Bone marrow monoclonal small B-cell infiltration (MSBC) associated with monoclonal B lymphocytosis (MBL), is present at a higher frequency in patients with diffuse large B-cell lymphoma (DLBCL). We have prospectively collected blood and bone marrow (BM) samples in patients with primary DLBCL at diagnosis to study the clonal relationship of MBL/MSBC with the paired DLBCL. MBL/MSBC were detected in 6 of 19 patients, of whom 5 presented DLBCL with activated B-cell origin (ABC) and one germinal center B-cell (GCB) origin. MBL/MSBC were clonally related to the paired DLBCL in 3 of 6 patients, as demonstrated by rearranged immunoglobulin heavy chain gene sequence analysis. MBL/MSBC in these 3 patients showed a chronic lymphocytic leukemia (CLL)-like, a non-CLL-like and a germinal center cell-like immunophenotype, respectively. The former two MBL/MSBC immunophenotypes were associated with DLBCL-ABC, and the latter with DLBCL-GCB. Similar, but not identical, rearranged immunoglobulin genes were demonstrated for the 3 patients with MBL/MSBC and paired DLBCL and these were not clonally related. These results suggest that a subset of DLBCL may arise from MBL/MSBC.
The most common type of DLBCL, DLBCL not otherwise specified (NOS) is subdivided according to cell of origin, either from activated B cells or from germinal center B cells.1,2 DLBCL-ABC is genetically different to DLBCL-GCB and is characterized by chronic active B-cell receptor and Toll-like receptor signaling pathways that may be targeted with novel drugs.
We previously reported a high incidence of MSBC in BM and MBL in the blood of DLBCL patients, but due to the lack of materials, the clonal relationship between the two lymphoproliferative diseases could only be studied in one case.3 DLBCL-ABC showed a higher frequency of MBL/MSBC than DLBCL-GCB: 28.2% versus 3.7%, respectively.3 In addition, MBL/MSBC was more frequently of non-chronic lymphocytic leukemia (CLL) type than of CLL type, the latter being the most frequent MBL type in the general population.4 MBL has been detected in elderly patients, with an incidence rate of over 10%.5 CLL-type MBL is a precursor lesion for CLL.6,7 However, most patients with CLL-type MBL do not develop CLL. Non-CLL-type MBL has so far not been demonstrated to be a precursor lesion for lymphoma or leukemia, but was proposed in one study to represent a low-grade lymphoma of BM.8
Bone marrow (10 mL) and blood samples (20 mL) of patients with primary DLBCL at diagnosis were prospectively collected to detect MBL/MSBC and to study whether the latter cells were clonally related to the paired DLBCL. Samples were ultimately collected for 19 patients. All patients were treated at the Norwegian Radium Hospital, Norway (Online Supplementary Table S1). Diagnostic lymphoma tissue and BM trephine biopsy was available for all patients. The study was approved by the Regional Committee for Medical and Health Professional Research Ethics of South-East Norway (reference number 2010/3241).
All lymphoma and BM trephine biopsies were reviewed and appropriate immunohistochemical analysis was performed in all cases. Lymphomas were diagnosed according to the WHO classification, and the DLBCL cell of origin was studied using the Hans algorithm.1,9
Eight-color flow cytometry analysis was used with the following antibody combinations labeled with Pacific Blue/ e450 (PB/e450), Krome Orange (KO), FITC/Pe/PercPCy5.5/ Phycoerithrin cyanine 7(PeCy7)/APC/ APC Hilite7 or APC/cyanine7 (APCH7/cy7): 1) CD20+CD4/CD45/CD8+Igλ/CD56+/Igκ/CD5/CD19+TCRγδ/CD38; 2) CD20/CD45/CD23/CD10/CD79b/CD19/CD200/CD43.
Flow cytometry analysis was performed on a LSRII instrument (Becton-Dickinson), using FACSDiva software (Becton-Dickinson). If monoclonal B cells were present, stained samples were subsequently sorted with high-pressure settings using a FACS Aria IIu High speed sorter (Becton Dickinson) equipped with 408 nm, 488 nm and 633 nm lasers. MBL/MSBC for sorting were selected with a Becton Dickinson FACSDiva software, starting with gating of viable cells using the forward scatter versus side scatter dot plot. T cells and B cells were then gated out using a CD5 versus CD19 dot plot. Finally, MBL/MSBC were separated from polyclonal B cells taking advantage of the aberrant B-cell phenotypes identified by flow cytometry analysis. Cells were collected in PBS or RLT plus lysis buffer (Qiagen, Germany). Six of the 19 patients with DLBCL showed MBL/MSBC. In accordance with our previous study, 5 of 6 patients had DLBCL-ABC and one had DLBCL-GCB. MSBC in the BM was associated with MBL in all patients (Online Supplementary Table S1). The close association between MSBC in the BM and MBL in peripheral blood has previously been demonstrated.10,11 Also, the BM trephine biopsies of the 6 patients showed in total one to three foci with small B-lymphoid cells with infiltration patterns as previously described in patients with MBL (Figure 1 and Online Supplementary Figures S1 and S2).10,11 None of the patients showed histological BM infiltration with large B-cell lymphoma. In addition, MBL/MSBC identified by flow cytometry showed a forward scatter overlapping with that of small polytypic B lymphocytes in the same sample, indicating the small size of these cells (Online Supplementary Figure S3). The BM trephine biopsies of the 13 patients without MBL/MSBC, as detected by flow cytometry, did not show small B-cell aggregates by immunohistology.
Figure 1.
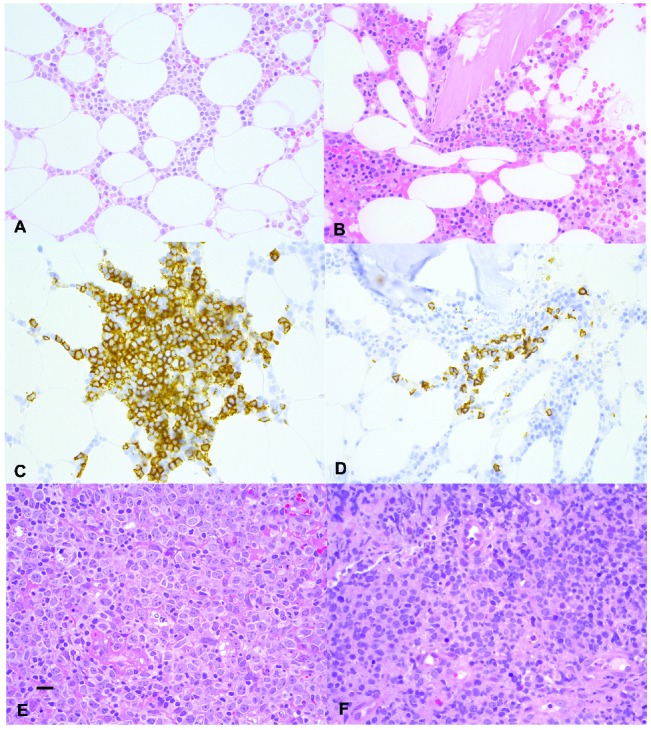
Bone marrow trephine sections of patients 1 and 2, respectively, show a small interstitial infiltrate with small lymphoid cells (A and B). H&E-stained sections (400x). The lymphoid cells are highlighted by anti-CD20 immunohistochemistry (C and D) respectively (400x). A rectal mucosa biopsy of patient 1 (E) H&E-stained section (400x) and a gastric mucosa biopsy of patient 2 (panel F, H&E-stained section (400x) shows diffuse infiltration with large B cell lymphoma. The scale bar indicates 50 micrometer.
In order to study the clonal relationship between MBL/MSBC and DLBCL paired samples, rearranged IGH gene sequences were analyzed. Rearranged IGH genes from sorted MBL/MSBC were amplified from DNA using the IGH Somatic Hypermutation Assay v.2.0 (Invivoscribe Inc., San Diego, CA, USA). For DLBCL samples, formalin-fixed tissue was analyzed using primers complimentary to IGHV framework 1, 2 and 3, as previously described.12 For one patient, frozen DLBCL tissue was available and was studied as for MBL/MSBC. The PCR products were subsequently sequenced using the BigDye® Terminator v.1.1 Cycle Sequencing Kit (Life Technologies, Carlsbad, CA, USA) and the primers from the IGH Somatic Hypermutation Assay v.2.0 kit (Invivoscribe Inc.). The International Immunogenetics Information System web-based software (www.imgt.org) was used to analyze the rearranged IGH sequences. The entire analysis was repeated twice. In addition, sequencing was repeated with IGHV family-specific primers.12 Rearranged IGHV sequences of MBL/MSBC and DLBCL revealed a clonal relationship in 3 of 6 paired samples. The samples of Patients 1 and 2 showed 94.27% and 94.59% homology to germ-line IGHV genes, respectively. Mutations were similar in paired MBL/MSBC and DLBCL samples. Patient 1 showed MBL/MSBC with a CLL immunophenotype (Table 1); the paired DLBCL of this patient showed an ABC immunophenotype without CLL immunophenotype. Patient 2 showed a non-CLL-type MBL/MSBC with a concordant DLBCL-ABC immunophenotype. Patient 6 showed shared, as well as divergent, somatic mutations in MBL/MSBC and DLBCL, with the latter showing more mutations (Table 1). The MBL/MSBC showed a GCB immunophenotype, as did the paired DLBCL. The GCB immunophenotype is consistent with the high mutation rate and the presence of divergent mutations, suggesting on-going mutation. Histological review of the DLBCL sample did not reveal concurrent follicular lymphoma, while only one focal paratrabecular small B-cell aggregate was seen in the BM. These findings were not diagnostic of concurrent follicular lymphoma. Parallel development of DLBCL from a common progenitor cell has previously been demonstrated for follicular lymphoma.13 Patient 6 in our series may represent a variant of the same process.
Table 1.
Immunophenotype and Immunoglobulin sequence characteristics of monoclonal B lymphocytosis/monoclonal small B-cell infiltration and diffuse large B-cell lymphoma.
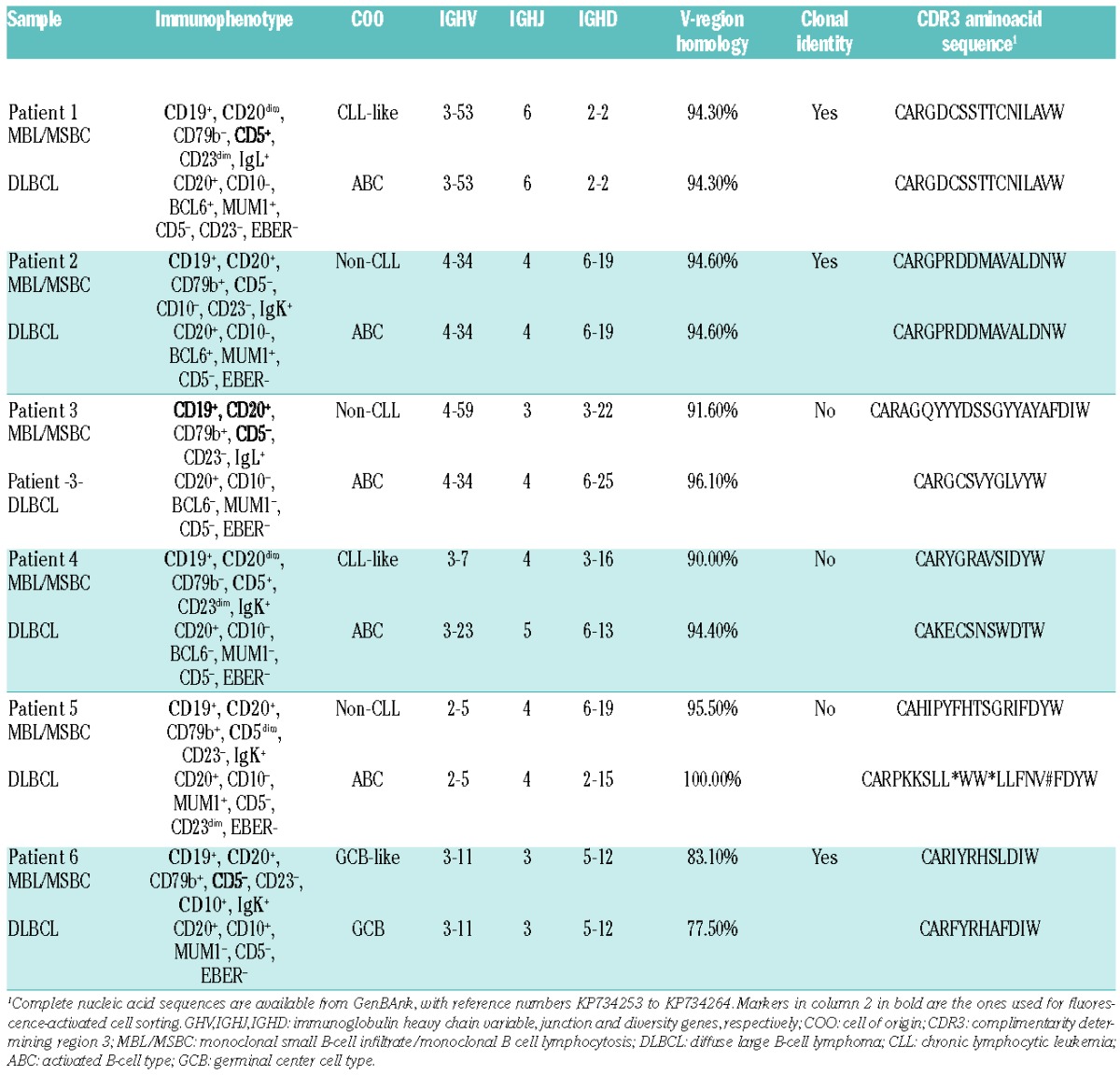
Taken together, a common clonal origin of MBL/MSBC and DLBCL was demonstrated in 3 of 6 cases, and strongly suggests that a subset of DLBCL may arise from small precursor cells, with either a CLL, non-CLL or GC immunophenotype. The former two are associated with DLBCL-ABC. One case of CLL-like MBL/MSBC, clonally related to DLBCL-ABC, had also been demonstrated in our earlier publication.3
IGHV gene usage was either the same or belonged to the same VH gene family for paired MBL/MSBC and DLBCL of the 3 cases without clonal relationship. Whether this indicates that MBL/MSBC and DLBCL arise through antigen-stimulation with subsequent selection of VH genes in these cases is an interesting hypothesis, but one that still needs to be demonstrated. Preferential VH gene use has previously been demonstrated in DLBCL and may support this hypothesis.14,15 More samples are currently being collected to study the molecular genetics of paired MBL/MSBC and DLBCL.
In conclusion, primary DLBCL in a subset of patients is clonally related to MBL/MSBC, indicating a common progenitor cell.
Footnotes
Funding: This study was supported by Health Region Authority South-East Norway and by the Norwegian Cancer Society.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Swerdlow SH, Campo E, Harris NL, et al. (Eds): WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC: Lyon: 2008. [Google Scholar]
- 2.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. [DOI] [PubMed] [Google Scholar]
- 3.Tierens AM, Holte H, Warsame A, et al. Low levels of monoclonal small B cells in the bone marrow of patients with diffuse large B-cell lymphoma of activated B-cell type but not of germinal center B-cell type. Haematologica. 2010;95(8):1334–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rawstron AC, Green MJ, Kuzmicki A, et al. Monoclonal B lymphocytes with the characteristics of “indolent” chronic lymphocytic leukemia are present in 3.5% ofadults with normal blood counts. Blood. 2002;100(2):635–639. [DOI] [PubMed] [Google Scholar]
- 5.Nieto WG, Almeida J, Romero A, et al. Primary Health Care Group of Salamanca for the Study of MBL. Increased frequency (12%) of circulating chronic lymphocytic leukemia-like B-cell clones in healthy subjects using a highly sensitive multicolor flow cytometry approach. Blood. 2009;114(1):33–37. [DOI] [PubMed] [Google Scholar]
- 6.Rawstron AC, Bennett FL, O’Connor SJ, et al. Monoclonal B-cell lymphocytosis andchronic lymphocytic leukemia. N Engl J Med. 2008;359(6):575–583. [DOI] [PubMed] [Google Scholar]
- 7.Landgren O, Albitar M, Ma W, et al. B-cell clones as early markers for chronic lymphocytic leukemia. N Engl J Med. 2009;360(7):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xochelli A, Kalpadakis C, Gardiner A, et al. Clonal B-cell lymphocy-tosisexhibiting immunophenotypic features consistent with a marginal-zone origin: isthis a distinct entity? Blood. 2014;123(8):1199–1206. [DOI] [PubMed] [Google Scholar]
- 9.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004; 103(1):275–282. [DOI] [PubMed] [Google Scholar]
- 10.Nelson BP, Abdul-Nabi A, Goolsby C, Winter J, Peterson L. Characterization of tissue findings in bone marrow biopsy specimens with small monoclonal B-cell populations. Am J Clin Pathol. 2014;141(5):687–696. [DOI] [PubMed] [Google Scholar]
- 11.Randen U, Tierens AM, Tjønnfjord GE, Delabie J. Bone marrow histology inmonoclonal B-cell lymphocytosis shows various B-cell infiltration patterns. Am J Clin Pathol. 2013;139(3):390–395. [DOI] [PubMed] [Google Scholar]
- 12.van Dongen JJ, Langerak AW, Brüggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98–3936. Leukemia. 2003;17(12):2257–2317. [DOI] [PubMed] [Google Scholar]
- 13.Pasqualucci L, Khiabanian H, Fangazio M, et al. Genetics of follicular lymphoma transformation. Cell Rep. 2014;6(1):130–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu FJ, Levy R. Preferential use of the VH4 Ig gene family by diffuse large-cell lymphoma. Blood. 1995;86(8):3072–3082. [PubMed] [Google Scholar]
- 15.Sebastián E, Alcoceba M, Balanzategui A, et al. Molecular characterization of immunoglobulin gene rearrangements in diffuse large B-cell lymphoma: antigen-driven origin and IGHV4-34 as a particular subgroup of the non-GCB subtype. Am J Pathol. 2012;181(5):1879–1888. [DOI] [PubMed] [Google Scholar]


