported to be 14% of patients treated with lenalidomide and dexamethasone in MM-009 and -010 trials88 and approximately 30% (grade 3 and 4) of patients treated with pomalidomide plus low-dose dexamethasone, mainly during the first three months of therapy.89 For this reason, routine antibiotic prophylaxis should be considered for the first three months of therapy with these IMiDs and is particularly recommended for patients with aggressive disease, history of infectious complications or neutropenia.88,89 The available data do not support the use of any specific antibiotic regimen to use and thus clinicians should follow their institutional guidelines for antibiotic prophylaxis. In patients who receive pomalidomide, quinolones must be used with caution due to common metabolic pathways that can increase pomalidomide exposure.
The use of prophylactic immunoglobulin replacement has shown no advantage in reducing infection rates in newly diagnosed myeloma patients.90 Regarding vaccinations, myeloma patients show suboptimal antibody responses to several vaccines; the responses seem to be worse for polysaccharide than protein antigens.91 In addition, all patients who undergo allogeneic SCT should receive vaccinations for Haemophilus influenzae type b, pertussis, pneumococci, meningococci, tetanus, diphtheria, hepatitis A and B, measles, mumps and rubella, influenza, poliomyelitis, varicella-zoster virus, human papilloma virus, and tick-borne encephalitis with a particular focus on vaccination of patients with active chronic graft-versus-host disease (GvHD).92
Recommendations: vaccination against influenza virus is appropriate and is recommended for both patients and their contacts. Moreover vaccination against Streptococcus pneumonia and Haemophilus influenzae is recommended, but efficacy for all vaccines is not guaranteed, due to suboptimal immune response (grade 1C). In general, live vaccines should be avoided in myeloma patients (grade 2C). Aciclovir or valacyclovir for herpes-zoster virus prophylaxis is recommended for patients receiving proteasome inhibitor-based therapies (grade 1A) or during ASCT/allogeneic-SCT, mainly in those with positive serology (grade 1C). Antiviral drugs should be continued for six weeks after discontinuation of the proteasome inhibitor. Due to increased infection rate during lenalidomide or pomalidomide administration, antibiotic prophylaxis is recommended at least for the first three months of therapy (grade 2C). Prophylactic immunoglobulin replacement is not routinely recommended; however, it may be useful in a subset of patients with severe, recurrent bacterial infections and hypogammaglobulinemia (grade 2C).
Venous thromboembolism
Myeloma itself, antimyeloma therapies, the presence of infections, the history of previous venous thromboembolism (VTE), immobility, obesity, paraplegia, ESA treatment, comorbidities, dehydration and renal failure are all important factors for the development of VTE. The incidence of VTE is approximately 8–22/1000 person years. Disease-related risk factors include the hyperviscosity, the inhibition of natural anticoagulants and the hypercoagulability status induced by inflammatory cytokines (i.e. increased von Willebrand factor, fibrinogen and factor VIII levels, acquired activated protein C resistance, decreased protein S levels, etc.).93 The incidence of VTE during front-line therapy is 1%–2% with conventional therapies such as melphalan and prednisone, and it is doubled by the use of doxorubicin or other chemotherapeutic agents, while the use of IMiDs in combination with dexamethasone or chemotherapeutic agents produces a VTE risk of up to 70% in the absence of anticoagulation.94 The risk for a VTE is higher during the first four months of therapy with lenalidomide or pomalidomide and then seems to be reduced.88,89 Aspirin and low molecular weight heparin (LMWH) have been used in myeloma patients under IMiDs. In a prospective, randomized, study, which compared aspirin 100 mg/day and enoxaparin 40 mg/day in 342 newly diagnosed patients who received lenalidomide and low-dose dexamethasone induction and melphalan-prednisone-lenalidomide consolidation, the incidence of VTE was 2.3% in the aspirin group and 1.2% in the enoxaparin group.95 Thus, aspirin can be considered as adequate anticoagulation therapy in patients who have no or one risk factor for VTE (i.e. hyperviscosity, personal or family history of VTE, obesity (Body Mass Index ≥30), co-morbidities: cardiac, diabetes, RI, chronic inflammatory disease, immobility, thrombophilias, concomitant presence of myeloproliferative disorders, hemoglobinopathies, recent surgery (within 6 weeks), medications: ESAs, hormone replacement therapy, tamoxifen/stilboestrol, doxorubicin, high-dose steroids (≥480mg of dexamethasone/month). Otherwise LMWH or full-dose warfarin can be used. The risk for bleeding has also to be taken into account in the choice of anticoagulation.94
Recommendations: patients who are due to start IMiD therapy should have a risk assessment for VTE and receive appropriate anticoagulation during the treatment duration (grade 1A). In these patients, aspirin (100 mg) is enough for VTE prophylaxis in low-risk patients (i.e. without risk factors, or only one myeloma/individual risk factor present), unless contraindicated (grade 1B). Otherwise, LMWH or full-dose warfarin has to be used (grade 1B). The use of LMWH has to be continued for at least four months and then patients may be switched to aspirin prophylaxis (grade 2C). Treatment of confirmed VTE has to be according to international or national guidelines96 (grade 1A). In cases of VTE, despite the use of full anticoagulation, the treating physician should consider the discontinuation of the responsible anti-myeloma drug (grade 2C).
Pain management recommendations
Pain is a considerable problem for many patients with MM. Regarding bone disease, the use of BPs along with anti-myeloma therapy, radiation or balloon kyphosplasty in specific indications may control the pain of the patients. The treating physician should take into account that, in several patients, pain, and especially back pain, may be due to other reasons, and not to myeloma itself. Regarding the relief of pain using pain killers or other drugs, on the basis of available data we suggest the following.97–100 Paracetamol can be administered at a dose of up to 1 g qid for the control of mild pain (grade 1B). In general, non-steroidal anti-inflammatory drugs should be avoided in MM (grade 2C). Oral tramadol or codeine can be given for the control of mild-moderate pain (grade 1C). In cases of chronic moderate to severe pain, fentanyl or buprenorphine patches or oxycodone are recommended (grade 1B). For severe chronic pain, nervous block with anesthetic drugs can be performed, while neurolytic block using chemicals, heat and freezing, may produce long-lasting blockade with pain relief for weeks, months or indefinitely. For the management of acute severe pain, subcutaneous opioid (i.e. oxycodone or morphine injection) can be used for the rapid relief of symptoms (grade 2B). Patients on opioids should be also given laxatives (grade 1A). All patients with chronic pain may be also considered as candidates for calcium channel blockers (gabapentin or pregabalin), for sodium channel blocker (lidocaine, oxcarbazepine) or a serotonin-norepinephrine reuptake inhibitors (duloxetine or amitryptiline) (grade 1B).
Conclusion
In conclusion, complications related to disease and/or anti-myeloma drugs contribute to increased mobility and mortality of myeloma patients. Furthermore, such complications also alter the performance status of the patients to define fit versus frail patients and consequently select and/or dose-reduce therapy (Figure 4). Therefore, the appropriate management of these complications is crucial for both patient quality of life and survival, and treating physicians must pay special attention to their management.
Figure 4.
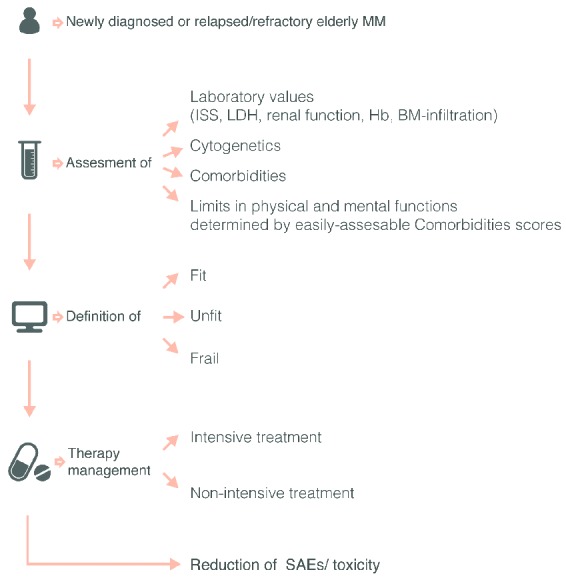
Special considerations prior to therapy in elderly or frail patients. In multiple myeloma patients with newly diagnosed or refractory disease a detailed geriatric and functional assessment helps to define more precisely ‘fit’ versus ‘frail’ patients and to evaluate patients’ risk for treatment toxicity and treatment discontinuation. These definitions of fit, unfit and frail patients are anticipated to influence selection of therapeutics, as well as the correct allocation to intensive or non-intensive treatment should reduce side-effects/SAEs and treatment toxicity.
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Terpos E, Dimopoulos MA. Myeloma bone disease: pathophysiology and management. Ann Oncol. 2005;16(8):1223–1231. [DOI] [PubMed] [Google Scholar]
- 2.Dimopoulos M, Terpos E, Comenzo RL, et al. International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple Myeloma. Leukemia. 2009;23(9):1545–1556. [DOI] [PubMed] [Google Scholar]
- 3.Terpos E, Moulopoulos LA, Dimopoulos MA. Advances in imaging and the management of myeloma bone disease. J Clin Oncol. 2011;29(14):1907–1915. [DOI] [PubMed] [Google Scholar]
- 4.Regelink JC, Minnema MC, Terpos E, et al. Comparison of modern and conventional imaging techniques in establishing multiple myeloma-related bone disease: a systematic review. Br J Haematol. 2013;162(1):50–61. [DOI] [PubMed] [Google Scholar]
- 5.Pianko MJ, Terpos E, Roodman GD, et al. Whole-body low-dose computed tomography and advanced imaging techniques for multiple myeloma bone disease. Clin Cancer Res. 2014;20(23):5888–5897. [DOI] [PubMed] [Google Scholar]
- 6.Walker R, Barlogie B, Haessler J, et al. Magnetic resonance imaging in multiple myeloma: diagnostic and clinical implications. J Clin Oncol. 2007;25(9):1121–1128. [DOI] [PubMed] [Google Scholar]
- 7.Waheed S, Mitchell A, Usmani S, et al. Standard and novel imaging methods for multiple myeloma: correlates with prognostic laboratory variables including gene expression profiling data. Haematologica. 2013;98(1):71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillengass J, Fechtner K, Weber MA, et al. Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol. 2010;28(9):1606–1610. [DOI] [PubMed] [Google Scholar]
- 9.Kastritis E, Moulopoulos LA, Terpos E, et al. The prognostic importance of the presence of more than one focal lesion in spine MRI of patients with asymptomatic (smoldering) multiple myeloma. Leukemia. 2014;28(12): 2402–2403. [DOI] [PubMed] [Google Scholar]
- 10.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–548. [DOI] [PubMed] [Google Scholar]
- 11.Moulopoulos LA, Dimopoulos MA, Christoulas D, et al. Diffuse MRI marrow pattern correlates with increased angiogenesis, advanced disease features and poor prognosis in newly diagnosed myeloma treated with novel agents. Leukemia. 2010;24(6): 1206–1212. [DOI] [PubMed] [Google Scholar]
- 12.Zamagni E, Patriarca F, Nanni C, et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011;118(23):5989–5995. [DOI] [PubMed] [Google Scholar]
- 13.Spinnato P, Bazzocchi A, Brioli A, et al. Contrast enhanced MRI and (1)(8)F-FDG PET-CT in the assessment of multiple myeloma: a comparison of results in different phases of the disease. Eur J Radiol. 2012;81(12): 4013–4018. [DOI] [PubMed] [Google Scholar]
- 14.Derlin T, Peldschus K, Munster S, et al. Comparative diagnostic performance of (1)(8)F-FDG PET/CT versus whole-body MRI for determination of remission status in multiple myeloma after stem cell transplantation. Eur Radiol. 2013;23(2):570–578. [DOI] [PubMed] [Google Scholar]
- 15.Kleber M, Udi J, Metzke B, et al. Challenging the current approaches to multiple myeloma-and other cancer-related bone diseases: from bisphosphonates to targeted therapy. Leuk Lymphoma. 2012;53(6):1057–1061. [DOI] [PubMed] [Google Scholar]
- 16.Berenson JR, Lichtenstein A, Porter L, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334(8):488–493. [DOI] [PubMed] [Google Scholar]
- 17.Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer. 2001;91(7):1191–1200. [DOI] [PubMed] [Google Scholar]
- 18.Rosen LS, Gordon D, Kaminski M, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J. 2001;7(5):377–387. [PubMed] [Google Scholar]
- 19.Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98(8):1735–1744. [DOI] [PubMed] [Google Scholar]
- 20.Gimsing P, Carlson K, Turesson I, et al. Effect of pamidronate 30 mg versus 90 mg on physical function in patients with newly diagnosed multiple myeloma (Nordic Myeloma Study Group): a double-blind, randomised controlled trial. Lancet Oncol. 2010;11(10): 973–982. [DOI] [PubMed] [Google Scholar]
- 21.Morgan GJ, Davies FE, Gregory WM, et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376(9757):1989–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan GJ, Child JA, Gregory WM, et al. Effects of zoledronic acid versus clodronic acid on skeletal morbidity in patients with newly diagnosed multiple myeloma (MRC Myeloma IX): secondary outcomes from a randomised controlled trial. Lancet Oncol. 2011;12(8):743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan GJ, Davies FE, Gregory WM, et al. Effects of induction and maintenance plus long-term bisphosphonates on bone disease in patients with multiple myeloma: the Medical Research Council Myeloma IX Trial. Blood. 2012;119(23):5374–5383. [DOI] [PubMed] [Google Scholar]
- 24.Mhaskar R, Redzepovic J, Wheatley K, et al. Bisphosphonates in multiple myeloma: a network meta-analysis. Cochrane Database Syst Rev. 2012;5:CD003188. [DOI] [PubMed] [Google Scholar]
- 25.Terpos E, Morgan G, Dimopoulos MA, et al. International Myeloma Working Group recommendations for the treatment of multiple myeloma-related bone disease. J Clin Oncol. 2013;31(18):2347–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berenson JR, Rosen L, Vescio R, et al. Pharmacokinetics of pamidronate disodium in patients with cancer with normal or impaired renal function. J Clin Pharmacol. 1997;37(4):285–290. [DOI] [PubMed] [Google Scholar]
- 27.Dimopoulos MA, Kastritis E, Anagnostopoulos A, et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: evidence of increased risk after treatment with zoledronic acid. Haematologica. 2006;91(7):968–971. [PubMed] [Google Scholar]
- 28.Dimopoulos MA, Kastritis E, Bamia C, et al. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol. 2009;20(1): 117–120. [DOI] [PubMed] [Google Scholar]
- 29.Hellstein JW, Adler RA, Edwards B, et al. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2011;142(11):1243–1251. [DOI] [PubMed] [Google Scholar]
- 30.Berenson J, Pflugmacher R, Jarzem P, et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol. 2011;12(3):225–235. [DOI] [PubMed] [Google Scholar]
- 31.Bhargava A, Trivedi D, Kalva L, Tumas M, Hooks M, Speth J. Managment of cancer-related vertebral compression fracture: Comparison of treatment options-A literature meta-analysis. J Clin Oncol. 2009;27(15S):e20529. [Google Scholar]
- 32.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33. [DOI] [PubMed] [Google Scholar]
- 33.König C, Kleber M, Ihorst G, et al. Prevalence of iron overload vs iron deficiency in multiple myeloma: resembling or different from MDS–and stem cell transplant (SCT)–patients? Clin Lymphoma Myeloma Leuk. 2013;13(6):671–680. [DOI] [PubMed] [Google Scholar]
- 34.Silvestris F, Cafforio P, Tucci M, Dammacco F. Negative regulation of erythroblast maturation by Fas-L(+)/TRAIL(+) highly malignant plasma cells: a major pathogenetic mechanism of anemia in multiple myeloma. Blood. 2002;99(4):1305–1313. [DOI] [PubMed] [Google Scholar]
- 35.Birgegard G, Gascon P, Ludwig H. Evaluation of anaemia in patients with multiple myeloma and lymphoma: findings of the European CANCER ANAEMIA SURVEY. Eur J Haematol. 2006;77(5):378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cazzola M, Messinger D, Battistel V, et al. Recombinant human erythropoietin in the anemia associated with multiple myeloma or non-Hodgkin’s lymphoma: dose finding and identification of predictors of response. Blood. 1995;86(12):4446–4453. [PubMed] [Google Scholar]
- 37.Dammacco F, Castoldi G, Rodjer S. Efficacy of epoetin alfa in the treatment of anaemia of multiple myeloma. Br J Haematol. 2001;113(1):172–179. [DOI] [PubMed] [Google Scholar]
- 38.Osterborg A, Brandberg Y, Molostova V, et al. Randomized, double-blind, placebo-controlled trial of recombinant human erythropoietin, epoetin Beta, in hematologic malignancies. J Clin Oncol. 2002;20(10):2486–2494. [DOI] [PubMed] [Google Scholar]
- 39.Hedenus M, Adriansson M, San Miguel J, et al. Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: a randomized, double-blind, placebo-controlled study. Br J Haematol. 2003;122(3):394–403. [DOI] [PubMed] [Google Scholar]
- 40.Beguin Y, Maertens J, De Prijck B, et al. Darbepoetin-alfa and intravenous iron administration after autologous hematopoietic stem cell transplantation: a prospective multicenter randomized trial. Am J Hematol. 2013;88(12):990–996. [DOI] [PubMed] [Google Scholar]
- 41.Osterborg A, Brandberg Y, Hedenus M. Impact of epoetin-beta on survival of patients with lymphoproliferative malignancies: long-term follow up of a large randomized study. Br J Haematol. 2005;129(2):206–209. [DOI] [PubMed] [Google Scholar]
- 42.Tonia T, Mettler A, Robert N, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev. 2012;12:CD003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richardson P, Schlag R, Khuageva N, et al. Characterization of haematological parameters with bortezomib-melphalan-prednisone versus melphalan-prednisone in newly diagnosed myeloma, with evaluation of long-term outcomes and risk of thromboembolic events with use of erythropoiesis-stimulating agents: analysis of the VISTA trial. Br J Haematol. 2011;153(2):212–221. [DOI] [PubMed] [Google Scholar]
- 44.Snowden JA, Ahmedzai SH, Ashcroft J, et al. Guidelines for supportive care in multiple myeloma 2011. Br J Haematol. 2011;154(1): 76–103. [DOI] [PubMed] [Google Scholar]
- 45.Katodritou E, Zervas K, Terpos E, Brugnara C. Use of erythropoiesis stimulating agents and intravenous iron for cancer and treatment-related anaemia: the need for predictors and indicators of effectiveness has not abated. Br J Haematol. 2008;142(1):3–10. [DOI] [PubMed] [Google Scholar]
- 46.Kleber M, Ihorst G, Deschler B, et al. Detection of renal impairment as one specific comorbidity factor in multiple myeloma: multicenter study in 198 consecutive patients. Eur J Haematol. 2009;83(6):519–527. [DOI] [PubMed] [Google Scholar]
- 47.Davenport A, Merlini G. Myeloma kidney: advances in molecular mechanisms of acute kidney injury open novel therapeutic opportunities. Nephrol Dial Transplant. 2012;27(10):3713–3718. [DOI] [PubMed] [Google Scholar]
- 48.Dimopoulos MA, Kastritis E, Rosinol L, Blade J, Ludwig H. Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia. 2008;22(8):1485–1493. [DOI] [PubMed] [Google Scholar]
- 49.Hutchison CA, Batuman V, Behrens J, et al. The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat Rev Nephrol. 2011;8(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang ZQ, Sanders PW. Biochemical interaction between Tamm-Horsfall glycoprotein and Ig light chains in the pathogenesis of cast nephropathy. Lab Invest. 1995;73(6):810–817. [PubMed] [Google Scholar]
- 51.Sengul S, Zwizinski C, Simon EE, Kapasi A, Singhal PC, Batuman V. Endocytosis of light chains induces cytokines through activation of NF-kappaB in human proximal tubule cells. Kidney Int. 2002;62(6):1977–1988. [DOI] [PubMed] [Google Scholar]
- 52.Ma CX, Lacy MQ, Rompala JF, et al. Acquired Fanconi syndrome is an indolent disorder in the absence of overt multiple myeloma. Blood. 2004;104(1):40–42. [DOI] [PubMed] [Google Scholar]
- 53.Bird JM, Owen RG, D’Sa S, et al. Guidelines for the diagnosis and management of multiple myeloma 2011. Br J Haematol. 2011;154(1):32–75. [DOI] [PubMed] [Google Scholar]
- 54.IMWG Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749–757. [PubMed] [Google Scholar]
- 55.Kleber M, Cybulla M, Bauchmuller K, Ihorst G, Koch B, Engelhardt M. Monitoring of renal function in cancer patients: an ongoing challenge for clinical practice. Ann Oncol. 2007;18(5):950–958. [DOI] [PubMed] [Google Scholar]
- 56.Dimopoulos MA, Terpos E, Chanan-Khan A, et al. Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol. 2010;28(33):4976–4984. [DOI] [PubMed] [Google Scholar]
- 57.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67(6):2089–2100. [DOI] [PubMed] [Google Scholar]
- 58.Terpos E, Christoulas D, Kastritis E, et al. The Chronic Kidney Disease Epidemiology Collaboration cystatin C (CKD-EPI-CysC) equation has an independent prognostic value for overall survival in newly diagnosed patients with symptomatic multiple myeloma; is it time to change from MDRD to CKD-EPI-CysC equations? Eur J Haematol. 2013;91(4):347–355. [DOI] [PubMed] [Google Scholar]
- 59.Kleber M, Koch B, Wäsch R, Engelhardt M. Estimation of GFR with different EGFR equations in multiple myeloma patients receiving lenalidomide treatment. Blood. 2013;122 (Abstract 5324). [Google Scholar]
- 60.Dimopoulos M, Terpos E, Symeonidis A, et al. Estimated Glomerular Filtration Rate Calculated By The CKD-EPI Formula Has Improved Prognostic Ability Over MDRD Formula In Patients With Newly Diagnosed, Symptomatic, Multiple Myeloma: Analysis In 1937 Patients.). Blood 2013;122 (Abstract 1867). [Google Scholar]
- 61.Burnette BL, Leung N, Rajkumar SV. Renal improvement in myeloma with bortezomib plus plasma exchange. N Engl J Med. 2011;364(24):2365–2366. [DOI] [PubMed] [Google Scholar]
- 62.Clark WF, Stewart AK, Rock GA, et al. Plasma exchange when myeloma presents as acute renal failure: a randomized, controlled trial. Ann Intern Med. 2005;143(11):777–784. [DOI] [PubMed] [Google Scholar]
- 63.Hutchison C, Bridoux F, Fermand JP. Renal improvement in myeloma with plasma exchange. N Engl J Med. 2011;365(11):1061; author reply 2. [DOI] [PubMed] [Google Scholar]
- 64.Heyne N, Denecke B, Guthoff M, et al. Extracorporeal light chain elimination: high cut-off (HCO) hemodialysis parallel to chemotherapy allows for a high proportion of renal recovery in multiple myeloma patients with dialysis-dependent acute kidney injury. Ann Hematol. 2012;91(5):729–735. [DOI] [PubMed] [Google Scholar]
- 65.Zannetti BA, Zamagni E, Santostefano M, et al. Bortezomib-based therapy combined with high cut-off hemodialysis is highly effective in newly diagnosed multiple myeloma patients with severe renal impairment. Am J Hematol. 2015; 10.1002/ajh.24035 [DOI] [PubMed] [Google Scholar]
- 66.Scheid C, Sonneveld P, Schmidt-Wolf IG, et al. Bortezomib before and after autologous stem cell transplantation overcomes the negative prognostic impact of renal impairment in newly diagnosed multiple myeloma: a subgroup analysis from the HOVON-65/GMMG-HD4 trial. Haematologica. 2014;99(1):148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dimopoulos MA, Richardson PG, Schlag R, et al. VMP (Bortezomib, Melphalan, and Prednisone) is active and well tolerated in newly diagnosed patients with multiple myeloma with moderately impaired renal function, and results in reversal of renal impairment: cohort analysis of the phase III VISTA study. J Clin Oncol. 2009;27(36):6086–6093. [DOI] [PubMed] [Google Scholar]
- 68.Badros AZ, Vij R, Martin T, et al. Carfilzomib in multiple myeloma patients with renal impairment: pharmacokinetics and safety. Leukemia. 2013;27(8):1707–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dimopoulos MA, Terpos E, Goldschmidt H, Alegre A, Mark T, Niesvizky R. Treatment with lenalidomide and dexamethasone in patients with multiple myeloma and renal impairment. Cancer Treat Rev. 2012;38(8): 1012–1019. [DOI] [PubMed] [Google Scholar]
- 70.Weisel K, Dimopoulos MA, Cavo M, et al. Pomalidomide + low-dose dexamethasone in patients with refractory or relapsed and refractory multiple myeloma and renal impairment: analysis of patients from the phase 3b Stratus trial (MM-010). Blood. 2014;124(21)(Abstract)4755. [Google Scholar]
- 71.Ramasamy K, Hazel B, Mahmood S, Corderoy S, Schey S. Bendamustine in combination with thalidomide and dexamethasone is an effective therapy for myeloma patients with end stage renal disease. Br J Haematol. 2011;155(5):632–634. [DOI] [PubMed] [Google Scholar]
- 72.Pönisch W, Moll B, Bourgeois M, et al. Bendamustine and prednisone in combination with bortezomib (BPV) in the treatment of patients with relapsed or refractory multiple myeloma and light chain-induced renal failure. J Cancer Res Clin Oncol. 2013;139(11):1937–1946. [DOI] [PubMed] [Google Scholar]
- 73.Dimopoulos MA, Roussou M, Gkotzamanidou M, et al. The role of novel agents on the reversibility of renal impairment in newly diagnosed symptomatic patients with multiple myeloma. Leukemia. 2013;27(2):423–429. [DOI] [PubMed] [Google Scholar]
- 74.Richardson PG, Delforge M, Beksac M, et al. Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia. 2012;26(4):595–608. [DOI] [PubMed] [Google Scholar]
- 75.Delforge M, Bladé J, Dimopoulos MA, et al. Treatment-related peripheral neuropathy in multiple myeloma: the challenge continues. Lancet Oncol. 2010;11(11):1086–1095. [DOI] [PubMed] [Google Scholar]
- 76.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12(5):431–440. [DOI] [PubMed] [Google Scholar]
- 77.Siegel D, Martin T, Nooka A, et al. Integrated safety profile of single-agent carfilzomib: experience from 526 patients enrolled in 4 phase II clinical studies. Haematologica. 2013;98(11):1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gay F, Palumbo A. Multiple myeloma: management of adverse events. Med Oncol. 2010;27(3):646–653. [DOI] [PubMed] [Google Scholar]
- 79.Richardson PG, Briemberg H, Jagannath S, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24(19):3113–3120. [DOI] [PubMed] [Google Scholar]
- 80.Ale A, Bruna J, Morell M, et al. Treatment with anti-TNF alpha protects against the neuropathy induced by the proteasome inhibitor bortezomib in a mouse model. Exp Neurol. 2014;253:165–173. [DOI] [PubMed] [Google Scholar]
- 81.Blimark C, Holmberg E, Mellqvist UH, et al. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica. 2015;100(1):107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tete SM, Bijl M, Sahota SS, Bos NA. Immune defects in the risk of infection and response to vaccination in monoclonal gammopathy of undetermined significance and multiple myeloma. Front Immunol. 2014;5:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nucci M, Anaissie E. Infections in patients with multiple myeloma. Semin Hematol. 2009;46(3):277–288. [DOI] [PubMed] [Google Scholar]
- 84.Kleber M, Ihorst G, Gross B, et al. Validation of the Freiburg Comorbidity Index in 466 multiple myeloma patients and combination with the international staging system are highly predictive for outcome. Clin Lymphoma Myeloma Leuk. 2013;13(5):541–551. [DOI] [PubMed] [Google Scholar]
- 85.Mehta J, Cavo M, Singhal S. How I treat elderly patients with myeloma. Blood. 2010;116(13):2215–2223. [DOI] [PubMed] [Google Scholar]
- 86.Eleutherakis-Papaiakovou E, Kostis E, Migkou M, et al. Prophylactic antibiotics for the prevention of neutropenic fever in patients undergoing autologous stem-cell transplantation: results of a single institution, randomized phase 2 trial. Am J Hematol. 2010;85(11):863–867. [DOI] [PubMed] [Google Scholar]
- 87.Vesole DH, Oken MM, Heckler C, et al. Oral antibiotic prophylaxis of early infection in multiple myeloma: a URCC/ECOG randomized phase III study. Leukemia. 2012;26(12): 2517–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dimopoulos MA, Palumbo A, Attal M, et al. Optimizing the use of lenalidomide in relapsed or refractory multiple myeloma: consensus statement. Leukemia. 2011;25(5): 749–760. [DOI] [PubMed] [Google Scholar]
- 89.Dimopoulos MA, Leleu X, Palumbo A, et al. Expert panel consensus statement on the optimal use of pomalidomide in relapsed and refractory multiple myeloma. Leukemia. 2014;28(8):1573–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Salmon SE, Samal BA, Hayes DM, Hosley H, Miller SP, Schilling A. Role of gamma globulin for immunoprophylaxis in multiple myeloma. N Engl J Med. 1967;277(25):1336–1340. [DOI] [PubMed] [Google Scholar]
- 91.Robertson JD, Nagesh K, Jowitt SN, et al. Immunogenicity of vaccination against influenza, Streptococcus pneumoniae and Haemophilus influenzae type B in patients with multiple myeloma. Br J Cancer. 2000;82(7):1261–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hilgendorf I, Freund M, Jilg W, et al. Vaccination of allogeneic haematopoietic stem cell transplant recipients: report from the international consensus conference on clinical practice in chronic GVHD. Vaccine. 2011;29(16):2825–2833. [DOI] [PubMed] [Google Scholar]
- 93.De Stefano V, Za T, Rossi E. Venous thromboembolism in multiple myeloma. Semin Thromb Hemost. 2014;40(3):338–347. [DOI] [PubMed] [Google Scholar]
- 94.Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22(2):414–423. [DOI] [PubMed] [Google Scholar]
- 95.Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood. 2012;119(4):933–939. [DOI] [PubMed] [Google Scholar]
- 96.Kuderer NM, Lyman GH. Guidelines for treatment and prevention of venous thromboembolism among patients with cancer. Thromb Res. 2014;133Suppl 2:S122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Davis MP, Walsh D, Lagman R, LeGrand SB. Controversies in pharmacotherapy of pain management. Lancet Oncol. 2005;6(9):696–704. [DOI] [PubMed] [Google Scholar]
- 98.Snowden JA, Ahmedzai SH, Ashcroft J, et al. Guidelines for supportive care in multiple myeloma 2011. Br J Haematol. 2011;154(1): 76–103. [DOI] [PubMed] [Google Scholar]
- 99.Yan PZ, Butler PM, Kurowski D, Perloff MD. Beyond neuropathic pain: gabapentin use in cancer pain and perioperative pain. Clin J Pain. 2014;30(7):613–629. [DOI] [PubMed] [Google Scholar]
- 100.Palumbo A, Rajkumar SV, San Miguel JF, et al. International myeloma working group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol. 2014; 32(6):587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]


