WHO and many other organisations are very interested in implementing treatment-as-prevention as a global policy to control the HIV pandemic.1 Widespread treatment of HIV-infected individuals with antiretroviral therapy will reduce HIV transmission, because it decreases viral load and hence infectiousness. To implement the rollout of treatment-as-prevention in an efficient manner, estimation of the number of HIV-infected individuals and where they live is needed. This assessment will be difficult to accomplish, particularly in areas of sub-Saharan Africa with severe HIV epidemics. We propose a solution to this problem by using geospatial statistical techniques and global positioning system (GPS) data.
To estimate the number of HIV-infected individuals in a particular area, a predictive map of the prevalence of infection could be constructed. This map would then be overlaid on a grid map that shows the geographical dispersion of the population. The size of the grid would determine the degree of spatial resolution of the overlay map (ie, the density-of-infection map). The density map would show the estimated number of HIV-infected individuals per km2 and their geographical distribution over the area of interest. The total number of HIV-infected individuals could be obtained by summing the estimates in each grid over the entire area.
All of the geospatial techniques needed to construct density-of-infection maps for HIV are techniques that have been used in studies of other infectious diseases— eg, dengue fever, influenza, malaria, rotavirus, and tuberculosis.2–8 Predictive prevalence maps have been constructed by using georeferenced prevalence data and spatial interpolation techniques. The most commonly used techniques are Bayesian geostatistical modelling and Kriging.7,8 Bayesian geostatistical models are constructed in the same manner as are Bayesian statistical models, but include additional parameters to allow for spatial dependency in the data. Bayesian geostatistical models have been used to generate predictive prevalence and risk maps for malaria and tuberculosis.7,8 Kriging uses semivariograms to model spatial dependency. The standard error of the estimated prevalence at any specific location is usually calculated, irrespective of whether Bayesian geostatistical modelling or Kriging is used for spatial interpolation. The standard error is then mapped to visualise the uncertainty in the prediction at any geographical location. The standard error is always largest in areas with the lowest density of sample sites. Kriging was developed by Danie Krige9 in the 1950s to identify the locations of gold mines by using georeferenced samples of mineral deposits. In 1992, Carrat and Valleron2 were the first to apply Kriging to the spatial analysis of an infectious disease. They used surveillance data from specific geographical locations and generated predictive surfaces to identify the spatial and temporal spread of the 1989–90 influenza epidemic in France. Kriging has since been used to generate predictive prevalence maps for dengue fever,3 rotavirus,4 and malaria.5–7
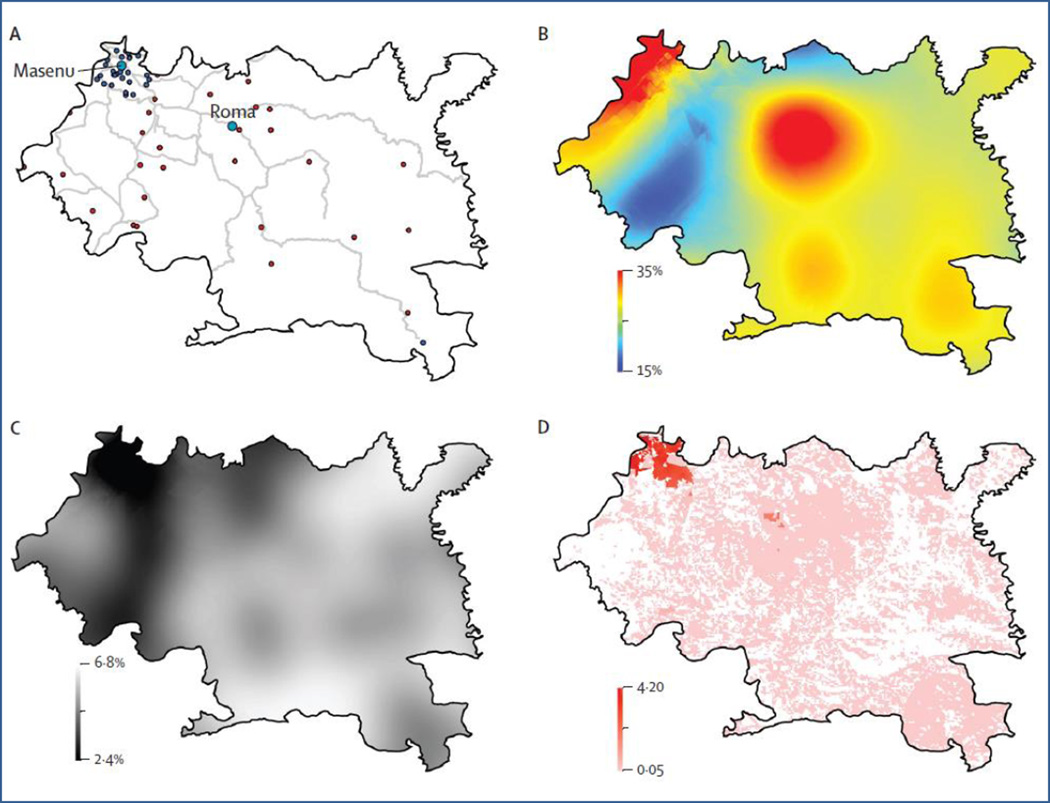
We propose that the same geospatial statistical techniques can be applied to HIV. We used these techniques to estimate the number of HIV-infected individuals in Maseru (a health-care district in Lesotho) and establish their geographical location. The district of Maseru is a relatively large area, about 4300 km2, and Lesotho has one of the most severe HIV epidemics in the world. We used HIV prevalence data collected in the 2009–10 Lesotho Demographic and Health Survey, which was based on cluster sampling.10 Handheld GPS devices were used to establish the geographical coordinates at each sample site. Of the Demographic and Health Survey sample sites in Maseru, 31 were in urban areas and 28 were in rural areas (figure part A).
Figure 1.
Geospatial mapping of Maseru, Lesotho
(A) Road networks (grey lines) and Demographic and Health Survey sample sites for urban (blue dots) and rural (red dots) locations. (B) Kriging map of HIV surface prevalence. Prevalence ranges from 11% (blue) to 35% (red). (C) Standard error map of Kriging estimates. (D) Density-of-infection map showing the number of HIV-infected individuals (aged 15–49 years) at a resolution of 100 m2.
A map of Kriging estimates (ie, prevalence predictions) for individuals aged 15–49 years, based on the georeferenced prevalence data, is shown in figure part B; spatial resolution is 100m2. The predictive map shows that prevalence is high (on average >20%) throughout Maseru, but that prevalence varies substantially with geography. Prevalence is predicted to be highest along the northwest border of the Maseru district where the city of Maseru (the capital of Lesotho) is located, and also in the centre of the district around the city of Roma. The standard error of the prediction estimates (figure part C) ranges from 2.4% (black shading) to 6.8% (white shading). Figure part D shows the geographical distribution of HIV-infected individuals and the density of infection; density ranges from 4.2 HIV-infected individuals per 100 m2 (red shading) to less than 0.05 HIV-infected individuals per 100 m2 (white shading). The map was used to determine that about 46 000 HIV-infected individuals aged 15–49 years live in the Maseru district.
Geospatial statistical techniques have been used for more than 40 years in studies of many infectious diseases. They have provided important new insights into epidemics and, more recently, have assisted in the design of health policies for dengue, influenza, malaria, rotavirus, and tuberculosis.2–8 Their use could greatly assist the design of health policies to fight HIV epidemics. We recommend that—to maximise efficiency and cost-effectiveness—a geospatial approach should be used in decisions about how to roll out treatment-as-prevention and other public-health interventions in sub-Saharan Africa. At a minimum, this geospatial approach could be used to find HIV-infected individuals in high-prevalence epidemics, establish where they live, and estimate the burden of disease.
Acknowledgments
This work was supported by National Institute of Health Award R01 AI041935.
Footnotes
We declare that we have no conflicts of interest.
References
- 1.World Health Organization. Antiviral therapy for prevention. [accessed July 22, 2013]; http://www.who.int/hiv/topics/artforprevention/en/
- 2.Carrat F, Valleron AJ. Epidemiologic mapping using the “kriging” method: application to an influenza-like illness epidemic in France. Am J Epidemiol. 1992;135:1293–1300. doi: 10.1093/oxfordjournals.aje.a116236. [DOI] [PubMed] [Google Scholar]
- 3.Duncombe J, Clements A, Davis J, Hu W, Weinstein P, Ritchie S. Spatiotemporal patterns of Aedes aegypti populations in Cairns, Australia: assessing drivers of dengue transmission. Trop Med Int Health. 2013;18:839–849. doi: 10.1111/tmi.12115. [DOI] [PubMed] [Google Scholar]
- 4.Torok TJ, Kilgore PE, Clarke MJ, Holman RC, Bresee JS, Glass RI. Visualizing geographic and temporal trends in rotavirus activity in the United States, 1991 to 1996. Pediatr Infect Dis J. 1997;16:941–946. doi: 10.1097/00006454-199710000-00007. Comment www.thelancet.com/lancetgh Vol 1 November 2013 e253. [DOI] [PubMed] [Google Scholar]
- 5.Gething PW, Atkinson PM, Noor AM, Gikandi PW, Hay SI, Nixon MS. A local space-time kriging approach applied to a national outpatient malaria data set. Comput Geosci. 2007;33:1337–1350. doi: 10.1016/j.cageo.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleinschmidt I, Bagayoko M, Clarke GPY, Craig M, Le Sueur D. A spatial statistical approach to malaria mapping. Int J Epidemiol. 2000;29:355–361. doi: 10.1093/ije/29.2.355. [DOI] [PubMed] [Google Scholar]
- 7.Clements AC, Reid HL, Kelly GC, Hay SI. Further shrinking the malaria map: how can geospatial science help to achieve malaria elimination? Lancet Infect Dis. 2013;13:709–718. doi: 10.1016/S1473-3099(13)70140-3. [DOI] [PubMed] [Google Scholar]
- 8.Randremanana RV, Richard V, Rakotomanana F, Sabatier P, Bicout DJ. Bayesian mapping of pulmonary tuberculosis in Antananarivo Madagascar. BMC Infect Dis. 2010;10:21. doi: 10.1186/1471-2334-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krige DG. A statistical approach to some basic mine valuation problems on the Witwatersrand. J Chem Metal Min Soc S Afr. 1951;52:119–139. [Google Scholar]
- 10.Measure Demographic and Health Surveys. Lesotho: standard DHS; 2009. [accessed July 22, 2013]. http://www.measuredhs.com/data/dataset/Lesotho_Standard-DHS_2009.cfm. [Google Scholar]