Leukemia cutis (LC), the infiltration of the epidermis, dermis, or subcutis with leukemia cells, complicates 5–10% of cases of acute myeloid leukemia (AML) in adults and is considered a marker of poor prognosis.1–3 While the association between AML with monocytic features and LC has been described, little is known about the association of other AML characteristics and LC.2,4,5 Recently, a number of recurrent gene mutations have been described in AML; however, the association of these mutations and LC has not been systematically investigated.6,7 Using amplicon-based next-generation sequencing (NGS) of a panel of recurrent, hematologic malignancy-associated mutations, we sought to determine the association between molecular markers and LC.
We identified 284 patients diagnosed with AML at the University of Pennsylvania (2001–2014) who had undergone targeted NGS analysis of 33 genes associated with hematologic malignancy;8 of these, 23 are recurrently mutated in AML and were studied (listed in Table 2). These 284 cases were identified either from the Hematologic Malignancies Tissue Bank at the University of Pennsylvania (2001–2013), or from a pathology database of patients tested in a clinical context (after February 2013). Using a clinical database of acute leukemia patients (February 2011 – August 2014) who were evaluated for NPM1 mutations by a targeted method, an additional 276 patients with known NPM1 status were identified. Redundant cases were excluded.
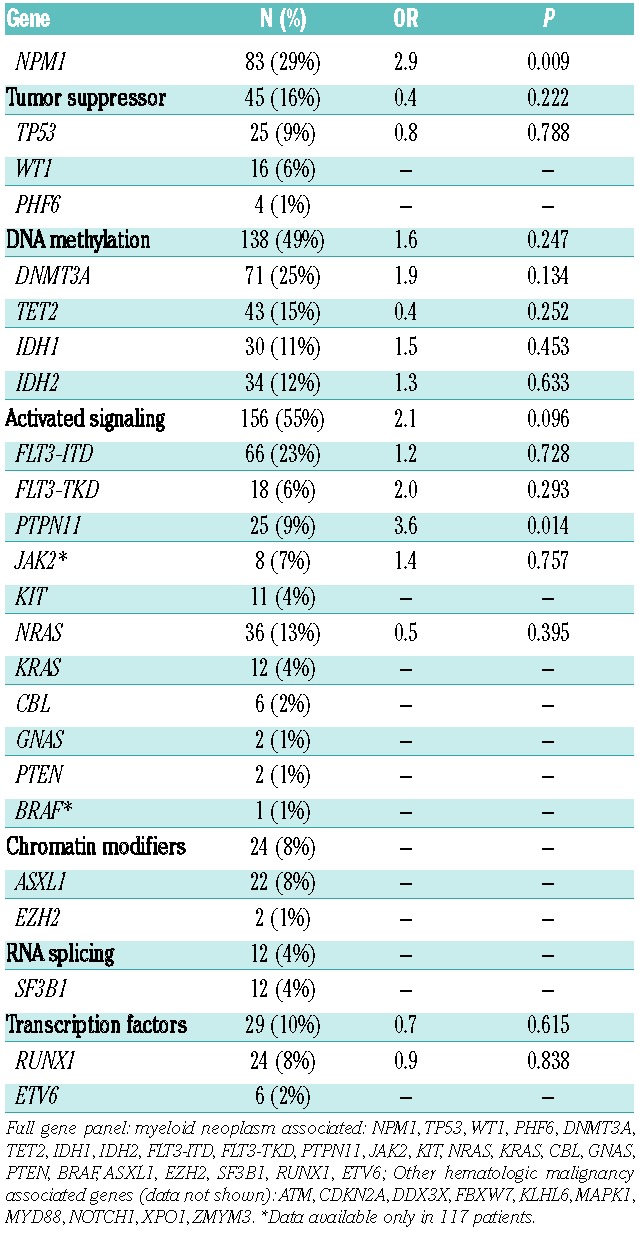
Table 2.
AML-Associated Somatic Mutations and Leukemia Cutis (n=284).
All cases of AML were confirmed by a hematopathologists review of the diagnostic material. The presence of monocytic features was determined by a combination of morphology and immunophenotypic analysis, as well as cytochemistry, as appropriate. For each AML patient, a dermatopathology database was reviewed to identify cases of skin biopsy-proven LC at any time during the disease course. Independent dermatopathology review was obtained for indeterminate cases of LC; cases still classified as indeterminate after re-review were excluded from the analysis. Information regarding clinical and disease characteristics was determined by review of the medical records. Targeted NGS testing of 33 genes associated with hematologic malignancies (including NPM1) was conducted by the Center for Personalized Diagnostics at the University of Pennsylvania. Average read depth was 3000X, minimal depth was 250x, and reporting frequency cutoff for variants was 5%.8 Mutations were classified into four categories: pathogenic, likely disease-associated, variant of uncertain significance (VUS), or likely benign based on review of publically available data; only pathogenic or likely disease-associated mutations were included in this analysis. Targeted NPM1 analysis was performed in the Department of Pathology at the University of Pennsylvania. The targeted NPM1 test consists of multiplex RT-PCR followed by detection on a liquid bead array. This assay allows for the simultaneous detection of the most common NPM1 mutations in exon 12 (type A, B, and D). The analytical sensitivity of the assay is approximately 0.01%. The Institutional Review Board of the University of Pennsylvania approved this research.
Patient and clinical characteristics were summarized by descriptive statistics. Association between the presence of mutation and LC was assessed by the chi-square test or logistic regression, stratified by monocytic subtype when appropriate. Only genes with a mutation frequency ≥ 5% (n=24) were assessed for association with LC. All statistical tests were two-sided, with P values <.05 considered statistically significant. All analyses were conducted in STATA 12 (StataCorp, College Station, TX).
We initially identified 284 AML patients with extended mutation testing; the molecular profiling was completed at AML diagnosis in most patients (86%, n=243) with the remainder undergoing assessment after initiation of therapy (persistent disease or relapse) (Table 1). The median age was 59 years (range 17–86) with the majority of patients having intermediate cytogenetics (12% favorable, 59% intermediate, 23% unfavorable, and 6% unknown). The 3 most common mutations were NPM1 (29%), DNMT3A (25%), and FLT3-ITD (23%). Biopsy-confirmed LC was present in 10% (n=27) of patients overall.
Table 1.
Patient and disease characteristics.
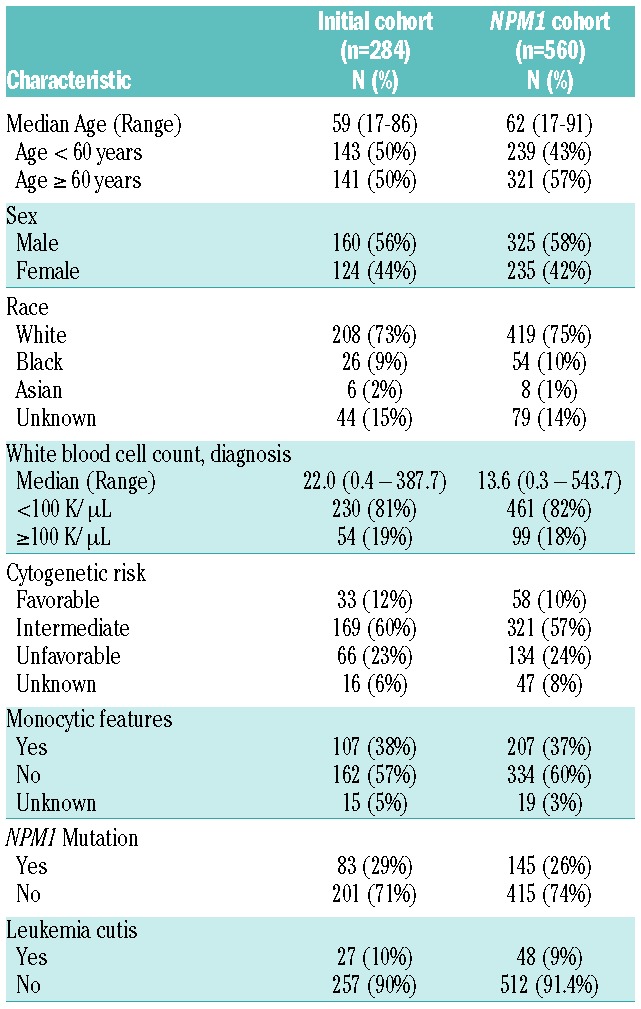
The presence of an NPM1 mutation was associated with LC (OR: 2.9; 95% CI: 1.3–6.6, P=0.009), as 14 out of 27 cases of LC had an NPM1 mutation (all the common exon 12 insertion). An association between PTPN11 and LC was also detected (OR: 3.6; P=0.014); however, 4 of the 6 cases of LC in patients with a PTPN11 mutation also had a concurrent NPM1 mutation. No association was detected between LC and the presence of any other myeloid malignancy-associated mutations or functional class of mutations (Table 2). We further examined the association of NPM1 mutation status and LC in an expanded cohort of patients with known NPM1 mutant status. Characteristics of the expanded cohort are similar to the initial cohort (Table 1). In this larger cohort, we confirmed the association of NPM1 mutations with LC (OR: 2.7; P=0.001). In total, 46% of cases of AML with LC were NPM1 mutant compared to 24% of AML cases not associated with LC (Table 3).
Table 3.
AML with NPM1 mutation is associated with leukemia cutis.
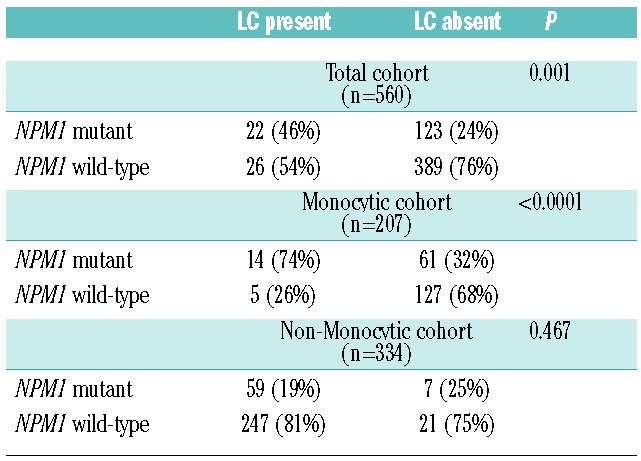
Since both NPM1 and LC have been associated with AML with monocytic features, we further examined the association of NPM1 and LC accounting for monocytic subtype. AML with monocytic features was enriched for the presence of NPM1 mutations compared to AML without monocytic features (36% versus 20%, P<0.0001). Among patients with monocytic AML, 14 out of 19 (74%) of those with LC had NPM1 mutations compared to 61 out of 188 (32%) of those without LC, suggesting that the presence of mutated NPM1 was significantly associated with the development of LC (OR: 5.8; P=0.001) in the monocytic subgroup. In contrast, among those with non-monocytic AML, 7 out of 21 (25%) of patients with LC were NPM1 mutant compared to 59 out of 306 (19%) without LC, indicating no association between NPM1 and LC in the non-monocytic subgroup (OR: 1.4; P=0.469). Interestingly, monocytic AML was not itself associated with LC in the NPM1 mutant (OR 1.9, P=0.185) or NPM1 wild-type cohort (OR: 0.46; P=0.131).
The true incidence of LC has been difficult to define as reports have been limited by small numbers of patients with varying demographic characteristics. This study is among the largest single-center reviews of LC in AML. Biopsy-proven LC was found in 10% of patients in this large cohort, which appears to be higher than some previously reported series in adult AML patients. This suggests that LC is a more common complication of adult AML than is sometimes reported.1–3 This higher incidence in our cohort may also be a reflection of the increased surveillance and clinician suspicion of the condition in our institution.
We identified a unique association between NPM1 mutation status and the presence of LC among patients with AML with monocytic features. We initially identified this finding in a cohort of 284 patients who had undergone extended mutation testing, and subsequently confirmed the association in an expanded cohort of 560 AML patients with known NPM1 mutant status. Interestingly, although we show an association between NPM1 and LC, we did not confirm an independent association between monocytic features and LC.
Our study is limited by its retrospective nature – not all patients were prospectively monitored, particularly from a dermatologic perspective, and therefore our data likely represents an under-ascertainment of LC. It is also possible that patients known to have AML with monocytic features may have been preferentially evaluated for LC. This partiality, however, would not be expected to bias our finding of an association between NPM1 mutant status and LC within the monocytic AML cohort. Our analysis is additionally limited by the use of a discrete gene panel, as some genes recurrently mutated in AML are challenging to identify using NGS techniques and therefore not included (e.g., CEBPA, partial tandem duplication of MLL). Additionally, mutation assessment was not always conducted contemporaneously with LC diagnosis. While this may have led to the misclassification of LC with regard to NPM1 status in a small number of cases, we expect this impact to be limited given the reported high rate of stability (>90%) of NPM1 status at diagnosis and relapse.9 A further limitation of our study is the lack of response and survival data in this cohort. NPM1-mutant AML in the absence of FLT3-ITD mutation is reported to have a favorable association in both younger and older patients, while the presence of LC has been associated with unfavorable outcome.2,10–12 The implication of having both of these prognostic features is unknown and should be the subject of further investigation.
The cellular mechanisms through which NPM1 mutations might alter leukemic myeloblasts homing to the skin require further study. Regardless of mechanism, our data support the World Health Organization’s provisional classification of NPM1-mutated AML as a distinct biological entity. We note that an association between NPM1 mutation and myeloid sarcoma has formerly been described, supporting the unique biology of NPM1-mutated AML.13 In summary, our data suggests that the previously described association between AML with monocytic features and LC may largely be explained by an association between NPM1 and LC.
Acknowledgments
Presented in abstract form at the 2014 American Society of Hematology Annual Meeting, San Francisco, CA, USA.
Footnotes
Funding: this work was supported by the following grants from the National Cancer Institute, National Institutes of Health: CA09679-22 (MRL) and 1K23CA141054 (AEP).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol. 2008;129(1):130–142. [DOI] [PubMed] [Google Scholar]
- 2.Agis H, Weltermann A, Fonatsch C, et al. A comparative study on demographic, hematological, and cytogenetic findings and prognosis in acute myeloid leukemia with and without leukemia cutis. Ann Hematol. 2002;81(2):90–95. [DOI] [PubMed] [Google Scholar]
- 3.Shaikh BS, Frantz E, Lookingbill DP. Histologically proven leukemia cutis carries a poor prognosis in acute nonlymphocytic leukemia. Cutis. 1987;39(1):57–60. [PubMed] [Google Scholar]
- 4.Ratnam KV, Khor CJ, Su WP. Leukemia cutis. Dermatol Clin. 1994;12(2):419–431. [PubMed] [Google Scholar]
- 5.Bakst RL, Tallman MS, Douer D, Yahalom J. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118(14):3785–3793. [DOI] [PubMed] [Google Scholar]
- 6.Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Research Investigators. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Eng J Med. 2013;368(22):2059–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sehgal AR, Gimotty PA, Zhao J, et al. DNMT3A mutational status affects the results of dose-escalated induction therapy in acute myelogenous leukemia. Clin Cancer Res. 2015;21(7):1614–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kronke J, Bullinger L, Teleanu V, et al. : Clonal evolution in relapsed NPM1- mutated acute myeloid leukemia. Blood. 2013;122(1):100–108. [DOI] [PubMed] [Google Scholar]
- 10.Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Eng J Med. 2008;358(18):1909–1918. [DOI] [PubMed] [Google Scholar]
- 11.Becker H, Marcucci G, Maharry K, et al. Favorable prognostic impact of NPM1 mutations in older patients with cytogenetically normal de novo acute myeloid leukemia and associated gene- and microRNA-expression signatures: a Cancer and Leukemia Group B study. J Clin Oncol. 2010;28(4):596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazenby M, Gilkes AF, Marrin C, Evans A, Hills RK, Burnett AK. The prognostic relevance of flt3 and npm1 mutations on older patients treated intensively or non-intensively: a study of 1312 patients in the UK NCRI AML16 trial. Leukemia. 2014;28(10):1953–1959. [DOI] [PubMed] [Google Scholar]
- 13.Falini B, Lenze D, Hasserjian R, et al. Cytoplasmic mutated nucleophosmin (NPM) defines the molecular status of a significant fraction of myeloid sarcomas. Leukemia. 2007;21(7):1566–1570. [DOI] [PubMed] [Google Scholar]


