Philadelphia chromosome negative myeloproliferative neoplasms (MPNs) are a group of clonal neoplastic diseases originating from the myeloid lineage. MPNs include polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF).1 Most MPNs are sporadic but familial clustering does occur.1–3 The gain of function mutation of the JAK2 tyrosine kinase gene (JAK2V617F), a key disease driver, is seen in both sporadic and familial cases in approximately 95% of PV, 60% of ET and 50% of PMF patients, respectively.3,4 In addition, other oncogenic mutations and cytogenetic alterations are frequently observed in MPNs, suggesting a significant genomic instability.4 An earlier report indicated that the JAK2V617F mutation was responsible for these genetic alterations, while other studies demonstrated the same scenarios in cells without JAK2V617F.3,4 Taken together, it is probable that there are as yet undiscovered common mechanisms, which trigger the genomic instability in MPNs regardless of JAK2 status.
Telomeres are nucleoprotein complexes mainly consisting of telomeric DNA and 6 shelterin proteins (TRF1, TRF2, TPP1, POT1, TIN2 and RAP1), essential for chromosome-end protection.5–8 Telomere dysfunction is an early event triggering genomic instability in tumorigenesis.5 MPNs exhibit genetic alterations with an indolent clinical course. Earlier studies have shown shorter telomeres in leukocytes of patients with MPNs.2,9–11 However, it is still not clear whether the shelterin protein expression is altered and associated with telomere shortening in MPNs. The present study was designed to address these issues.
We determined telomere length (TL), shelterin protein expression and telomerase activation in a cohort of 81 MPN patients and in 43 healthy adult controls. Characteristics of patients and healthy individuals are presented in Table 1. As this group of disorders involves the myeloid lineage, we collected both peripheral blood granulocytes and lymphoid cells from patients and healthy controls, and determined TL in these cells using flow-FISH (Figure 1A) (Online Supplementary Appendix).10,12 TL in granulocytes was significantly shorter in MPN patients than in healthy controls (mean±SD, same below: 7.27 kb±2.11 vs. 8.66 kb±1.82; P<0.001) (Figure 1B). As TL is highly age-dependent (r = −0.34; P<0.001) (Online Supplementary Figure S1), we made a further comparison after correcting for the influence of age, in which the significant difference in TL between patients and healthy controls was maintained (P=0.046). In contrast, there was no difference in TL of lymphoid cells between patients and healthy controls (8.38 kb±2.08 vs. 8.67 kb±1.89; P=0.437) (Figure 1C). Furthermore, patients’ granulocytes exhibited significantly shorter TL than that of their own lymphoid cells (P=0.001) (Figure 1D), whereas TL did not differ between controls’ granulocytes and lymphoid cells (8.66 kb±1.82 vs. 8.67 kb±1.89; P=0.976) (Figure 1E). These results suggest that accelerated telomere attrition was restricted to myeloid cells of MPN patients, consistent with previous reports.10,11
Table 1.
Summary of clinical features of patients with myeloproliferative neoplasm and healthy controls.
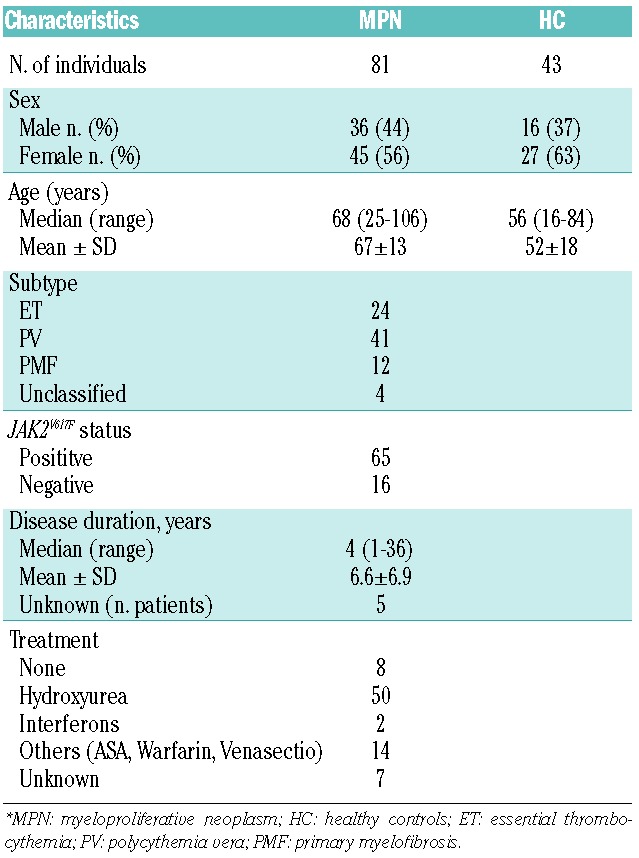
Figure 1.
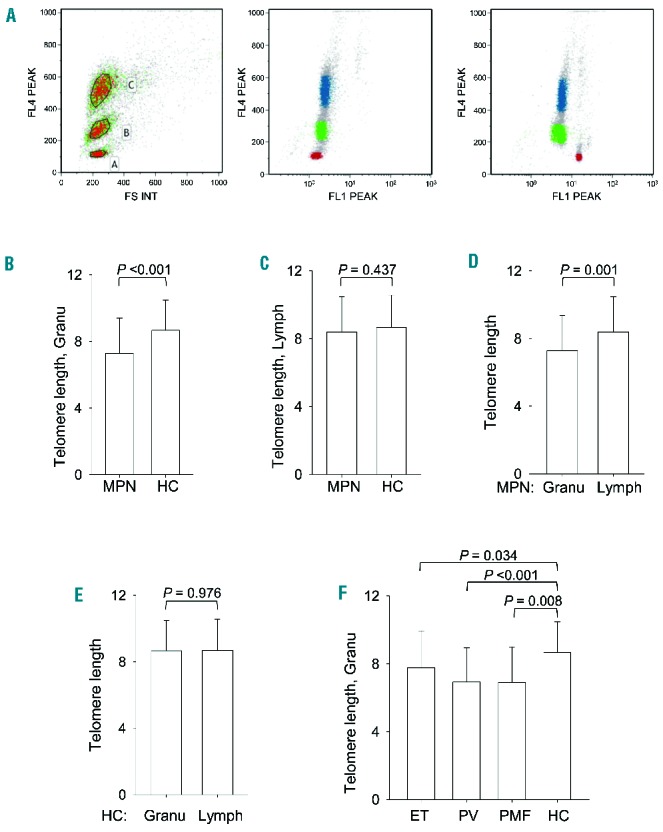
Flow-FISH assessment of telomere length in blood cells from MPN patients. (A) (Left) Forward scatter on the x-axis and cell cycle stain with LDS 751dsDNS staining, detectable in FL4, on the y-axis. Cell populations were gated as shown, where gate A includes calf thymocytes (red in middle and right panels), gate B human mononuclear cells (green in middle and right panels) and gate C human granulocytes (blue in middle and right panels). (Middle) Whole blood sample hybridized without telomeric probe. (Right) Whole blood sample hybridized with FITC-labeled telomeric probe. (B–F) Telomere length (kB) in granulocytes (Granu) and lymphoid cells (Lymph) derived from patients with a myeloproliferative neoplasm (MPN) and healthy controls (HC). Telomere length (kilobase, KB).
To validate the TL assay using FLOW-FISH, we further employed a quantitative PCR (qPCR) to determine TL in granulocytes derived from 26 patients or healthy individuals (Online Supplementary Appendix). FLOW-FISH and qPCR showed highly consistent TL results in all 26 samples (r = 0.811; P<0.001) (Online Supplementary Figure S2). When TL in granulocytes was compared among MPN subtypes, PV patients had significantly shorter telomeres in their granulocytes compared to controls (6.92 kb±2.02 vs. 8.66 kb±1.82; P<0.001) (Figure 1F). Similarly, significantly shorter granulocyte telomeres were seen in ET and PMF patients (7.77 kb±2.17 vs. 8.66 kb±1.82 and 6.90 kb±2.09 vs. 8.66 kb±1.82; P=0.034 and 0.008, respectively) (Figure 1F). In PV patients, TL was still significantly reduced compared to controls (P=0.025) after adjustment for age. However, the difference in TL between controls and ET and PMF patients, respectively, did not reach statistical significance after age adjustment. Other clinical variables, such as sex and disease duration, were not associated with granulocyte TL in MPN patients, while TL was negatively associated with JAK2V617F burdens (Online Supplementary Figure S3) (P<0.001). There was no significant difference in TL among PV, ET and PMF patients.
We then determined whether the expression of shelterin factors was altered in patient granulocytes. mRNA levels of all 6 shelterin proteins were analyzed using qPCR (Figure 2) (Online Supplementary Appendix). There were no differences in TRF1 or TRF2 mRNA expression between healthy individuals and MPN patients (1.14±1.62 vs. 1.0±1.27, P=0.997 for TRF1 and 1.0 ± 0.43 vs. 0.93 ± 0.26, P=0.733 for TRF2). There was a higher expression of POT1 (1.54±0.56 vs. 1.0±0.47; P<0.001) and TIN2 (1.20±0.33 vs. 1.0±0.31; P<0.001) in the granulocytes of MPN patients compared to those of healthy controls (Figure 2A and B). When dividing patients into subtypes, POT1 expression remained significantly higher in all the 3 subtypes compared to healthy controls (see Figure 2A and B for comparison with ET, PV and PMF; P=0.03, <0.001 and <0.001, respectively). TIN2 expression was significantly higher in PV and PMF patients (P<0.001 and P<0.03, respectively), but not in patients with ET (Figure 2B). In contrast, RAP1 expression was down-regulated in MPNs as compared to controls (0.69±0.18 vs. 1.0 ± 0.35; vs. <0.001). RAP1 mRNA levels were reduced in all 3 MPN subtypes (P<0.001, P<0.001, and P=0.015 for ET, PV and PMF, respectively) (Figure 2C). TPP1 expression was significantly lower in patients with PV as compared to controls (0.78±0.28 vs. 1.0±0.39; P=0.004) (Figure 2D). In addition, hydroxyurea treatment was negatively associated with a lower expression of TPP1 but not others (Online Supplementary Figure S4). We further analyzed the protein expression of POT1, TIN2 and TPP1 in a subset of patients and controls (Online Supplementary Appendix). POT1 protein levels were significantly higher in granulocytes from MPN patients than in those from healthy individuals (P=0.007) (Figure 2E and G). TIN2 protein expression seems higher in patients; however, its expression in the controls was too weak to perform a reliable quantitative comparison (Figure 2G). The protein expression of TPP1 was lower in MPN patients (P<0.001) (Figure 2F and G). These results demonstrate concordant mRNA and protein alterations of shelterin factors in MPN patients. We observed that the JAK2 mutation was positively associated with POT1 expression, whereas it did not correlate with the expression of the other five shelterin proteins. POT1 mRNA levels were higher in patients carrying the JAK2V617F mutation (P=0.038) (Figure 2H). Furthermore, POT1 expression tended to correlate with JAK2V617F allele burdens (Figure 2I) (P=0.08) based on the analysis of a limited patient number; this should be confirmed in a larger patient group. To determine whether the elevated POT1 expression was driven by the JAK2V617F, we performed experiments with a JAK2 inhibitor on the JAK2V617F+/+ cell line HEL. Significantly diminished levels of POT1 mRNA were observed in HEL cells treated with LY2784544 (P=0.001), accompanied by its protein downregulation (Figure 2J and K). In contrast, LY2784544 did not inhibit POT1 expression in wt JAK2-carrying leukemic K562 cells.
Figure 2.
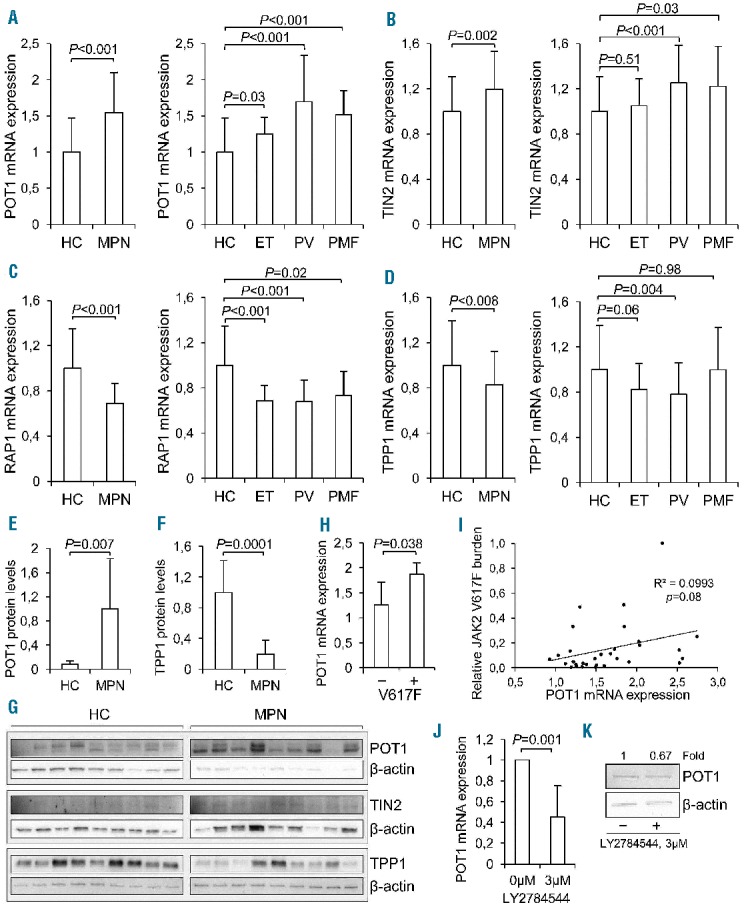
mRNA expression of POT1, TIN2, RAP1 and TPP1 in granulocytes derived from patients with myeloproliferative neoplasm (MPN) and JAK2V617F-dependent POT1 upregulation. (A and D) mRNA levels of each shelterin factor were analyzed with quantitative PCR and expressed arbitrarily based on the ratio of the target and β2-microglobin CT values. (A) POT1. (B) TIN2. (C) RAP1. (D) TPP1. (Left) Comparison between all MPNs and healthy controls, and right panels: Comparison between each subtype of MPNs and healthy controls. (E and F) Protein expression analyzed with western blot and signal quantified in ImageJ, (E) POT1, (F) TPP1. (G) Original protein blots of POT1, TIN2 and TPP1, and β–actin as loading reference. (H) POT1 mRNA in granulocytes from MPN patients with and without JAK2V617F mutation. (I) The correlation between JAK2V617F allele burdens and POT1 mRNA expression. (J–K) POT1 mRNA expression and protein expression in HEL erythroleukemia cell line (JAK2V617F+/+) cells treated with the JAK2-inhibitor LY2784544. Cells were exposed to LY2784544 (3 μM) for 24 hrs, RNA and protein was extracted and POT1 levels were then determined using qPCR and Western blotting, respectively. HC: healthy controls; MPN: myeloproliferative neoplasm; ET: essential thrombocythemia; PV: polycythemia vera; and PMF: primary myelofibrosis.
The induction of telomerase reverse transcriptase (TERT) and activation of telomerase, an RNA-dependent DNA polymerase synthesizing telomeric DNA, are essential in malignant transformation, and whether this happens in MPNs with an indolent course is controversial.13 We thus determined TERT mRNA levels using qPCR. There was no difference in TERT mRNA expression in granulocytes derived from MPN patients compared to those from healthy controls (1.29±1.54 vs. 1.0±0.88; P=0.35). Our finding suggests that telomerase remains silent in MPN cells.
In addition, KU80 is a DNA repair factor that has been shown to localize to telomeres, affect telomere length maintaining and prevent end joining.14 Therefore, we determined its expression in patient granulocytes. KU80 mRNA expression was found to be significantly higher in granulocytes derived from patients with MPN than in those from healthy individuals (1.28±0.31 vs. 1.0±0.28; P<0.001). KU80 mRNA levels were significantly positively correlated to TRF2, TPP1, POT1 and TIN2 expression, but negatively correlated to TL (data not shown).
In summary, we found a significantly reduced TL in granulocytes from patients with MPNs compared to healthy controls. More importantly, levels of POT1 and TIN2 expression, two shelterin proteins negatively regulating TL, were significantly elevated, whereas two other shelterin proteins, TPP1 and RAP1 having a telomere-lengthening function, were down-regulated in MPN granulocytes. POT1 expression was positively correlated with the presence of JAK2V617F in MPN patients and JAK2 inhibitors suppressed POT1 expression in JAK2V617F -harboring cells. These results collectively reveal widespread dysregulation of shelterin factors in MPNs. The abnormal expression of shelterin factors may contribute to accelerated telomere erosion, thereby driving genetic aberrations and pathogenesis of MPNs. Recently, imetelstat, a small oligonucleotide that mimics telomeric DNA sequences and thereby targets telomere structure and telomerase activity, has been used to treat MPNs and the efficacy is very promising.15 Given the present finding, it is worth investigating whether expression changes in shelterin proteins may predict MPN patient response to imetelstat treatment or have other therapeutic implications.
Acknowledgments
We would like to thank the research nurses Petra Janeld and Sarianna Cortes-Wiig at the hematology centre, Karolinska University hospital for drawing blood from the patients, and to the technical staff Margareta Andersson and Selina Parvin in the hematology laboratory for preparing the blood upon arrival. We also thank the butchery Ö-slakt AB in Värmdö, Sweden, for kindly donating calf thymuses for this study. The study was supported by grants from the Adolf H. Lundin Charitable foundation, the regional agreement on medical training and clinical research between Stockholm County Council and Karolinska Institutet, Swedish Research Council, Swedish Cancer Society and Cancer Society in Stockholm.
Footnotes
The online version of this article has a Supplementary Appendix.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Fourth Edition. Lyon, France: IARC; 2008. [Google Scholar]
- 2.Rumi E, Passamonti F, Della Porta MG, et al. Familial chronic myeloproliferative disorders: clinical phenotype and evidence of disease anticipation. J Clin Oncol. 2007;25(35):5630–5635. [DOI] [PubMed] [Google Scholar]
- 3.Tefferi A, Skoda R, Vardiman JW. Myeloproliferative neoplasms: contemporary diagnosis using histology and genetics. Nat Rev Clin Oncol. 2009;6(11):627–637. [DOI] [PubMed] [Google Scholar]
- 4.Klampfl T, Harutyunyan A, Berg T, et al. Genome integrity of myeloproliferative neoplasms in chronic phase and during disease progression. Blood. 2011;118(1):167–176. [DOI] [PubMed] [Google Scholar]
- 5.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009; 361(24):2353–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xin H, Liu D, Songyang Z. The telosome/shelterin complex and its functions. Genome Biol. 2008;9(9):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinto A, Li H, Nicholls C, Liu J. Telomere protein complexes and interactions with telomerase in telomere maintenance. Front Biosci. 2011;16(1):187–207. [DOI] [PubMed] [Google Scholar]
- 8.Kong F, Zheng C, Xu D. Telomerase as a “stemness” enzyme. Sci China Life Sci. 2014;57(6):564–570. [DOI] [PubMed] [Google Scholar]
- 9.Bernard L, Belisle C, Mollica L, et al. Telomere length is severely and similarly reduced in JAK2V617F-positive and -negative myeloproliferative neoplasms. Leukemia. 2009;23(2):287–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elena C, Rumi E, Portolan M, Della Porta MG, Pascutto C, Passamonti F. Flow-FISH evaluation of telomere length in Philadelphia-negative myeloproliferative neoplasms. Haematologica. 2011;96(8):1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terasaki Y, Okumura H, Ohtake S, Nakao S. Accelerated telomere length shortening in granulocytes: a diagnostic marker for myeloproliferative diseases. Exp Hematol. 2002;30(12):1399–1404. [DOI] [PubMed] [Google Scholar]
- 12.Roos G, Hultdin M. Flow cytometric determination of telomere length. Cytometry. 2001;45(1):79–80. [DOI] [PubMed] [Google Scholar]
- 13.Bock O, Serinsoz E, Schlue J, Kreipe H. Different expression levels of the telomerase catalytic subunit hTERT in myeloproliferative and myelodysplastic diseases. Leuk Res. 2004;28(5):457–460. [DOI] [PubMed] [Google Scholar]
- 14.Hsu HL, Gilley D, Galande SA, et al. Ku acts in a unique way at the mammalian telomere to prevent end joining. Genes Dev. 2000; 14(22):2807–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams CS. No end in sight for telomerase-targeted cancer drugs. Nat Med. 2013; 19(1):6. [DOI] [PubMed] [Google Scholar]


