Abstract
Patients with refractory or relapsed and refractory multiple myeloma who no longer receive benefit from novel agents have limited treatment options and short expected survival. del(17p) and t(4;14) are correlated with shortened survival. The phase 3 MM-003 trial demonstrated significant progression-free and overall survival benefits from treatment with pomalidomide plus low-dose dexamethasone compared to high-dose dexamethasone among patients in whom bortezomib and lenalidomide treatment had failed. At an updated median follow-up of 15.4 months, the progression-free survival was 4.0 versus 1.9 months (HR, 0.50; P<0.001), and median overall survival was 13.1 versus 8.1 months (HR, 0.72; P=0.009). Pomalidomide plus low-dose dexamethasone, compared with high-dose dexamethasone, improved progression-free survival in patients with del(17p) (4.6 versus 1.1 months; HR, 0.34; P <0.001), t(4;14) (2.8 versus 1.9 months; HR, 0.49; P=0.028), and in standard-risk patients (4.2 versus 2.3 months; HR, 0.55; P<0.001). Although the majority of patients treated with high-dose dexamethasone took pomalidomide after discontinuation, the overall survival of patients treated with pomalidomide plus low-dose dexamethasone or high-dose dexamethasone was 12.6 versus 7.7 months (HR, 0.45; P=0.008) in patients with del(17p), 7.5 versus 4.9 months (HR, 1.12; P=0.761) in those with t(4;14), and 14.0 versus 9.0 months (HR, 0.85; P=0.380) in standard-risk subjects. The overall response rate was higher in patients treated with pomalidomide plus low-dose dexamethasone than in those treated with high-dose dexamethasone both among standard-risk patients (35.2% versus 9.7%) and those with del(17p) (31.8% versus 4.3%), whereas it was similar in patients with t(4;14) (15.9% versus 13.3%). The safety of pomalidomide plus low-dose dexamethasone was consistent with initial reports. In conclusion, pomalidomide plus low-dose dexamethasone is efficacious in patients with relapsed/refractory multiple myeloma and del(17p) and/or t(4;14). This study is registered at ClinicalTrials.gov as NCT01311687 and with EudraCT as 2010-019820-30.
Introduction
The current standard of care for patients with multiple myeloma involves treatment with proteasome inhibitors such as bortezomib and immunomodulatory drugs such as lenalidomide.1,2 With the recent advances in treatment, the 5-and 10-year relative survival rates for multiple myeloma are now 40% and 21%, respectively.3 However, most patients with multiple myeloma will relapse several times during the course of their disease.4 There are few established treatments available for those who have exhausted novel agents. The median overall survival for patients in whom bortezomib or immunomodulatory drugs have failed is only 9 months, demonstrating the clear, unmet need for further treatment options for such patients.4
The chromosomal aberrations del(17p) and t(4;14) (found in approximately 11% and 14% of multiple myeloma patients, respectively) are associated with an adverse prognosis, reducing median event-free survival from diagnosis to 20.6 months (versus 36.5 months; P<0.001) and 15 months (versus 35 months; P<0.001), respectively.5,6 The presence of t(4;14) was also found to predict shorter survival in patients with advanced relapsed or refractory disease treated with lenalidomide and dexamethasone (9.4 versus 15.4 months; P=0.005).7 The (4;14) translocation results in increased expression of the oncogenes fibroblast growth factor receptor 3 (FGFR3) and multiple myeloma set domain (MMSET).8–10 The chromosomal abnormality del(17p) is frequently associated with mutations in the TP53 gene or alterations in the p53 pathway.11 Few studies have analyzed the impact of these cytogenetic features or other baseline characteristics specifically in advanced relapsed/refractory multiple myeloma.4,12,13
Pomalidomide is a distinct immunomodulatory drug with direct antimyeloma, stromal support–inhibitory, and immunomodulatory effects.14 The open-label, randomized phase 2 MM-002 trial reported an overall response rate of 33% to pomalidomide + low-dose dexamethasone (LoDEX), with a median progression-free survival of 4.2 months and median overall survival of 16.5 months. These results were consistent even in patients who were refractory to lenalidomide as the most recent line of prior therapy.15 Additionally, pomalidomide + LoDEX was effective in patients presenting with the cytogenetic abnormalities del(17p) and/or t(4;14).15 Favorable results with pomalidomide + LoDEX in patients with del(17p) were also seen in the IFM 2010-02 trial.16 The international, multicenter, open-label, randomized, phase 3 trial MM-003 compared pomalidomide + LoDEX (n=302) with high-dose dexamethasone (HiDEX; n=153) treatment in patients with multiple myeloma refractory to their last prior treatment in whom bortezomib and lenalidomide had previously failed. Initial results at a median follow-up of 10 months showed significant improvements in progression-free survival [(4.0 versus 1.9 months; hazard ratio (HR), 0.48; P<0.0001)] and overall survival (12.7 versus 8.1 months; HR, 0.74; P=0.0285) with pomalidomide + LoDEX, with similar efficacy across various populations of patients, including those refractory to lenalidomide as last prior treatment.17 The most common grade 3/4 adverse events reported were hematologic (neutropenia, anemia, and thrombocytopenia).
This updated analysis of the MM-003 trial presented here is based on a median follow-up of 15.4 months. Analyses of baseline characteristics predictive of long-term benefit and assessments of the impact of high-risk cytogenetic abnormalities are also reported.
Methods
Study design and patients
A full description of the study design, inclusion and exclusion criteria, and treatment protocol has been published elsewhere.17 Briefly, MM-003 was an open-label, randomized, phase 3 trial conducted in 93 centers in the European Union, Russia, Switzerland, Australia, Canada, and the USA. Patients enrolled had to have refractory or relapsed and refractory multiple myeloma treated with two or more prior antimyeloma regimens, have been refractory to their last prior treatment, failed prior treatment with bortezomib and lenalidomide (following ≥2 previous consecutive cycles of each, alone or in combination), and have received adequate prior alkylator therapy. Failure of bortezomib and lenalidomide therapy was defined as progressive disease on or within 60 days of completing treatment, progressive disease ≤6 months after achieving a partial response, or intolerance when achieving no more than a minimal response (bortezomib only). Additional details regarding the selection of patients have been described elsewhere.17
All patients provided written informed consent to participate in the study, which was approved by institutional review boards or independent ethics committees at all participating centers and performed in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Guidelines on Good Clinical Practice. This trial is registered with ClinicalTrials.gov (NCT01311687) and EudraCT (2010-019820-30). All authors and the sponsor were involved in the data collection, analysis, review, and interpretation and writing of the report.
A total of 455 patients were randomized in a 2:1 ratio to receive 28-day cycles of pomalidomide (4 mg/day orally on days 1–21) + LoDEX (40 mg/day orally on days 1, 8, 15, and 22) or HiDEX (40 mg/day orally on days 1–4, 9–12, and 17–20). In both arms of the study, dexamethasone was administered at a dose of 20 mg/day for patients aged ≥75 years. Treatment was continued until progression or unacceptable toxicity. Thromboprophylaxis, consisting of the physician’s choice of low-dose aspirin or low-molecular-weight heparin, was required for all patients who received pomalidomide and those at high risk of developing thrombosis.
Assessments and statistical analyses
The primary endpoint was progression-free survival and secondary endpoints included overall survival, rate of overall responses defined by International Myeloma Working Group (IMWG)18 and European Group for Blood and Marrow Transplant (EBMT)19 criteria, time to progression, duration of response, duration of treatment, safety, and quality of life. Progression-free survival and response were based on investigator assessment. For cytogenetic analyses, samples were collected at the time of screening the patients and analyzed by a central laboratory, as described previously.20 Briefly, enrichment of CD138+ plasma cells was followed by interphase fluorescence in situ hybridization (FISH) for TP53 deletion [del(17p)] and/or fibroblast growth factor receptor 3/immunoglobulin heavy locus gene rearrangement [t(4;14)]. Any detectable presence of mutation was considered a positive result.
Progression-free survival, overall survival, and duration of response were estimated using the Kaplan-Meier product-limit method and compared between treatment groups using log-rank tests stratified by age, disease population, and number of prior antimyeloma therapies. Overall response rate was compared between treatment arms using the Fisher exact test. Progression-free survival and overall survival for patients within each treatment arm were analyzed by cytogenetic abnormality status using an unstratified log-rank test. Patients in the pomalidomide + LoDEX arm who survived >12 or ≤12 months were descriptively compared with respect to demographics and baseline characteristics to identify potential factors that may contribute to longer survival. Then, these identified potential factors were used in a Cox regression model selection process for overall survival in all subjects in the pomalidomide + LoDEX arm. The forward selection method was used to identify significant factors.
Results
Patients’ characteristics
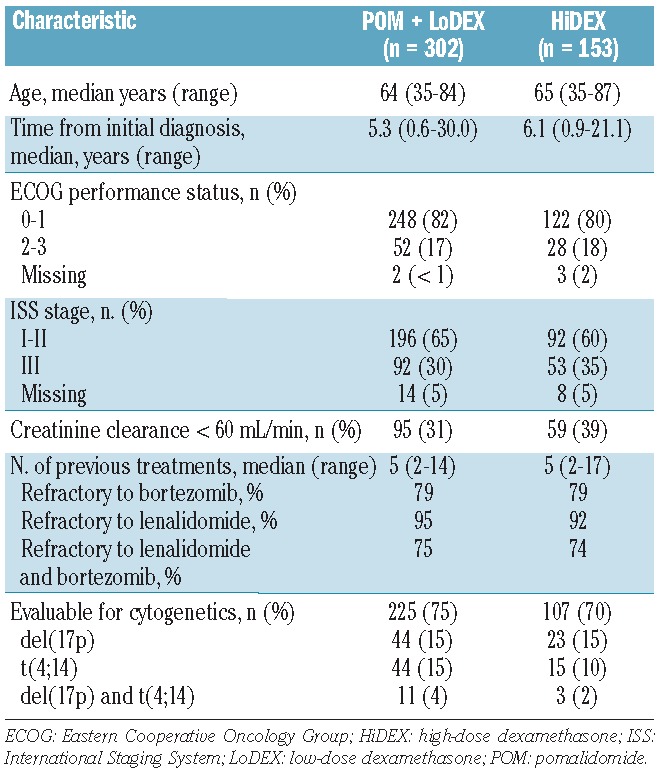
The data cutoff for this report was September 1, 2013, for a median follow-up of 15.4 months. The treatment arms were well balanced. Patients were heavily pretreated, with a median of five lines of prior therapy in both arms (Table 1). A total of 225 patients (75%) treated with pomalidomide + LoDEX and 107 patients (70%) treated with HiDEX had evaluable cytogenetics. Chromosomal abnormalities were balanced between arms: 15% of patients in each treatment arm were positive for del(17p) and 15% (pomalidomide + LoDEX) and 10% (HiDEX) were positive for t(4;14).
Table 1.
Patients’ baseline characteristics.
Updated survival (intent-to-treat population)
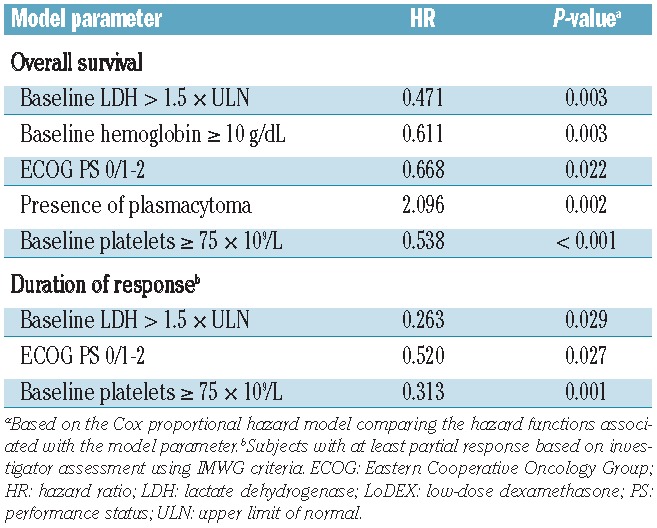
With extended follow-up, the median progression-free survival remained significantly longer with pomalidomide + LoDEX versus HiDEX (4.0 versus 1.9 months; HR, 0.50; P<0.001). The median overall survival was also significantly longer with pomalidomide + LoDEX versus HiDEX (Figure 1; 13.1 versus 8.1 months; HR, 0.72; P=0.009). This overall survival advantage was observed despite 85 patients (56%) on the HiDEX arm receiving subsequent pomalidomide-based treatment. Multivariate analysis of the pomalidomide + LoDEX treatment arm identified several baseline characteristics significantly associated with longer overall survival: normal lactate dehydrogenase level, higher level of hemoglobin or platelets, lower Eastern Cooperative Oncology Group (ECOG) performance status, and absence of plasmacytoma (Table 2). Normal lactate dehydrogenase level, higher platelet count, and lower ECOG performance status were also significantly associated with longer duration of response.
Figure 1.
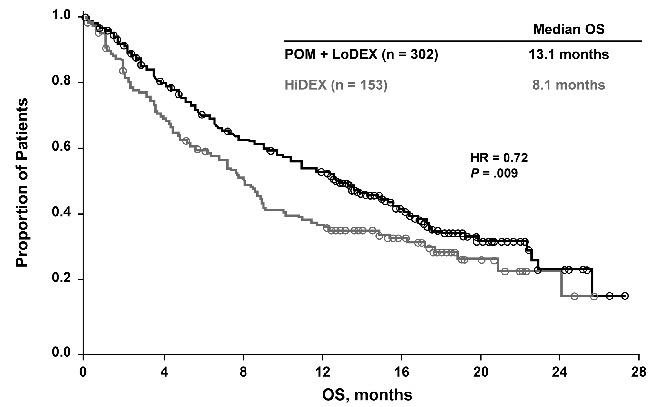
Kaplan-Meier curve of overall survival (OS) for the intent-to-treat population. HiDEX: high-dose dexamethasone; HR: hazard ratio; LoDEX: low-dose dexamethasone; POM: pomalidomide.
Table 2.
Multivariate Cox proportional hazards analysis of baseline characteristics predictive of overall survival and duration of response in patients treated with pomalidomide + LoDEX.
Safety
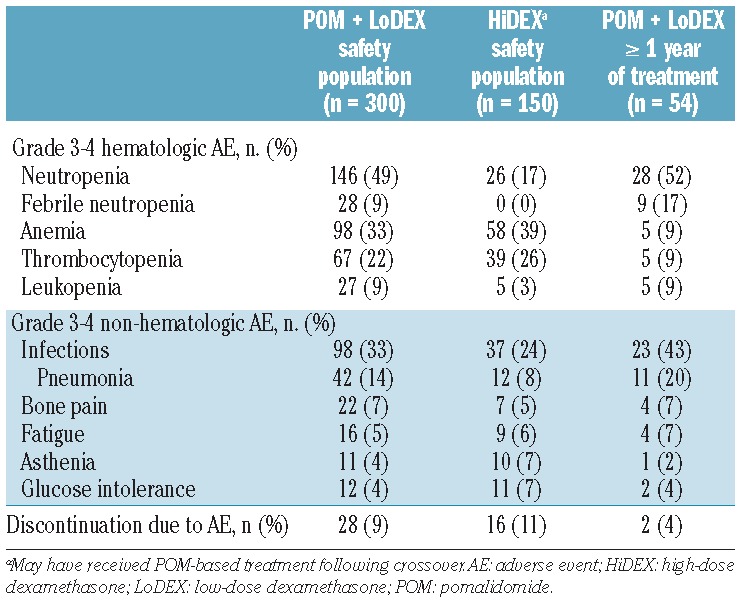
The updated safety profile of this analysis was consistent with that reported in the original publication (Table 3).17 The most common grade 3/4 adverse events were neutropenia (49% versus 17%), anemia (33% versus 39%), and infections (33% versus 24%) for the pomalidomide + LoDEX versus HiDEX safety populations, respectively. The rates of discontinuation due to adverse events were low considering the heavily pretreated condition of this population of patients and were comparable between the two treatment arms (9% versus 10%). In the 54 pomalidomide + LoDEX patients who were treated for 1 year or longer, the most frequent grade 3/4 adverse events were neutropenia (52%) and infections (43%). These patients experienced anemia and thrombocytopenia notably less frequently (both 9%) compared with the pomalidomide + LoDEX safety population.
Table 3.
Most commonly reported grade 3/4 adverse events for safety populations and patients treated with pomalidomide (POM) + LoDEX for 1 year or more.
Cytogenetic subanalyses
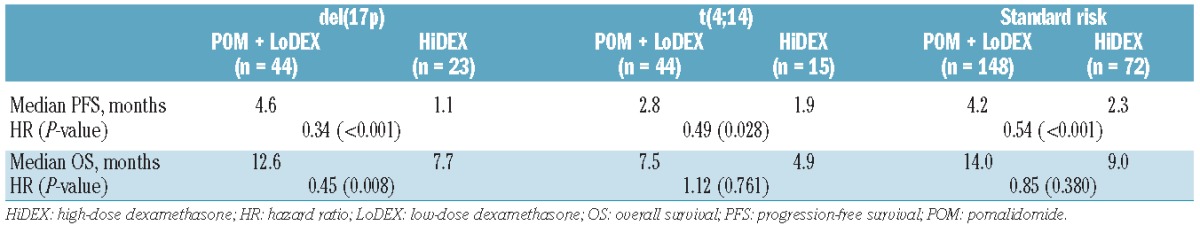
A significant progression-free survival benefit of pomalidomide + LoDEX treatment versus HiDEX was observed in all cytogenetic subgroups (Table 4): del(17p) (4.6 versus 1.1 months; HR, 0.34; P<0.001), t(4;14) (2.8 versus 1.9 months; HR, 0.49; P=0.028), and standard-risk (4.2 versus 2.3 months; HR, 0.55; P<0.001). The overall survival of patients treated with pomalidomide + LoDEX was longer than that of patients treated with HiDEX in all groups: the difference was statistically significant for patients with del(17p) (12.6 versus 7.7 months; HR, 0.45; P=0.008) and showed a trend for those with t(4;14) (7.5 versus 4.9 months; HR, 1.12; P=0.761) and standard-risk patients (14.0 versus 9.0 months; HR, 0.85; P=0.380). The overall survival benefit was achieved despite high numbers of HiDEX patients subsequently receiving pomalidomide: 46% of del(17p)/t(4;14) and 64% of standard-risk patients.
Table 4.
Progression-free and overall survival by cytogenetic risk group.
An analysis of the impact of del(17p) and t(4;14) within the HiDEX arm showed that these cytogenetic abnormalities are associated with worsened outcomes. The median progression-free survival was reduced from 2.3 months for standard-risk patients to 1.1 months for those with del(17p) or 1.9 months for those with t(4;14) (Figure 2B; P=0.023 and 0.161, respectively). The median overall survival was similarly reduced, from 9.0 months for standard-risk patients to 7.7 months for those with del(17p) or 4.9 months for patients with t(4;14) (Figure 3B; P=0.019 and 0.516, respectively).
Figure 2.
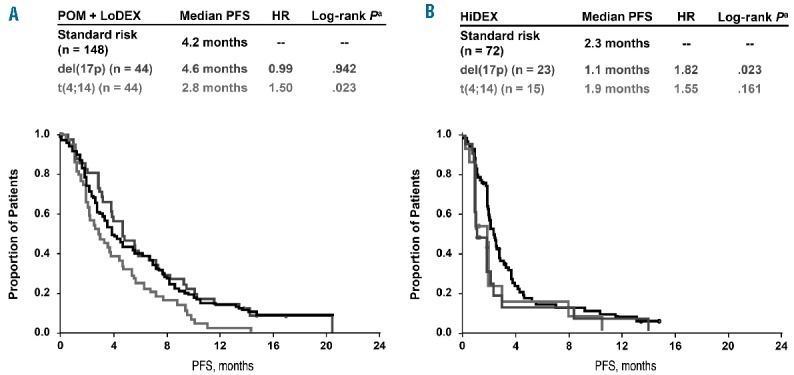
Kaplan-Meier curves of progression-free survival by cytogenetic risk groups for patients treated with (A) POM + LoDEX and (B) HiDEX. aLog-rank P vs. standard risk. HiDEX: high-dose dexamethasone; HR: hazard ratio; LoDEX: low-dose dexamethasone; PFS: progression-free survival; POM: pomalidomide.
Figure 3.
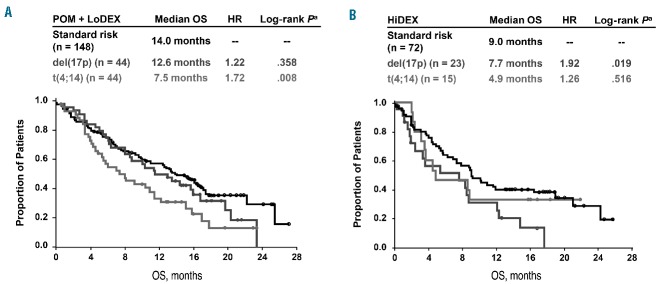
Kaplan-Meier curves of overall survival (OS) by cytogenetic risk groups for patients treated with (A) POM + LoDEX and (B) HiDEX. aLog-rank P vs. standard risk. HiDEX: high-dose dexamethasone; HR: hazard ratio; LoDEX: low-dose dexamethasone; POM: pomalidomide.
Among the patients treated with pomalidomide + LoDEX, the outcomes in those with del(17p) are similar to those in patients with standard-risk, with a progression-free survival of 4.6 months versus 4.2 months (HR, 0.99; P=0.942) and overall survival of 12.6 months versus 14.0 months (HR, 1.22; P=0.358). The presence of t(4;14) was a significant negative predictor, shortening progression-free survival to 2.8 months (HR, 1.50; P=0.023 versus standard-risk) and overall survival to 7.5 months (HR, 1.72; P=0.008 versus standard-risk).
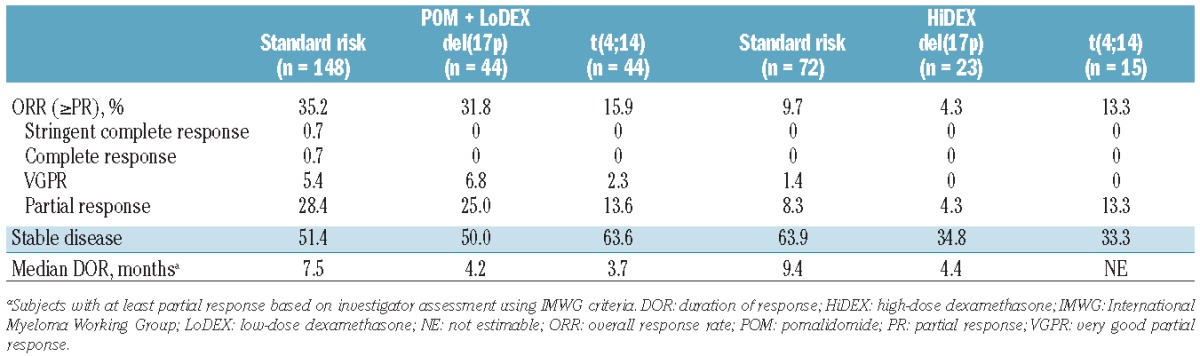
Response to pomalidomide + LoDEX treatment differed dramatically between cytogenetic risk groups (Table 5). Patients with del(17p) treated with pomalidomide + LoDEX had an overall response rate similar to that in patients with standard cytogenetic risk (31.8% in comparison with 35.1%), whereas the response rate was much lower in patients with t(4;14) (15.9%). The rate of response to HiDEX was somewhat lower in patients with del(17p) (4.3%) than in patients with standard cytogenetic risk (9.7%), while patients with t(4;14) were slightly more likely to respond (13.3%). Response to HiDEX was numerically lower than to pomalidomide + LoDEX in each cytogenetic risk group.
Table 5.
Response to treatment (IMWG criteria).
Subsequent treatments
Approximately half (33/63) of the patients treated with pomalidomide + LoDEX who progressed following achievement of a partial response or better were treated with at least one subsequent antimyeloma therapy. The median time from progression to next therapy was 1.8 months (range, 0.02–7.8 months). The most common treatment regimens were bortezomib (30%), lenalidomide (21%), and thalidomide (15%); a wide variety of therapies were used demonstrating that a standard of care is not well established for these patients with advanced disease. Further survival was generally short, with only half (32/63) surviving 6 months following progression on pomalidomide + LoDEX.
Discussion
MM-003 was the first randomized phase 3 clinical trial in refractory or relapsed and refractory multiple myeloma patients who had failed therapy with bortezomib and lenalidomide. This updated analysis with 15.4 months of follow-up confirmed the previously reported overall survival benefit with pomalidomide + LoDEX compared with HiDEX (median 13.1 versus 8.1 months; P=0.009).17 Importantly, with the extended follow-up, the Kaplan-Meier overall survival curves continued to diverge in favor of pomalidomide + LoDEX, even though 56% of patients randomized to the HiDEX arm subsequently received pomalidomide-based therapy. These results support early use of pomalidomide + LoDEX following exhaustion of bortezomib- and lenalidomide-based options.17 In this long-term follow-up, the median overall survival of 13.1 months in patients treated with pomalidomide + LoDEX is consistent with that recently reported with pomalidomide + LoDEX in the MM-002 trial (16.5 months),15 compares favorably with historical controls (9 months),4 and is similar to that reported with carfilzomib (15.6 months)21 in comparable populations of patients.
The updated safety profile of pomalidomide + LoDEX is consistent with that reported in the initial MM-003 publication and previous trials.15,17,22 The extended data demonstrate that adverse events are manageable after long-term treatment with pomalidomide + LoDEX. Patients who continue treatment for 1 year or longer exhibit notably lower rates of grade 3/4 anemia and thrombocytopenia, which is consistent with the finding that higher hemoglobin and platelet levels were associated with longer survival in multivariate analysis.
Features associated with worse prognosis have not been thoroughly evaluated in advanced relapsed/refractory multiple myeloma. A study conducted by the IMWG found that higher β2-microglobulin and lower serum albumin levels correlated with poorer prognosis in a multivariate model.4 Multivariate analysis of patients treated with pomalidomide + LoDEX within MM-003 showed that normal lactate dehydrogenase level, higher hemoglobin and platelet levels, lower ECOG performance status, and absence of plasmacytoma were significantly associated with longer survival and that normal lactate dehydrogenase level, higher platelet count, and lower ECOG performance status were also significantly associated with longer duration of response. These results confirmed that patients’ characteristics can significantly predict outcomes even in heavily pretreated patients.
The importance of high-risk cytogenetic features in advanced relapsed/refractory multiple myeloma has not been clearly defined. In patients in whom novel agent therapy failed, the IMWG found that t(4;14) at diagnosis contributed to shorter overall survival (HR, 2.14; P=0.086) but did not find that del(17p) was specifically associated with shorter overall survival or event-free survival.4 It is important to note that limited data regarding cytogenetic status and FISH results were available for the IMWG study.4 In particular, information regarding the prognostic value of cytogenetic status at the time of relapse is currently lacking. The large advanced relapsed/refractory multiple myeloma population included in MM-003 makes it a valuable source to assess the impact of cytogenetic abnormalities. By Kaplan-Meier analysis, del(17p) was found here to be a significant negative predictor of progression-free survival and overall survival in patients treated without newer agents (ie, HiDEX); t(4;14) also appeared to predict shortened progression-free survival and overall survival in these patients, although the effect was not statistically significant.
This analysis has shown that pomalidomide + LoDEX was of benefit to patients in the in MM-003 trial regardless of their cytogenetic risk profile. The progression-free survival advantages observed with pomalidomide + LoDEX versus HiDEX in patients with del(17p) or t(4;14) (HR, 0.34 and 0.49, respectively) were similar to those for patients with standard-risk cytogenetics (HR, 0.55) and the overall study population (HR, 0.49). The benefits of pomalidomide + LoDEX treatment were particularly dramatic in patients with del(17p), who achieved outcomes statistically indistinguishable from standard-risk patients (progression-free survival: 4.6 versus 4.2 months; P=0.942; overall survival: 12.6 versus 14.0 months; P=0.358). The French IFM 2010-02 trial of pomalidomide + LoDEX in patients with del(17p) and/or t(4;14) reported similar findings.16 In this study, the median time to progression (7.3 versus 2.8 months) and overall survival (12 versus 9.2 months) appeared longer for patients with del(17p) than in those with t(4;14).16 However, 75% of the patients in the MM-003 trial were refractory to both bortezomib and lenalidomide, whereas 54% were so in the French trial.17,23
Clinical trials of newer agents suggest that these may overcome the poor prognosis associated with specific cytogenetic abnormalities. A recent single-arm trial of carfilzomib analyzed patients with relapsed/refractory multiple myeloma and high-risk cytogenetic abnormalities [defined as del(13) or hypodiploidy by metaphase cytogenetic analysis and/or del(17p13), t(4;14), or t(14;16) by interphase FISH]. In this study, progression-free survival was not significantly reduced compared with that for standard-risk patients (median 3.5 versus 4.6 months; P=0.06).12 It is notable that the cytogenetic profile predictive of benefiting from carfilzomib differs from that reported for pomalidomide + LoDEX.12 The median progression-free survival and overall survival were shorter in carfilzomib-treated patients with del(17p) than in patients with t(4;14) (median progression-free survival, 2.8 versus 4.1 months; overall survival, 7.0 versus 11.8 months),12 whereas pomalidomide + LoDEX–treated patients with del(17p) fared better than those with t(4;14). The percentages of patients in the carfilzomib trial positive for del(17p) and t(4;14) (18% and 11%, respectively) were similar to those in the MM-003 trial.12
Data regarding the interactions of del(17p) or t(4;14) with bortezomib treatment are inconsistent. In a small pilot study of patients with early relapsed or refractory multiple myeloma, t(4;14) had no impact on response to bortezomib treatment, but the effect of del(17p) was not examined.24 In newly diagnosed multiple myeloma, bortezomib-containing induction regimens were shown to significantly improve prognosis and survival for patients with t(4;14) but not those with del(17p) in IFM 2005-01;25 however, the HOVON-65 trial showed that the addition of bortezomib to induction was beneficial in the presence of del(17p) but not t(4;14).26 The landmark GIMEMA trial of bortezomib-thalidomide-dexamethasone induction found that patients with t(4;14) benefited from the addition of bortezomib, but the trial did not contain enough del(17p) patients for analysis.27 In contrast, in the Total Therapy 3 protocol, the addition of bortezomib to thalidomide and dexamethasone negated the adverse consequences of del(17p) in newly diagnosed multiple myeloma patients who were low-risk according to their gene expression profile.28 By comparison, results from MM-003 and IFM 2010-02 are consistent in demonstrating that pomalidomide may overcome the poor outcomes typically associated with del(17p).16
In conclusion, these updated results for MM-003 confirm the previous observations that pomalidomide + LoDEX significantly improved progression-free survival, overall survival, and response compared with HiDEX in patients with refractory or relapsed and refractory multiple myeloma. Patients with standard-risk cytogenetic profiles or those with del(17p) or t(4;14) all gain significantly extended progression-free survival and some degree of overall survival benefit from pomalidomide + LoDEX vs HiDEX. These results support the use of pomalidomide + LoDEX as a standard of care in patients with relapsed/refractory multiple myeloma following exhaustion of treatment options with bortezomib and lenalidomide, including patients with the high-risk cytogenetic abnormalities del(17p) and t(4;14).
Acknowledgments
This study was funded by Celgene Corporation. The authors would like to thank the patients who volunteered to participate in this study, staff members at the study sites who cared for them, and the representatives of the sponsors who were involved in data gathering and analyses. We also thank Richard Balzer, PhD, and Nicola Hanson, PhD, from MediTech Media for writing assistance, which was funded by Celgene Corporation.
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Palumbo A, Rajkumar SV, San Miguel JF, et al. International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol. 2014;32(6):587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelhardt M, Terpos E, Kleber M, et al. European Myeloma Network recommendations on the evaluation and treatment of newly diagnosed patients with multiple myeloma. Haematologica. 2014;99(2):232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pulte D, Gondos A, Brenner H. Improvement in survival of older adults with multiple myeloma: results of an updated period analysis of SEER data. Oncologist. 2011;16(11):1600–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter International Myeloma Working Group study. Leukemia. 2012;26(1):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avet-Loiseau H, Attal M, Moreau P, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myélome. Blood. 2007;109(8):3489–3495. [DOI] [PubMed] [Google Scholar]
- 6.Avet-Loiseau H, Hulin C, Campion L, et al. Chromosomal abnormalities are major prognostic factors in elderly patients with multiple myeloma: the Intergroupe Francophone du Myélome experience. J Clin Oncol. 2013;31(22):2806–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avet-Loiseau H, Soulier J, Fermand JP, et al. Impact of high-risk cytogenetics and prior therapy on outcomes in patients with advanced relapsed or refractory multiple myeloma treated with lenalidomide plus dexamethasone. Leukemia. 2010;24(3):623–628. [DOI] [PubMed] [Google Scholar]
- 8.Chesi M, Nardini E, Brents LA, et al. Frequent translocation t(4;14)(p16.3;q32.3) in multiple myeloma is associated with increased expression and activating mutations of fibroblast growth factor receptor 3. Nat Genet. 1997;16(3):260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chesi M, Nardini E, Lim RS, Smith KD, Kuehl WM, Bergsagel PL. The t(4;14) translocation in myeloma dysregulates both FGFR3 and a novel gene, MMSET, resulting in IgH/MMSET hybrid transcripts. Blood. 1998;92(9):3025–3034. [PubMed] [Google Scholar]
- 10.Chesi M, Brents LA, Ely SA, et al. Activated fibroblast growth factor receptor 3 is an oncogene that contributes to tumor progression in multiple myeloma. Blood. 2001;97(3):729–736. [DOI] [PubMed] [Google Scholar]
- 11.Lode L, Eveillard M, Trichet V, et al. Mutations in TP53 are exclusively associated with del(17p) in multiple myeloma. Haematologica. 2010;95(11):1973–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakubowiak AJ, Siegel DS, Martin T, et al. Treatment outcomes in patients with relapsed and refractory multiple myeloma and high-risk cytogenetics receiving single-agent carfilzomib in the PX-171-003-A1 study. Leukemia. 2013;27(12):2351–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein U, Jauch A, Hielscher T, et al. Chromosomal aberrations +1q21 and del(17p13) predict survival in patients with recurrent multiple myeloma treated with lenalidomide and dexamethasone. Cancer. 2011;117(10):2136–2144. [DOI] [PubMed] [Google Scholar]
- 14.Quach H, Ritchie D, Stewart AK, et al. Mechanism of action of immunomodulatory drugs (IMiDs) in multiple myeloma. Leukemia. 2010;24(1):22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson PG, Siegel DS, Vij R, et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood. 2014;123(12):1826–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leleu X, Karlin L, Macro M, et al. Pomalidomide plus low-dose dexamethasone in multiple myeloma with deletion 17p and/or translocation (4;14): IFM 2010-02 trial results. Blood. 2015;125(9):1411–1417. [DOI] [PubMed] [Google Scholar]
- 17.San Miguel J, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(11):1055–1066. [DOI] [PubMed] [Google Scholar]
- 18.Durie BG, Harousseau J, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9): 1467–1473. [DOI] [PubMed] [Google Scholar]
- 19.Bladé J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102(5): 1115–1123. [DOI] [PubMed] [Google Scholar]
- 20.Hartmann L, Biggerstaff JS, Chapman DB, et al. Detection of genomic abnormalities in multiple myeloma: the application of FISH analysis in combination with various plasma cell enrichment techniques. Am J Clin Pathol. 2011;136(5):712–720. [DOI] [PubMed] [Google Scholar]
- 21.Siegel DS, Martin T, Singhal S, et al. Response rates to single-agent carfilzomib in patients refractory or intolerant to both bortezomib and immunomodulators in trial PX-171-003-A1. J Clin Oncol. 2012;30(15 suppl):518s Abstract 8035. [Google Scholar]
- 22.Leleu X, Attal M, Arnulf B, et al. Pomalidomide plus low dose dexamethasone is active and well tolerated in bortezomib and lenalidomide-refractory multiple myeloma: Intergroupe Francophone du Myélome 2009–02. Blood. 2013;121(11): 1968–1975. [DOI] [PubMed] [Google Scholar]
- 23.Leleu X, Karlin L, Macro M, et al. Pomalidomide plus low-dose dexamethasone in relapsed or refractory multiple myeloma (RRMM) with deletion (del)17p and/or translocation t(4;14). Blood. 2013;122(21):Abstract 689. [Google Scholar]
- 24.Chang H, Trieu Y, Qi X, Xu W, Stewart KA, Reece D. Bortezomib therapy response is independent of cytogenetic abnormalities in relapsed/refractory multiple myeloma. Leuk Res. 2007;31(6):779–782. [DOI] [PubMed] [Google Scholar]
- 25.Avet-Loiseau H, Leleu X, Roussel M, et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p). J Clin Oncol. 2010;28(30):4630–4634. [DOI] [PubMed] [Google Scholar]
- 26.Neben K, Lokhorst HM, Jauch A, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119(4):940–948. [DOI] [PubMed] [Google Scholar]
- 27.Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376(9758):2075–2085. [DOI] [PubMed] [Google Scholar]
- 28.Shaughnessy JD, Zhou Y, Haessler J, et al. TP53 deletion is not an adverse feature in multiple myeloma treated with total therapy 3. Br J Haematol. 2009;147(3):347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]


