Although environmental factors such as cytokines and stroma are recognized to influence the growth and chemosensitivity of acute myeloid leukemia (AML) cells, little is known about the effect of physical forces such as pressure. The pressure in the marrow of AML patients can be 10–20 fold higher compared to patients with solid tumors or non-malignant conditions,1 possibly due to increased cellularity and fibrosis. Therefore, we sought to establish whether the pressure in the marrow is another environmental factor that can influence chemosensitivity. Using AML cell lines and primary patient samples, we show that high pressure promotes a transition to a gel-like plasma membrane that reduces the intracellular accumulation of daunorubicin in AML cells. Therefore, biomechanical stimulus such as pressure is another environmental factor that can influence chemosensitivity.
We designed and manufactured pressure chambers to deliver different levels of physiological pressure to AML cells while maintaining continuous gas exchange (Online Supplementary Figure S1). Two AML cell lines, HL60 and TEX, were cultured at pressures up to 310 mmHg above atmospheric pressure (atm). A pressure of 310 mmHg above atm corresponds to less than 0.5% of the maximum pressure acting on the head of the femur of an upright 160 lb person, and therefore, would be physiologically relevant as erosion of the trabeculae from leukemic infiltration increases sensitivity to external forces.1,2 We found that the growth and viability of these cell lines did not change at high pressure (Figure 1A). Next, we explored the impact of increased pressure on the sensitivity of these cells to three anthracyclines used in the treatment of AML: daunorubicin, idarubicin, and mitoxantrone.3,4 HL60 and TEX cells grown at increased static pressure were more resistant to daunorubicin, (Figure 1B), but not idarubicin (Figure 1C) or mitoxantrone (Figure 1D and Online Supplementary Figure S2). Furthermore, the effects of increased pressure on chemosensitivity were reversible.
Figure 1.
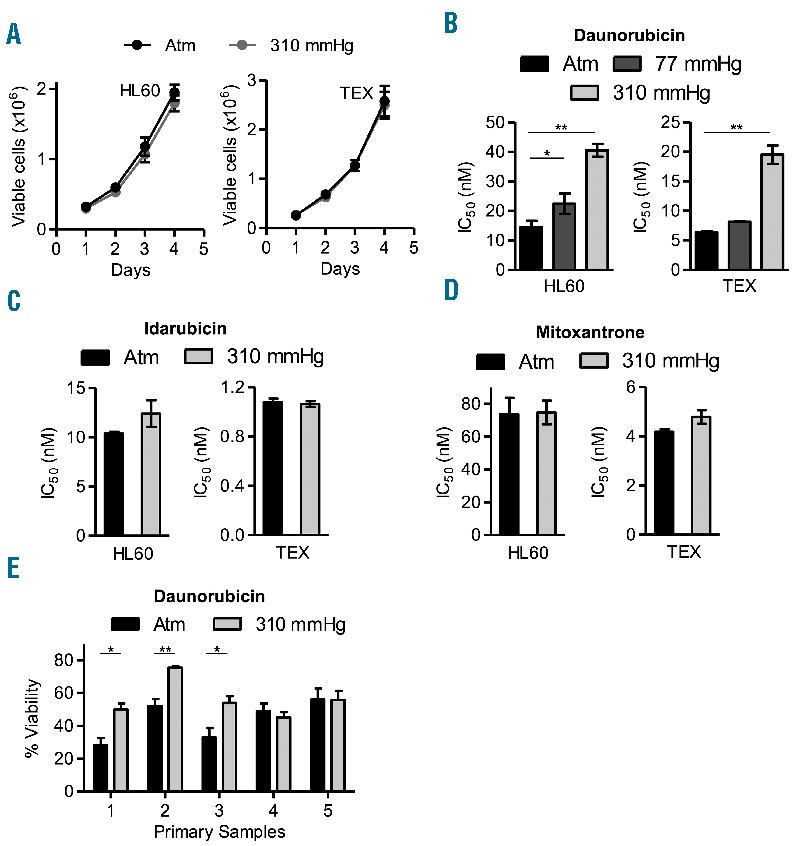
Acute myeloid leukemia (AML) cells at increased pressure display chemoresistance to danorubicin. (A). Cell viability measured by trypan blue exclusion assay of HL60 and TEX leukemia cells cultured over 4 days at 37°C at atmospheric pressure (atm) and 310 mmHg above atm. (B). HL60 and TEX cells were cultured at 37°C at atm and 77 mmHg and 310 mmHg above atm for three days, and then treated with increasing concentrations of daunorubicin at indicated pressures for another three days. Cell viability was determined by Celltiter Fluor assay. *P<0.05 and **P<0.0001 as determined by one-way ANOVA with Bonferroni post-test. (C and D). HL60 and TEX cells were cultured at 37°C at atm and 310 mmHg above atm for three days, and then treated with increasing concentrations of idarubicin (D) and mitoxantrone (E) at the same pressures for another three days. Cell viability was determined by Celltiter Fluor assay and IC50 were calculated. (E) Patient samples were cultured at 37°C at atm and 310 mmHg above atm for three days, and then treated with 250 nM daunorubicin for three days at the same pressure levels. Cell viability was determined by Celltiter Fluor assay. *P<0.01 and **P<0.001 as determined by Student t-test. In all panels, data represent the mean ± standard deviation of three independent experiments.
We also tested the impact of increased static pressure on samples from 5 patients with acute leukemia (4=AML, 1=T-ALL) that were sensitive to daunorubicin (patients’ characteristics are shown in Online Supplementary Table S1). Similar to the cell lines, increased pressure did not alter the growth and viability of the primary cells, but 3 of 5 samples (3 of 4 AML samples) had reduced sensitivity to daunorubicin at high pressure (Figure 1E and Online Supplementary Figure S3).
To investigate the biological effects of increased pressure that may influence chemosensitivity, we measured the intracellular accumulation of daunorubicin. Compared to cells at atm, cells cultured at increased pressure accumulated less daunorubicin (Figure 2A and Online Supplementary Figure S4). In contrast, we observed no difference in the accumulation of [3H] mitoxantrone in TEX cells treated at increased pressure (Figure 2B).
Figure 2.
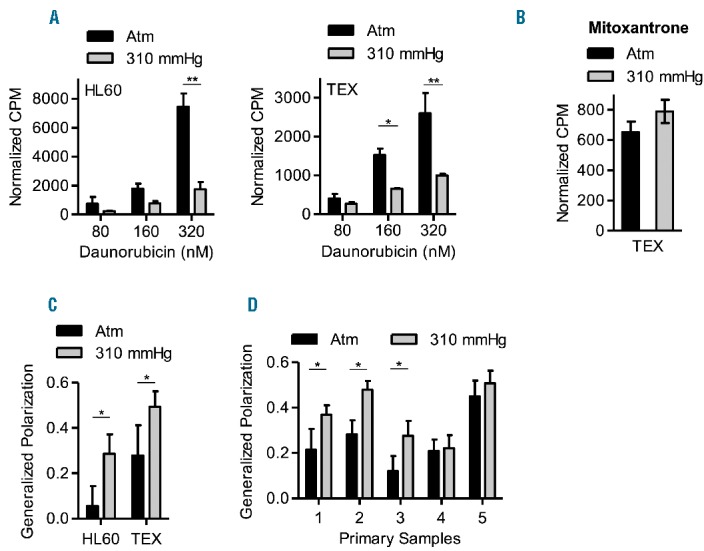
AML cells have reduced daunorubicin accumulation at increased pressure. (A). HL60 and TEX cells were cultured at 37°C at atm and 310 mmHg above atm for 3 days and then treated with 80, 160 and 320 nM of [3H] daunorubicin for 3 hours at the same pressure levels. Following treatment, intracellular levels of radiolabeled daunorubicin were measured using a scintillation counter. *P<0.01 and **P<0.0001 as determined by two-way ANOVA with Bonferroni post-tests. Mean ± SD, n=3. (B) TEX cells were cultured at 37°C at atm and 310 mmHg above atm for 3 days and then treated with 8 nM of [3H] mitoxantrone for 3 hours at the same pressure levels. Following treatment, intracellular levels of radiolabeled mitoxantrone were measured using a scintillation counter. Mean ± SD, n=3. (C–D). HL60, TEX, and primary AML patient cells (1 × 106 cells) were cultured at 37°C at atm and 310 mmHg above atm for 3 days, and then stained with 10 μM of the lipophilic probe, laurdan for 30 minutes. The emission state of laurdan was assessed using two-photon microscopy at 37°C, atm and within 10 minutes of removing from the pressure chamber. The fluorescence spectrum of laurdan is sensitive to the physical state of membrane phospholipids; therefore, when excited, laurdan emits at 440 nm when the membrane is in a gel-like state and at 490 nm when the membrane is in a liquid-crystalline phase. The generalized polarization (GP) factor was calculated as described in the Supplemental data. *P<0.0001 as determined by Student’s t-test. Data represent the mean ± SD of GP values from 30–60 cells.
No prior studies have investigated the impact of static pressure on plasma membrane dynamics in cancer cells. However, studies on red blood cells,5,6 bacterial membranes7 and synthetic lipid vesicles8,9 demonstrate that increased pressure alters membrane dynamics and influences drug and particle uptake. In these systems, the acyl chains of the phospholipids in the membrane straighten under increased pressure causing the membrane to become thicker and transition from a fluid liquid-crystalline state to a more solid gel-like state. The thicker gel-like membrane can reduce the permeability of the membrane to small molecules and can also impair the activity of membrane-associated drug uptake channels. Notably, these changes in membrane dynamics are rapidly reversible upon returning to normal pressure.
To determine if plasma membrane dynamics are altered in AML cells at increased pressure, HL60, TEX, and primary patient AML cells were cultured at high pressure and stained with the lipophilic probe, laurdan (6-dodecanoyl-2-dimethylaminonaphthalene) (see Online Supplementary Appendix for description of method). The fluorescence spectrum of laurdan is sensitive to the physical state of membrane phospholipids, and has been used to evaluate plasma membrane dynamics in red blood cells exposed to increased pressure6 and in K562 leukemia cells exposed to increased temperatures.10 Using two- photon microscopy at 37°C, atm, and within 10 min of removing from the pressure chamber, we calculated the generalized polarization (GP) factor of laurdan and observed an increase in the gel-like state of the membrane in both HL60 and TEX cells cultured at high pressure (Figure 2C). Moreover, primary AML cells from patients 1, 2, and 3 that demonstrated a change in chemosensitivity to daunorubicin at higher pressure also showed an increase in the gel-like state in their membrane at increased pressure. In contrast, primary cells from patients 4 (T-ALL) and 5 (AML) that did not display a change in chemosensitivity at increased pressure also showed no change in membrane dynamics at increased pressure (Figure 2D and Online Supplementary Figure S5). Thus, in a subset of AML cells, increased pressure promotes a transition to a gel-like membrane that can reduce the intracellular accumulation of select anthracyclines.
Cholesterol is an important regulator of plasma membrane structure and consistency, and the amount of cholesterol in the membrane can vary over 10-fold between cells. Prior studies have evaluated the impact of cholesterol content on membrane dynamics after exposure to biomechanical forces. For example, addition of cholesterol to endothelial cells decreased membrane fluidity and dampened the impact of sheer stress on the plasma membrane.11 Similarly, using synthetic lipid vesicles, as the amount of cholesterol in the vesicle increased, the membrane became more gel-like and little further change was observed upon exposure to increased pressure.12 Conversely, membranes with the least amount of cholesterol are the most liquid and show the greatest change in their membrane dynamics upon exposure to increased pressure. However, it is unknown whether cells alter their membrane composition in response to changes in biomechanical pressure.
It is worthy of note that the tested anthracyclines vary in their hydrophobicity. Of the three agents, daunorubicin has the highest logP (logarithm of partition coefficient) value of 1.83 indicating that it is the most hydrophobic, while idarubicin and mitoxantrone have lower logP values of 0.2 and -3.1, respectively. Changes in membrane dynamics would, therefore, preferentially affect the more hydrophobic daunorubicin compared to the other agents.
Our study is not without limitations. First, membrane phase transitions are generally rapidly reversible and the imaging was done at atm. Although we imaged the cells within 10 min of removal from the pressure chamber, some of the changes in membrane dynamics may have reversed before imaging could be completed. Second, the increased pressure could have decreased daunorubicin solubility and thus resulted in decreased sensitivity to the drug, although this is very unlikely as we did not detect precipitation of daunorubicin.
In summary, our study shows that increased static pressure renders AML cells resistant to daunorubicin by reducing intracellular drug levels, but does not affect sensitivity to idarubicin and mitoxantrone. Thus, these results highlight biomechanical stimuli such as pressure as another environmental factor that can influence chemosensitivity. Moreover, it suggests that some AML patients might benefit from chemotherapeutic regimens that contain anthracyclines such as idarubicin or mitoxantrone rather than daunorubicin.
Acknowledgments
We thank Jill Flewelling (Princess Margaret Cancer Centre) for administrative assistance.
Footnotes
Funding: this work was supported by the Ontario Institute of Cancer Research with funding provided by the Ontario Ministry of Research and Innovation, the Princess Margaret Cancer Centre Foundation, and the Ministry of Long Term Health and Planning in the Province of Ontario. A.D.S holds the Barbara Baker Chair in Leukemia and Related Diseases.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Petrakis NL. Bone marrow pressure in leukemic and non-leukemic patients. J Clin Invest. 1954;33(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodge WA, Fijan RS, Carlson KL, Burgess RG, Harris WH, Mann RW. Contact pressures in the human hip joint measured in vivo. Proc Natl Acad Sci U S A. 1986;83(9):2879–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohtake S, Miyawaki S, Fujita H, et al. Randomized study of induction therapy comparing standard-dose idarubicin with high-dose daunorubicin in adult patients with previously untreated acute myeloid leukemia: the JALSG AML201 Study. Blood. 2011;117(8):2358–2365. [DOI] [PubMed] [Google Scholar]
- 4.Gardin C, Chevret S, Pautas C, et al. Superior long-term outcome with idarubicin compared with high-dose daunorubicin in patients with acute myeloid leukemia age 50 years and older. J Clin Oncol. 2013;31(3):321–327. [DOI] [PubMed] [Google Scholar]
- 5.Barshtein G, Bergelson L, Dagan A, Gratton E, Yedgar S. Membrane lipid order of human red blood cells is altered by physiological levels of hydrostatic pressure. Am J Physiol. 1997;272(1 Pt 2):H538–H543. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez G, Celedon G, Escobar M, et al. Red cell membrane lipid changes at 3,500 m and on return to sea level. High Alt Med Biol. 2005;6(4):320–326. [DOI] [PubMed] [Google Scholar]
- 7.Ulmer HM, Herberhold H, Fahsel S, Ganzle MG, Winter R, Vogel RF. Effects of pressure-induced membrane phase transitions on inactivation of HorA, an ATP-dependent multidrug resistance transporter, in Lactobacillus plantarum. Appl Environ Microbiol. 2002;68(3):1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Periasamy N, Winter R. The effects of temperature, pressure and peptide incorporation on ternary model raft mixtures–a Laurdan fluorescence spectroscopy study. Biochim Biophys Acta. 2006;1764(3):398–404. [DOI] [PubMed] [Google Scholar]
- 9.Nicolini C, Kraineva J, Khurana M, Periasamy N, Funari SS, Winter R. Temperature and pressure effects on structural and conformational properties of POPC/SM/cholesterol model raft mixtures–a FT-IR, SAXS, DSC, PPC and Laurdan fluorescence spectroscopy study. Biochim Biophys Acta. 2006;1758(2):248–258. [DOI] [PubMed] [Google Scholar]
- 10.Balogh G, Maulucci G, Gombos I, et al. Heat stress causes spatially-distinct membrane re-modelling in K562 leukemia cells. PLoS One. 2011;6(6):e21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto K, Ando J. Endothelial cell and model membranes respond to shear stress by rapidly decreasing the order of their lipid phases. J Cell Sci. 2013;126(Pt 5):1227–1234. [DOI] [PubMed] [Google Scholar]
- 12.Winter R, Jeworrek C. Effect of pressure on membranes. Soft Matter. 2009;5:3157–3173. [Google Scholar]


