Abstract
Left ventricular assist device (LVAD) therapy improves survival, hemodynamic status, and end-organ perfusion in patients with refractory advanced heart failure. Hospital readmission is an important measure of the intensity of LVAD support care.
We analyzed readmissions of 148 patients (mean age, 53.6 ± 12.7 yr; 83% male) who received a HeartMate II LVAD from April 2008 through June 2012. The patients had severe heart failure; 60.1% were in Interagency Registry for Mechanically Assisted Circulatory Support class 1 or 2. All patients were observed for at least 12 months, and readmissions were classified as planned or unplanned. Descriptive and multivariate regression analyses were used to identify predictors of unplanned readmission.
Twenty-seven patients (18.2%) had no readmissions or 69 planned readmissions, and 121 patients (81.8%) had 460 unplanned readmissions. The LVAD-related readmissions were for bleeding, thrombosis, and anticoagulation (n=103; 49.1%), pump-related infections (n=60; 28.6%), and neurologic events (n=28; 13.3%). The readmission rate was 2.1 per patient-year. Unplanned readmissions were for comorbidities and underlying cardiac disease (54.3%) or LVAD-related causes (45.7%). In the unplanned-readmission rate, there was no significant difference between bridge-to-transplantation and destination-therapy patients. Unplanned readmissions were associated with diabetes mellitus (odds ratio [OR]=3.3; P=0.04) and with shorter mileage from residence to hospital (OR=0.998; P=0.046).
Unplanned admissions for LVAD-related sequelae and ongoing comorbidities were common. Diabetes mellitus and shorter distance from residence to hospital were significant predictors of readmission. We project that improved management of comorbidities and of anticoagulation therapy will reduce unplanned readmissions of LVAD patients in the future.
Keywords: Comorbidity, diabetes complications/epidemiology/mortality/surgery, heart failure/therapy, heart-assist devices, hospital readmission, hospitalization/statistics & numerical data, left ventricular assist device, patient readmission/statistics & numerical data, retrospective studies, surgical wound infections, thrombosis, treatment outcome
Circulatory support with left ventricular assist devices (LVADs) has emerged as a powerful therapy that can improve outcomes in patients who have advanced heart failure (HF) refractory to medical therapy.1–3 The scarcity of donor organs severely limits transplantation as an option for patients with advanced HF; moreover, transplant patients need lifelong immunosuppression, the medications for which can have their own serious side effects. The newest generation of LVADs comprises continuous-flow (CF) pumps, which use axial or centrifugal technology, deliver flows of up to 10 L/min,2,4 and are smaller and more durable than previous models. Currently, these LVADs are implanted either as a bridge to transplantation (BTT) or as destination therapy (DT), which offers a permanent alternative to transplantation.
Recently, patients with end-stage HF were given new options when the U.S. Food and Drug Administration (FDA) approved 2 continuous-flow LVADs: the Heart-Mate® II (Thoratec Corporation; Pleasanton, Calif) and the HeartWare® Ventricular Assist System (HeartWare Inc.; Framingham, Mass). The HeartMate II was approved for BTT in 2008 and for DT in 2010, and the HeartWare was approved for BTT in 2012. These milestones initiated modern LVAD therapy, enabling this treatment to become available to a larger population of patients.5–9 Consequently, LVAD use has dramatically increased throughout the world, particularly for DT, and growing numbers of medical centers are offering device therapy.9–11 This trend has been bolstered by reports that LVAD use, in HF patients 70 years of age or older, is associated with good functional recovery, quality of life, and survival.8 After recovering from the implantation procedure, most patients have improved hemodynamic values and end-organ perfusion.4,12,13 Improvements in survival rates are even better than those documented in the pivotal clinical trials (1-year survival rate, 68%–74% before FDA approval vs 85% after approval).14
Like transplantation, LVAD therapy requires vigilant ongoing management of various distinct problems, including increased gastrointestinal and cerebral bleeding, the risk of driveline infection, pump thrombosis, right-sided HF, and arrhythmias.15 Readmission to the hospital is an important measure of the need for more intensive management of such problems. The mean number of readmissions per patient-year is considered the measure that best relates the disease burden to individual patients.16
We hypothesized that the longitudinal need for hospital readmission (after the index admission for LVAD implantation) is influenced by preimplantation factors that are patient-related, operative, and perioperative. To test this hypothesis in the modern LVAD real-world (not clinical-trial) setting, we performed a retrospective chart review of patients who received the HeartMate II continuous-flow LVAD at our center from April 2008 through June 2012.
Patients and Methods
Patients. At our center, LVAD candidates are comprehensively evaluated by means of laboratory analysis, cardiopulmonary testing (if the patient is fit enough), imaging studies (chest radiology; computed tomographic scans of the head, abdomen, and pelvis; and carotid ultrasonography), electrocardiography, echocardiography, pulmonary function testing, right-sided heart catheterization, angiography, and psychosocial evaluation—all in accordance with contemporary guidelines.15 In selected cases, palliative-care consultation is provided to assist in medical decision-making.17 If necessary, additional specialty consultation is performed to evaluate comorbidities that could affect LVAD placement and function. Candidates are then presented to a multidisciplinary medical review board for LVAD approval.
Postoperative follow-up care of LVAD recipients who have been discharged from our hospital involves routine and protocol-driven outpatient visits and telephone contact with our LVAD coordinators. Outpatient-clinic visits are scheduled weekly for the first 4 weeks, then monthly thereafter. Laboratory testing includes a complete blood count, complete metabolic profile, and measurement of lactate dehydrogenase levels. Device interrogations are performed by circulatory support technicians during all follow-up visits. Patients are instructed to clean and dress their driveline exit sites daily, in accordance with our institutional protocol. Telephone assistance is available around the clock from an LVAD coordinator who provides the first point of contact for any patient-related issues that occur outside scheduled office hours. If patients require hospitalization, we recommend that they come to our institution. If the need for hospitalization is urgent, the patient should go to the nearest hospital for evaluation and stabilization; we will then arrange for transportation to our center, where the patient's condition can be managed by our dedicated multidisciplinary LVAD team.
All LVAD patients are routinely treated with aspirin (81 mg/d) and warfarin to achieve an international normalized ratio of 2 to 3. If a major bleeding episode occurs, anticoagulation is withheld until the source of the bleeding is determined and treatment strategies are selected.
Methods. We retrospectively reviewed our hospital's medical records and identified 220 patients who had undergone HeartMate II device implantation at our center from April 2008 through June 2012. Seventy-two of those patients were excluded from the analysis because they did not survive to hospital discharge (n=55), their device was explanted before hospital discharge (n=6), or they originally had a HeartMate Extended Vented Electric (XVE) pump that was exchanged for a Heart-Mate II (n=11). The final series comprised 148 patients whose indication for LVAD implantation was BTT in 65 cases (43.9%) and DT in 83 cases (56.1%). For the most recent patients in the cohort, the follow-up period lasted for a minimum of 12 months. The median follow-up time was 20 months (interquartile range, 12–28 mo) for patients with unplanned readmissions and 13 months (interquartile range, 6–21 mo) for patients with planned readmissions.
Before beginning the study, we obtained permission from our institutional review board (IRB), which waived consent for the use of unidentified patient data in the analysis. All data were obtained in compliance with protocols approved by the IRB. Sources included electronic medical records, clinic charts, records maintained in the office of our LVAD coordinator, and data from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS). Distance from the hospital to the patient's residence was calculated with the use of Google Maps.
To determine the number of readmissions for each patient and the reason for each readmission, we examined baseline patient variables (demographic traits, comorbidities, and laboratory variables), operative factors (duration of cardiopulmonary bypass, the device implanted, and delayed chest closure), and perioperative factors (length of intensive care, length of the index postoperative hospitalization, and hospital disposition).
To identify the primary cause of readmission, we consulted the primary diagnoses in the hospital progress and discharge notes. We then categorized the various causes in terms of planned versus unplanned readmissions. The planned readmissions were for elective surgical or diagnostic procedures, transplantation, or pump explantation. The unplanned readmissions were subdivided into those involving LVAD-related causes (bleeding, thrombosis, pump-related infection, other pump or driveline problems, neurologic events, and management of anticoagulation) versus non–LVAD-related causes, either cardiac (right-sided HF, arrhythmias with shock, arrhythmias without shock, and chest pain) or noncardiac (infection, gastrointestinal and biliary complications, hematologic sequelae, syncope due to hypovolemia, renal and urologic problems, respiratory complications, and neurologic, rheumatologic, psychiatric, and miscellaneous other conditions).
Statistical Analysis
Statistical analyses were performed by using R statistical software for Windows, version 3.0.2 (R Development Core Team; Auckland, New Zealand). Descriptive analysis was performed by presenting the mean ± SD for continuous data. Differences between 2 groups (that is, unplanned vs planned or no readmissions) of independent, normally distributed continuous variables were evaluated by using the Student t test. Variables that were not normally distributed were evaluated with use of the Wilcoxon rank sum test. Normality was checked with use of the Shapiro-Wilk test, and differences in categorical variables were evaluated with use of the Fisher exact test. Statistical comparisons were 2-sided, and the level of significance was set at P <0.05.
For multivariable regression analysis, we used a parsimonious, stepwise model to determine independent predictors of readmission. Marginally significant univariate variables (P <0.1) were considered.
Survival was analyzed with use of the Kaplan-Meier method, and patients were censored for explantation or transplantation. Survival rates for groups with 0 to 1 readmissions, 2 to 5 readmissions, and more than 5 readmissions were compared by using the log-rank test. The readmission rate was calculated in accordance with the number of readmissions per patient-year.
Results
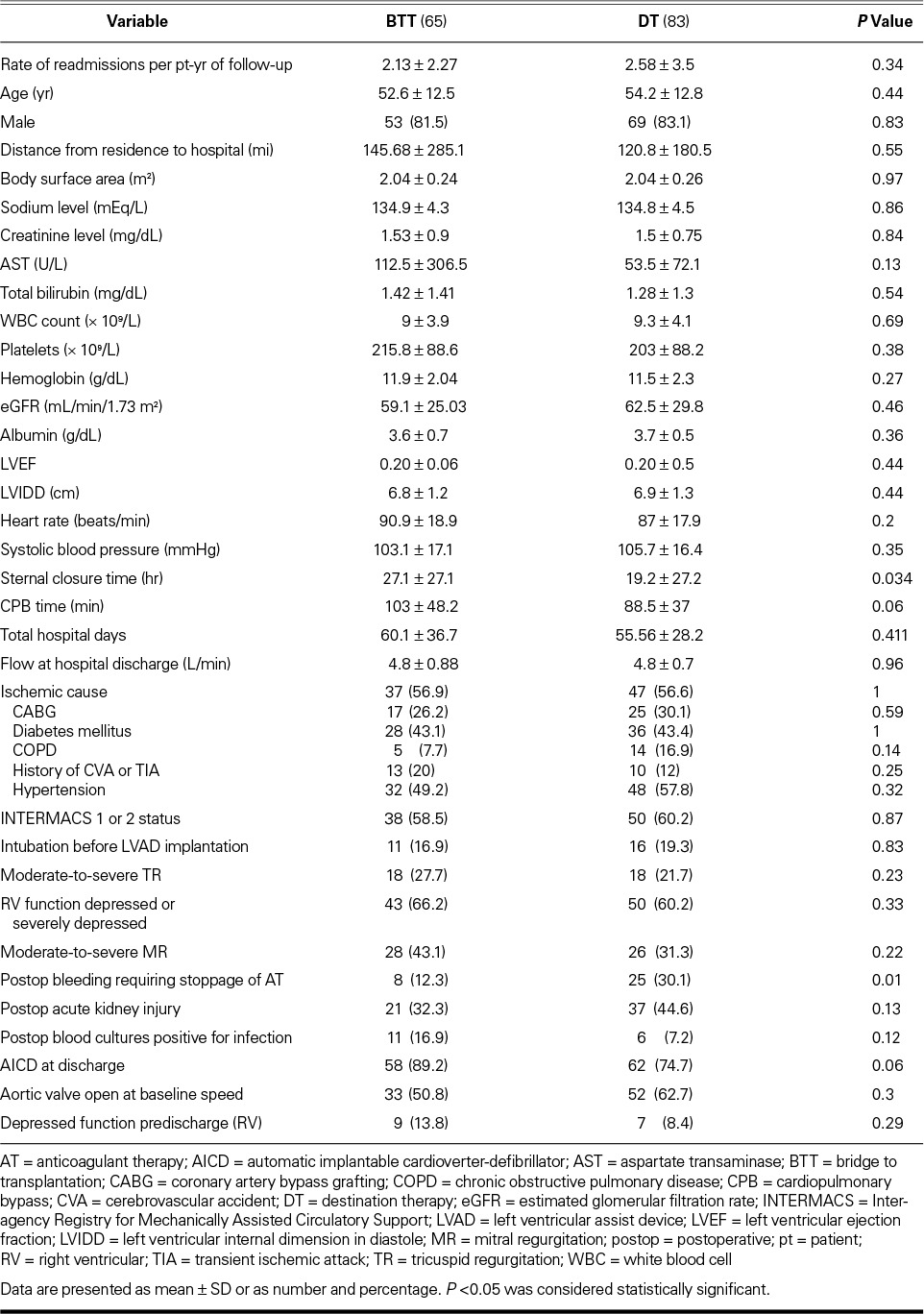
From April 2008 through June 2012, we implanted HeartMate II LVADs in 220 patients at our institution. Of these patients, 55 did not survive to hospital discharge, 6 had their device explanted before discharge, and 11 initially had a HeartMate XVE LVAD that was exchanged for a HeartMate II. Those 72 patients were excluded from the study, leaving 148 patients, whose demographic characteristics are shown in Table I. The patients' mean age was 53.6 ± 12.7 years, 123 were men (83%), and 85 (57.4%) had ischemic HF. Most of the patients had a low left ventricular ejection fraction (LVEF) (mean value, 0.20 ± 0.05). Ninety-four patients (63.5%) also had a depressed right ventricular ejection fraction as identified qualitatively on the baseline echocardiogram. Eighty-nine patients (60.1%) were in INTERMACS class 1 or 2 at device implantation. The indication for LVAD implantation was BTT in 65 cases (43.9%) and DT in 83 cases (56.1%). We compared these 2 populations to determine differences that could have affected subsequent readmission (Table II). The only significant differences were that the BTT group had an excess of delayed sternal closure times (P=0.01) and the DT group had more bleeding (P=0.01) that necessitated the cessation of anticoagulation.
TABLE I.
Patient Demographic, Operative, and Perioperative Characteristics, Stratified by Readmission Status
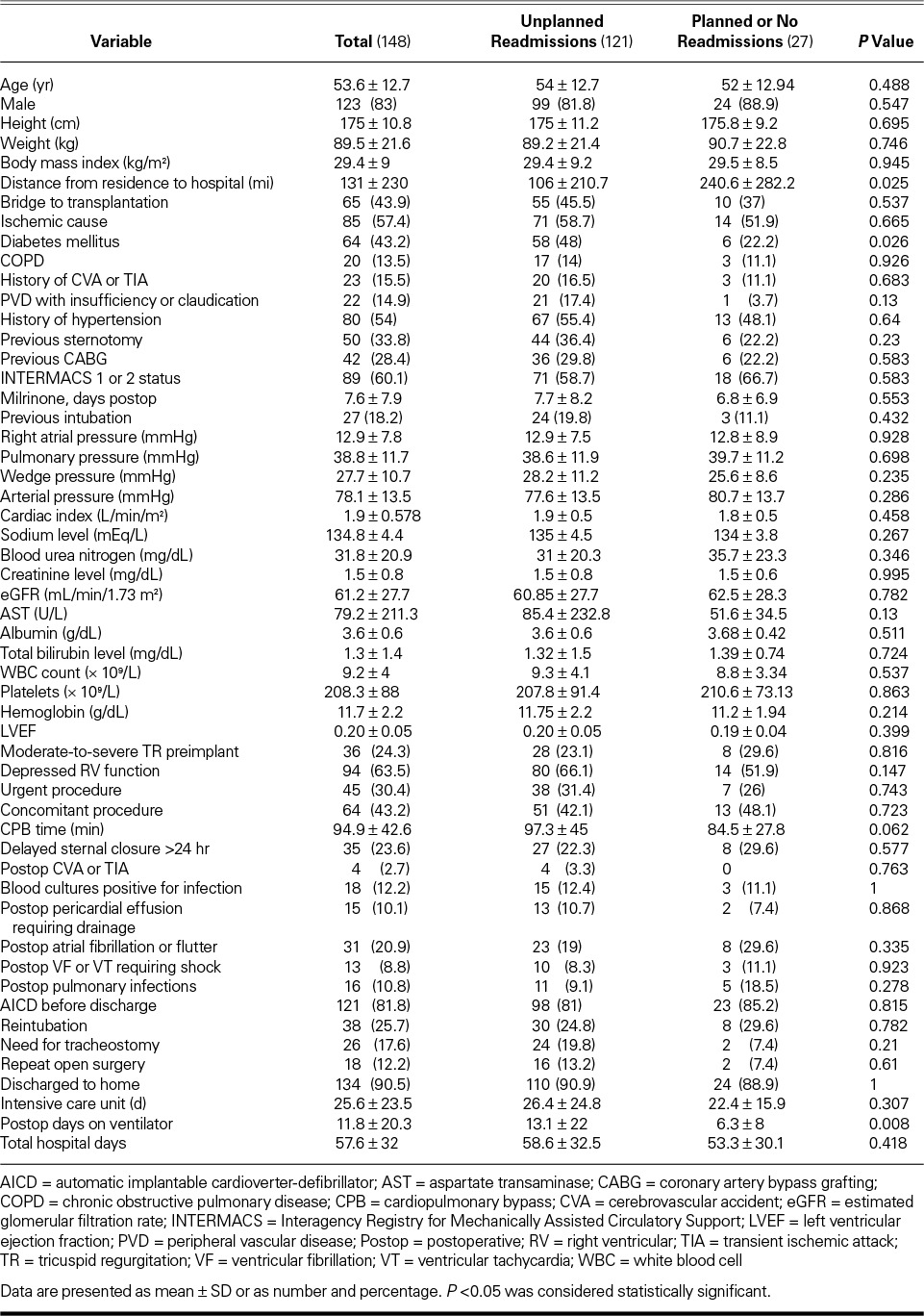
TABLE II.
Comparison between Bridge-to-Transplantation and Destination-Therapy Populations
Readmission
Twenty-seven patients (18.2%) had either no admissions or planned readmissions only (mean follow-up time, 14 ± 9 mo). The planned readmissions were for transplant-related procedures (39 readmissions; 56.5%), elective non–LVAD-related procedures (25 readmissions; 36.2%), or LVAD explantation because of recovery from HF (5 readmissions; 7.2%).
The other 121 patients (81.8%) had 460 unplanned readmissions (mean follow-up time, 21 ± 12 mo). These admissions were categorized as either LVAD-related (n=210; 45.7%) or non–LVAD-related (n=250; 54.3%). Figure 1 shows the specific causes of hospital readmissions. In order of frequency, the reasons for the LVAD-related readmissions were infection (n=60; 28.6%), bleeding (n=57; 27.1%), neurologic events (cardiovascular accident, transient ischemic attack, or intracranial bleeding) (n=28; 13.3%), thrombosis (n=23; 11%), management of anticoagulation issues (n=23; 11%), and pump and driveline events (n=19; 9%). The reasons for non–LVAD-associated unplanned readmissions were noncardiac comorbidities (n=139; 55.6%) and cardiac disease progression (n=111; 44.4%).
Fig. 1.
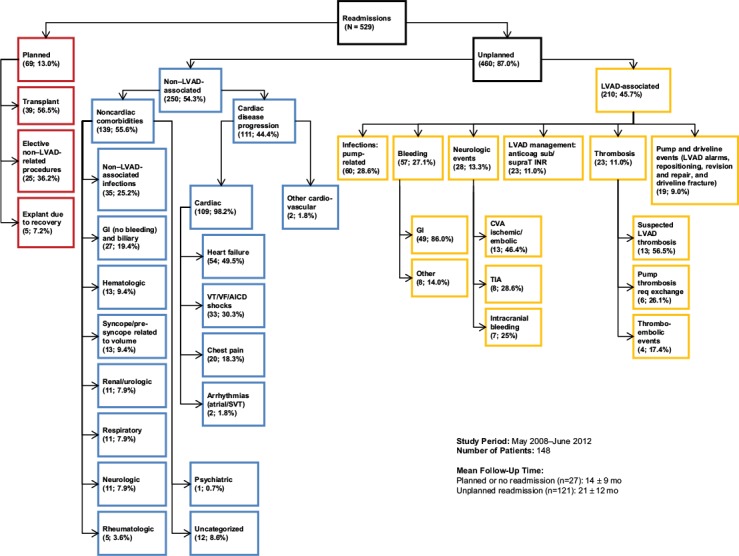
Chart shows primary causes of hospital readmissions in 148 patients with a HeartMate II LVAD.
Anticoag = anticoagulation; CVA = cardiovascular accident; GI = gastrointestinal; AICD = automatic implantable cardioverter-defibrillator; INR = international normalized ratio; LVAD = left ventricular assist device; req = requiring; sub/supraT = subtherapeutic or supratherapeutic anticoagulation; SVT = supraventricular tachycardia; TIA = transient ischemic attack; VF = ventricular fibrillation; VT = ventricular tachycardia
Of the unplanned readmissions related to cardiac-disease progression, HF accounted for 54 (49.5%), ventricular tachycardia for 33 (30.3%), chest pain for 20 (18.3%), and other arrhythmias for 2 (1.8%).
Of the unplanned non–LVAD-associated readmissions for noncardiac reasons, infections were the leading cause (n=35; 25.2%), followed by nonbleeding gastrointestinal complications (n=27; 19.4%).
The cumulative rate of unplanned readmissions was 2.1 per patient-year. There was a trend toward more unplanned readmissions in the DT group (1.02 per patient-year) than in the BTT group (0.84 per patient-year), but this difference was not significant (P=0.34).
Patterns of Readmission
The cumulative number of unplanned readmissions per patient was 0.5, 1.2, 1.59, and 1.87 at 3, 6, 9, and 12 months, respectively. Twenty-five patients had 1 unplanned readmission, 64 patients had 2 to 4 unplanned readmissions, and 32 patients had 5 or more unplanned readmissions; 1 patient had 15 unplanned readmissions (Fig. 2).
Fig. 2.
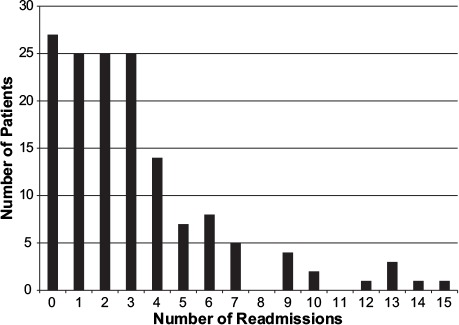
Graph shows number of readmissions per patient.
The time-effect rates of readmission were highest in the first 6 months after LVAD implantation, decreased between 6 and 12 months, and increased again after 12 months (0.82 per patient-year of follow-up observation).
Predictive Factors for Readmission
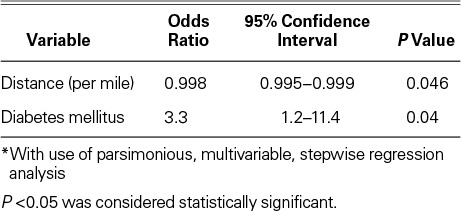
Univariate and multivariate analysis identified demographic, operative, and perioperative factors as significantly associated with the need for subsequent readmission. Unplanned readmission was associated with diabetes mellitus (hazard ratio [HR]=3.3; P=0.04) and with a shorter distance from the patient's residence to the hospital (HR=0.998; P=0.046) (Table III), as well as with the number of days the patient had been on the ventilator after LVAD placement (P=0.008). The strongest predictor of unplanned readmission was diabetes (HR=3.3; odds ratio [OR]=3.3; 95% confidence interval [CI], 0.995–0.999; P=0.04).
TABLE III.
Independent Predictors of Readmission *
Survival Data
During the follow-up period, 31 patients (25%) died. All deaths were in the unplanned-readmission group. Using a Kaplan-Meier curve, we compared the differences between patients who had 0 to 1 admissions after LVAD implantation and patients who had 2 to 4 admissions or 5 or more admissions. The results suggest that increased need for hospital readmission after LVAD implantation is associated with diminishing survival. The survival curves for all 3 groups trended together initially, then separated approximately 1.75 years after device implantation (Fig. 3).
Fig. 3.
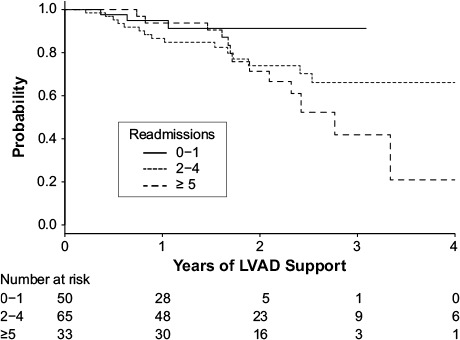
Kaplan-Meier curve shows differences between patients who had 0–1 admissions versus 2–4 admissions versus 5 or more admissions after left ventricular assist device (LVAD) implantation.
Discussion
For HF patients, hospital readmission is an important marker of disease worsening, and increased numbers of readmissions are associated with an increased risk of death.18 This risk is driven not only by worsening of pump failure and malignant arrhythmic events but also by a common panoply of comorbidities, including atherosclerotic coronary artery disease, diabetes mellitus, renal failure, and other serious conditions.19,20 After the index hospital admission for HF management, the rate of readmission within 30 days approaches 25%.21–23
Gaining an understanding of readmission patterns and trends outside clinical trials, in a real-world LVAD population, is crucial, because hospital readmission has adverse quality-of-life consequences for patients and their caregivers after successful LVAD implantation. Broader cost and resource-utilization issues also affect clinical care delivery to these patients.24 Furthermore, understanding hospital patterns and trends toward readmission might help us answer an important underlying question: does implanting an LVAD into a patient who already has a complex, multimorbid condition make that patient less likely to need repeated hospitalizations? Clearly, LVAD therapy improves survival rates and the quality of life; the question is whether or not—by improving cardiac output—LVAD therapy can ultimately simplify the care of these patients.
Our single-center study reveals contemporary trends in LVAD therapy. The time frame is crucial because LVAD therapy has evolved rapidly during the past few years. The FDA approved the HeartMate II for BTT in April 2008 and for DT in January 2010. The Heart-Ware device was approved for BTT in November 2012 and is currently available for DT patients only within the confines of an ongoing clinical trial. Therefore, the dates chosen for this analysis (April 2008–June 2012) afford us a look at “real-world” patients selected outside the strict bounds of clinical trials—those who are beneficiaries of the broadest possible availability of LVAD therapy. Three other single-center reports25–27 describe patients whose implants date back to 2006. Those reports include data concerning both clinical-trial patients and investigational devices that never became available outside trials; therefore, these populations might not be representative of contemporary cohorts.
We hypothesized that the baseline demographic traits, severity of the illness, and complexity of the postoperative course would become apparent in the subsequent need for repeated hospital readmission of LVAD recipients. However, we found no association between these 3 variables and readmission except for geography (the patient's relative closeness to the hospital), the presence of diabetes mellitus, prolonged postoperative ventilator use, and longer cardiopulmonary bypass time. All of these factors were associated with an increased likelihood of readmission. We did not attempt to confirm earlier reports28 that hemoglobin level predicted rehospitalization. Neither did we measure brain natriuretic peptide (BNP) levels, as did Hasin and co-authors,27 who found that elevations of this biomarker predicted readmission.
Our series confirmed that a shorter distance to the hospital is associated with an increased frequency of readmission. Our finding is contrary to that of the Mayo Clinic investigators: in their series, a longer distance to the medical center was associated with an increased need for hospital admission.27 Our finding probably is a product of the urban and suburban nature of our institution, and of local referral and transfer patterns.
The strong association between diabetes and readmission after LVAD implantation is novel to our study. Outcomes after coronary artery bypass grafting (CABG), the most frequently performed cardiac operation, have been thoroughly studied and provide insights into our finding. Patients with diabetes who undergo CABG are known to have poor outcomes both immediately after revascularization and at every stage thereafter.29,30 The reasons for this finding include increases in platelet aggregation, platelet adhesion, and thrombogenesis, which potentially contribute to premature graft failure.31 Outcomes are linked to preoperative control of blood-sugar levels, as indicated by the hemoglobin A1c test (HbA1c). Halkos and colleagues32 showed that HbA1c is a powerful predictor of postoperative in-hospital death and morbidity. In addition, both diabetes and the duration of mechanical ventilation have been shown to be independent risk factors for postoperative infection in cardiac patients.33 In another large series of CABG patients, Hudson and colleagues34 showed that patients with diabetes and elevated HbA1c values had a significant increase in postoperative acute kidney injury (OR=1.148; 95% CI, 1.003–1.313; P=0.04). If the HbA1c is elevated to >8.6%, the post-CABG mortality rate is quadrupled compared with that of diabetic patients who have HbA1c levels of <8.6% (indicative of better intermediate-term glycemic control). In elective situations, it has been proposed that CABG should be delayed until glycemic control can be achieved.35 If these results are replicated in an LVAD cohort, closer blood-sugar control is probably warranted, particularly for patients undergoing elective or semielective LVAD implantation.
Hospital readmission after device implantation is frequent even in the current decade of LVAD therapy, and only a small minority of patients do not need readmission for further device management. We report a high rate of unplanned admissions at 81.8%; this figure exceeds those cited in earlier reports (55%–68%)36 but is only slightly higher than the rates cited in subsequent single-center reports, in which 72.4% to 79% of LVAD patients had at least one readmission (usually unplanned).26,27 Table IV summarizes the findings from the other single-center reports.25–27 The centers vary in regard to the time frames studied and the proportion of BTT patients in the study populations, but the relatively high rates of hospitalization after LVAD implantation and the primary causes for readmission are remarkably homogeneous. In providing details on readmission trends that involved 2,507 LVAD implantations from 2006 through 2011, a Medicare database report37 showed that the mean number of hospitalizations after LVAD implantation was stable at 1.8 ± 2.1 and that readmission rates were not affected by center volume.
TABLE IV.
Comparison of Left Ventricular Assist Device Studies from Single Centers
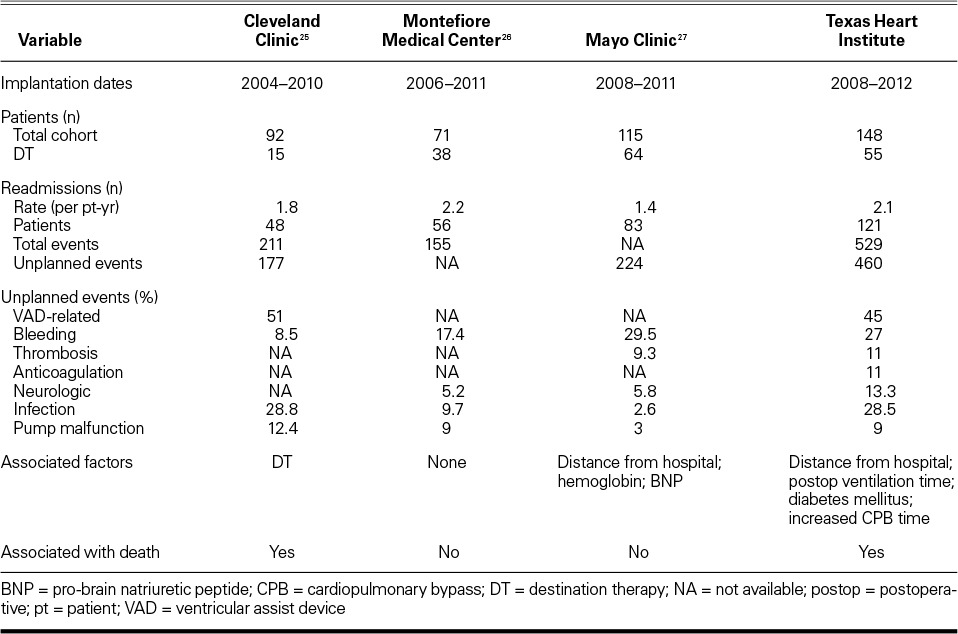
The leading single cause of readmission in our series was LVAD (driveline and pump) infection, which accounted for 28.6% of all LVAD-related admissions—a finding consistent with previous reports.38,39 The combination of bleeding, anticoagulation management, and thrombosis accounted for 49.1% of our LVAD-related readmissions. This finding is again consistent with previous reports that cite bleeding as the most common perioperative event in these patients.40 The 3rd leading cause of readmission was neurologic events, which occurred in 28 patients, or 13.3% of the LVAD-related admissions. Technological innovations designed to eliminate the driveline and to provide more biocompatible surfaces are clearly needed.
Two hundred fifty (54.3%) of our patients were admitted for non–LVAD-related causes. Of that group, more than half (n=139; 55.6%) were readmitted for management of noncardiac conditions, which indicates the highly comorbid state of most patients with advanced HF. In the remaining 111 readmitted patients (44.4% of the non–LVAD-related group), cardiac causes included progressive right-sided HF, arrhythmias, automatic implantable cardioverter-defibrillator shocks, and chest-pain evaluations. Cleveland Clinic investigators25 reported a similar proportion of non–LVAD-related readmissions (49%) despite the fact that their population was largely composed of BTT patients (84.8%)—in contrast to our series, which had predominantly DT patients (55.8%). We did not find a significant difference between our DT and BTT populations in regard to their need of subsequent hospitalization. Conversely, the Cleveland Clinic investigators found a far greater need for readmission in the DT population.25 Our results suggest that within the context of current LVAD therapy, DT and BTT patients are subject to similar LVAD- and comorbidity-related episodes of destabilization. If this finding is validated at other institutions, it provides justification for moving away from the arbitrary and artificial distinctions between BTT and DT that are part of the regulatory and funding models for circulatory support in the United States.
Our analysis of mortality rates agrees with the Cleveland Clinic's report,25 which suggested that increased numbers of admissions are associated with an increased mortality rate.
Limitations. The limitations of our study include those inherent in any retrospective single-center analysis. Our institution is a large-volume tertiary-care center in Houston, Texas, and our patient population does not necessarily resemble LVAD populations in other regions of the country. For example, our center aggressively promotes LVAD implantation in patients who have very complex disease, and we routinely receive referrals of patients who have been deemed unacceptable candidates for LVAD therapy at other centers. This fact might well affect the readmission rates that we have reported. Therefore, our study could lack breadth of application, and analysis of larger cohorts could possibly identify other predictors of readmission.
Conclusions. Unplanned admissions for LVAD-related sequelae and ongoing comorbidities were common. Diabetes mellitus and shorter distance from the residence to the hospital were significant predictors of readmission. We project that improved management of comorbidities and of anticoagulation therapy will reduce unplanned readmissions of LVAD patients in the future.
Mechanical circulatory support for the failing heart is a relatively young therapy for advanced disease, with a history only several decades long. Enormous strides have been made since the early years, when device recipients were initially confined to the intensive care unit; within a short time, these devices have become smaller and implantable, enabling patients to live independently at home, to travel freely, and to pursue activities that would otherwise have been curtailed by symptoms or altogether excluded by a reduced lifespan. The authors hope that this report provides not only a measure by which to judge the clinical impact of current LVAD therapy but also the vision through which further innovations might evolve in this rapidly advancing field.
Footnotes
From: Center for Cardiac Support (Drs. Frazier, Hernandez, Mallidi, and Meyers; and Mr. Hoang), Department of Cardiopulmonary Transplantation (Drs. Frazier, Mallidi, and Singh), and Department of Biostatistics and Epidemiology (Dr. Elayda and Mr. Ali), Texas Heart Institute, Houston, Texas 77030; and Cornell University (Mr. Hoang), Ithaca, New York 14853
Dr. Hernandez is now at St. John's Episcopal Hospital, Far Rockaway, New York.
References
- 1.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357(9):885–96. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 2.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 3.Aaronson KD, Slaughter MS, Miller LW, McGee EC, Cotts WG, Acker MA et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012;125(25):3191–200. doi: 10.1161/CIRCULATIONAHA.111.058412. [DOI] [PubMed] [Google Scholar]
- 4.Rogers JG, Aaronson KD, Boyle AJ, Russell SD, Milano CA, Pagani FD et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55(17):1826–34. doi: 10.1016/j.jacc.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 5.John R, Naka Y, Smedira NG, Starling R, Jorde U, Eckman P et al. Continuous flow left ventricular assist device outcomes in commercial use compared with the prior clinical trial. Ann Thorac Surg. 2011;92(4):1406–13. doi: 10.1016/j.athoracsur.2011.05.080. [DOI] [PubMed] [Google Scholar]
- 6.Terracciano CM, Miller LW, Yacoub MH. Contemporary use of ventricular assist devices. Annu Rev Med. 2010;61:255–70. doi: 10.1146/annurev.med.032309.063018. [DOI] [PubMed] [Google Scholar]
- 7.Kamdar F, Boyle A, Liao K, Colvin-Adams M, Joyce L, John R. Effects of centrifugal, axial, and pulsatile left ventricular assist device support on end-organ function in heart failure patients. J Heart Lung Transplant. 2009;28(4):352–9. doi: 10.1016/j.healun.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Adamson RM, Stahovich M, Chillcott S, Baradarian S, Chammas J, Jaski B et al. Clinical strategies and outcomes in advanced heart failure patients older than 70 years of age receiving the HeartMate II left ventricular assist device: a community hospital experience. J Am Coll Cardiol. 2011;57(25):2487–95. doi: 10.1016/j.jacc.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 9.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA et al. The Fourth INTERMACS Annual Report: 4,000 implants and counting. J Heart Lung Transplant. 2012;31(2):117–26. doi: 10.1016/j.healun.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Moazami N, Fukamachi K, Kobayashi M, Smedira NG, Hoercher KJ, Massiello A et al. Axial and centrifugal continuous-flow rotary pumps: a translation from pump mechanics to clinical practice. J Heart Lung Transplant. 2013;32(1):1–11. doi: 10.1016/j.healun.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32(2):141–56. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Russell SD, Rogers JG, Milano CA, Dyke DB, Pagani FD, Aranda JM et al. Renal and hepatic function improve in advanced heart failure patients during continuous-flow support with the HeartMate II left ventricular assist device. Circulation. 2009;120(23):2352–7. doi: 10.1161/CIRCULATIONAHA.108.814863. [DOI] [PubMed] [Google Scholar]
- 13.Sandner SE, Zimpfer D, Zrunek P, Rajek A, Schima H, Dunkler D et al. Renal function and outcome after continuous flow left ventricular assist device implantation. Ann Thorac Surg. 2009;87(4):1072–8. doi: 10.1016/j.athoracsur.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Mehra MR, Stewart GC, Uber PA. The vexing problem of thrombosis in long-term mechanical circulatory support. J Heart Lung Transplant. 2014;33(1):1–11. doi: 10.1016/j.healun.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Slaughter MS, Pagani FD, Rogers JG, Miller LW, Sun B, Russell SD et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29(4 Suppl):S1–39. doi: 10.1016/j.healun.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Metcalfe C, Thompson SG, Cowie MR, Sharples LD. The use of hospital admission data as a measure of outcome in clinical studies of heart failure. Eur Heart J. 2003;24(1):105–12. doi: 10.1016/s0195-668x(02)00384-6. [DOI] [PubMed] [Google Scholar]
- 17.Brush S, Budge D, Alharethi R, McCormick AJ, MacPherson JE, Reid BB et al. End-of-life decision making and implementation in recipients of a destination left ventricular assist device. J Heart Lung Transplant. 2010;29(12):1337–41. doi: 10.1016/j.healun.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260–6. doi: 10.1016/j.ahj.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 19.Alba AC, Braga J, Gewarges M, Walter SD, Guyatt GH, Ross HJ. Predictors of mortality in patients with an implantable cardiac defibrillator: a systematic review and meta-analysis. Can J Cardiol. 2013;29(12):1729–40. doi: 10.1016/j.cjca.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Chamberlain AM, McNallan SM, Dunlay SM, Spertus JA, Redfield MM, Moser DK et al. Physical health status measures predict all-cause mortality in patients with heart failure. Circ Heart Fail. 2013;6(4):669–75. doi: 10.1161/CIRCHEARTFAILURE.112.000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernheim SM, Grady JN, Lin Z, Wang Y, Wang Y, Savage SV et al. National patterns of risk-standardized mortality and readmission for acute myocardial infarction and heart failure. Update on publicly reported outcomes measures based on the 2010 release. Circ Cardiovasc Qual Outcomes. 2010;3(5):459–67. doi: 10.1161/CIRCOUTCOMES.110.957613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krumholz HM, Merrill AR, Schone EM, Schreiner GC, Chen J, Bradley EH et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2(5):407–13. doi: 10.1161/CIRCOUTCOMES.109.883256. [DOI] [PubMed] [Google Scholar]
- 23.Bradley EH, Curry L, Horwitz LI, Sipsma H, Wang Y, Walsh MN et al. Hospital strategies associated with 30-day readmission rates for patients with heart failure. Circ Cardiovasc Qual Outcomes. 2013;6(4):444–50. doi: 10.1161/CIRCOUTCOMES.111.000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers JG, Bostic RR, Tong KB, Adamson R, Russo M, Slaughter MS. Cost-effectiveness analysis of continuous-flow left ventricular assist devices as destination therapy. Circ Heart Fail. 2012;5(1):10–6. doi: 10.1161/CIRCHEARTFAILURE.111.962951. [DOI] [PubMed] [Google Scholar]
- 25.Smedira NG, Hoercher KJ, Lima B, Mountis MM, Starling RC, Thuita L et al. Unplanned hospital readmissions after HeartMate II implantation: frequency, risk factors, and impact on resource use and survival. JACC Heart Fail. 2013;1(1):31–9. doi: 10.1016/j.jchf.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Forest SJ, Bello R, Friedmann P, Casazza D, Nucci C, Shin JJ et al. Readmissions after ventricular assist device: etiologies, patterns, and days out of hospital. Ann Thorac Surg. 2013;95(4):1276–81. doi: 10.1016/j.athoracsur.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 27.Hasin T, Marmor Y, Kremers W, Topilsky Y, Severson CJ, Schirger JA et al. Readmissions after implantation of axial flow left ventricular assist device. J Am Coll Cardiol. 2013;61(2):153–63. doi: 10.1016/j.jacc.2012.09.041. [DOI] [PubMed] [Google Scholar]
- 28.Jennings DL, Wagner JL, To L, Nemerovski CW, Kalus JS, Morgan JA, Lanfear DE. Epidemiology and outcomes associated with anemia during long-term support with continuous-flow left ventricular assist devices. J Card Fail. 2014;20(6):387–91. doi: 10.1016/j.cardfail.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Carson JL, Scholz PM, Chen AY, Peterson ED, Gold J, Schneider SH. Diabetes mellitus increases short-term mortality and morbidity in patients undergoing coronary artery bypass graft surgery. J Am Coll Cardiol. 2002;40(3):418–23. doi: 10.1016/s0735-1097(02)01969-1. [DOI] [PubMed] [Google Scholar]
- 30.Alserius T, Hammar N, Nordqvist T, Ivert T. Improved survival after coronary artery bypass grafting has not influenced the mortality disadvantage in patients with diabetes mellitus. J Thorac Cardiovasc Surg. 2009;138(5):1115–22. doi: 10.1016/j.jtcvs.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman DM, Dimitrova KR, Decastro H, Friedmann P, Geller CM, Ko W, Tranbaugh RF. Improving long term outcome for diabetic patients undergoing surgical revascularization by use of the radial artery conduit: a propensity matched study. J Cardiothorac Surg. 2013;8:27. doi: 10.1186/1749-8090-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halkos ME, Puskas JD, Lattouf OM, Kilgo P, Kerendi F, Song HK et al. Elevated preoperative hemoglobin A1c level is predictive of adverse events after coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2008;136(3):631–40. doi: 10.1016/j.jtcvs.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 33.Lola I, Levidiotou S, Petrou A, Arnaoutoglou H, Apostolakis E, Papadopoulos GS. Are there independent predisposing factors for postoperative infections following open heart surgery? J Cardiothorac Surg. 2011;6:151. doi: 10.1186/1749-8090-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hudson CC, Welsby IJ, Phillips-Bute B, Mathew JP, Lutz A, Chad-Hughes G et al. Glycosylated hemoglobin levels and outcome in non-diabetic cardiac surgery patients. Can J Anaesth. 2010;57(6):565–72. doi: 10.1007/s12630-010-9294-4. [DOI] [PubMed] [Google Scholar]
- 35.Tennyson C, Lee R, Attia R. Is there a role for HbA1c in predicting mortality and morbidity outcomes after coronary artery bypass graft surgery? Interact Cardiovasc Thorac Surg. 2013;17(6):1000–8. doi: 10.1093/icvts/ivt351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pagani FD, Miller LW, Russell SD, Aaronson KD, John R, Boyle AJ et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54(4):312–21. doi: 10.1016/j.jacc.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 37.Khazanie P, Hammill BG, Patel CB, Eapen ZJ, Peterson ED, Rogers JG et al. Trends in the use and outcomes of ventricular assist devices among Medicare beneficiaries, 2006 through 2011. J Am Coll Cardiol. 2014;63(14):1395–404. doi: 10.1016/j.jacc.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon RJ, Quagliarello B, Lowy FD. Ventricular assist device-related infections. Lancet Infect Dis. 2006;6(7):426–37. doi: 10.1016/S1473-3099(06)70522-9. [DOI] [PubMed] [Google Scholar]
- 39.Holman WL, Pamboukian SV, McGiffin DC, Tallaj JA, Cadeiras M, Kirklin JK. Device related infections: are we making progress? J Card Surg. 2010;25(4):478–83. doi: 10.1111/j.1540-8191.2010.01034.x. [DOI] [PubMed] [Google Scholar]
- 40.Bunte MC, Blackstone EH, Thuita L, Fowler J, Joseph L, Ozaki A et al. Major bleeding during HeartMate II support. J Am Coll Cardiol. 2013;62(23):2188–96. doi: 10.1016/j.jacc.2013.05.089. [DOI] [PubMed] [Google Scholar]


