Abstract
We report the case of a 62-year-old woman who presented with classic symptoms of stable angina. Cardiac images and catheterization results revealed absent pulmonary valve syndrome and compression of the left main coronary artery by a massively dilated pulmonary artery aneurysm. The patient's anginal symptoms were relieved after pulmonary arterioplasty.
Others have described proximal left main coronary artery compression in the presence of a dilated and hypertensive pulmonary artery. To our knowledge, this is the first case in which a pulmonary artery aneurysm caused left main coronary insufficiency in the absence of pulmonary hypertension—a clinically important complication of congenital pulmonary valve-related pulmonary arteriopathy.
Keywords: Angina pectoris/etiology; coronary vessel anomalies/complications; heart defects, congenital/complications/diagnosis/radiography; heart valves/abnormalities; pulmonary artery/abnormalities/pathology/surgery; treatment outcome
In absent pulmonary valve (PV) syndrome, the PV leaflets are hypoplastic and dysmorphic, resulting in severe pulmonary incompetence and (less often) stenosis. Absent PV can occur in isolation, in association with tetralogy of Fallot, and occasionally in association with patent ductus arteriosus and a hypoplastic right heart.1–3 The main and branch pulmonary arteries (PAs) are characteristically markedly enlarged and might compress the bronchi or esophagus.4 We describe the apparently unique case of a patient with this syndrome who presented with exertional angina.
Case Report
In September 2010, a 62-year-old woman presented with a 6-month history of left-sided substernal chest heaviness, which reliably occurred after 20 to 30 minutes of brisk walking on a treadmill or on inclines. The symptoms resolved with rest. The patient reported no respiratory symptoms or dysphagia. Her medical history included a congenitally abnormal PV with an associated massive PA aneurysm. On examination, her vital signs and weight were normal. No findings suggested Noonan syndrome. The patient's jugular venous pressure was normal, and there were no prominent A waves. Auscultation revealed clear lungs, a normal S1, a single S2, a mid-peaking grade 2/6 systolic ejection murmur over the left upper sternal border with a short grade 3/4 low-pitched diastolic murmur, and no systolic ejection click. There was a palpable right ventricular (RV) lift and no PA pulsation.
An electrocardiogram showed sinus rhythm, incomplete right bundle branch block, precordial T-wave inversions, and a normal QTc interval. An echocardiogram revealed biatrial enlargement, a trileaflet aortic valve without stenosis, hypoplastic and thickened pulmonic leaflets consistent with a congenitally abnormal PV, a peak pulmonic gradient of 41 mmHg (mean, 22 mmHg), severe pulmonic insufficiency, main PA dilation of 7.9 cm, an estimated RV systolic pressure of 38 mmHg (assuming a right atrial pressure of 5 mmHg), severe RV dilation without hypertrophy or dysfunction, mild tricuspid regurgitation, and an intact ventricular septum.
Given the patient's history of exertional chest discomfort, she underwent cardiac computed tomographic angiography. This revealed compression of the proximal left main coronary artery (LMCA) by the severely enlarged main PA. Immediately distal to the origin of the LMCA was narrowing—with an obliquely oriented, slit-like course, secondary to compression by the massively dilated main PA (Figs. 1–3). The RV end-diastolic volume was severely enlarged at 225 cc/m2 (normal, <90 cc/m2). An incidental finding was an anomalous origin of the right coronary artery from the noncoronary cusp. Coronary angiograms revealed a 50% tapering stenosis in the proximal third of the LMCA, secondary to compression by the dilated main PA (Fig. 4). Right-sided heart catheterization yielded a right atrial pressure of 6 mmHg, a normal PA pressure of 26/13 mmHg, a pulmonary capillary wedge pressure of 7 mmHg, and a mean transpulmonary valve gradient of 6 mmHg.
Fig. 1.
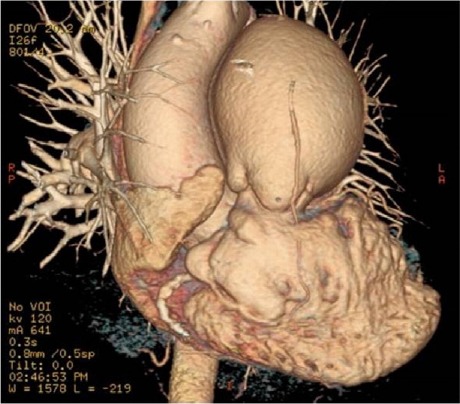
Computed tomographic angiogram (3-dimensional volume-rendering with electrocardiographic gating) shows massive enlargement of the main pulmonary artery at the level of the pulmonary valve.
Fig. 4.
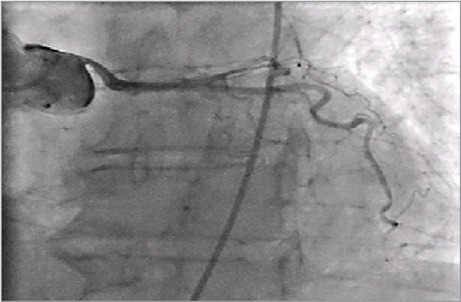
Coronary angiogram shows compression of the left main coronary artery by the enlarged main pulmonary artery.
Fig. 2.
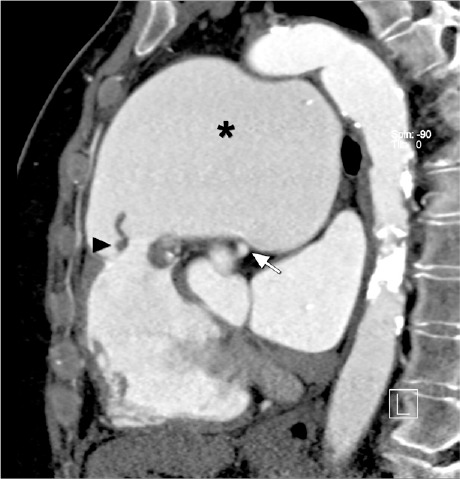
Computed tomographic angiogram (oblique sagittal multiplanar reconstruction) shows markedly thickened pulmonary valve leaflets (arrowhead), and a massively dilated main pulmonary artery (asterisk) that extrinsically compresses the nonatherosclerotic left main coronary artery (arrow).
Fig. 3.
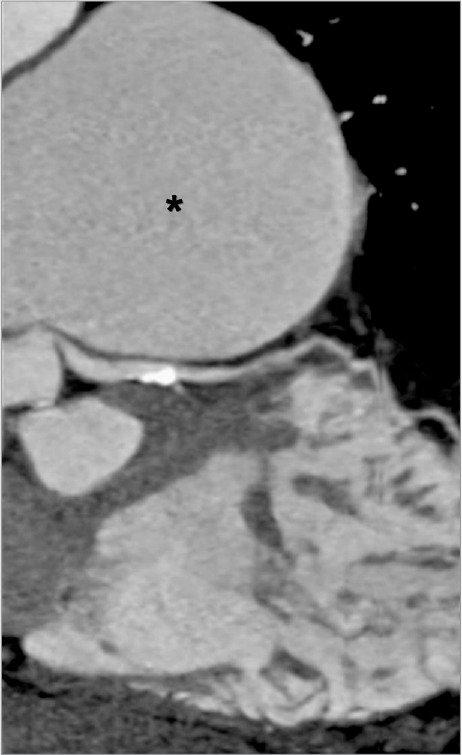
Computed tomographic angiogram (curved multiplanar reconstruction, along the course of the left main and proximal left anterior descending coronary arteries) shows extrinsic compression of the proximal left main coronary artery by the enlarged pulmonary artery (asterisk). Mild distal atherosclerosis (partially calcified) without significant luminal narrowing is noted.
The patient underwent surgical PA aneurysm repair by means of branch and main pulmonary arterioplasty, along with PV replacement with use of a 27-mm bioprosthetic pericardial valve. Of note, upon intraoperative inspection, we found the PV to be trileaflet with rudimentary, thickened, and dysmorphic leaflets. Follow-up computed tomographic angiograms revealed a substantial interval decrease in the size of the PA aneurysm and complete resolution of the LMCA compression (Figs. 5 and 6). The patient recovered uneventfully. As of July 2015, she had no recurrent chest discomfort and reported that she vigorously exercised daily with no functional limitations or chest symptoms.
Fig. 5.
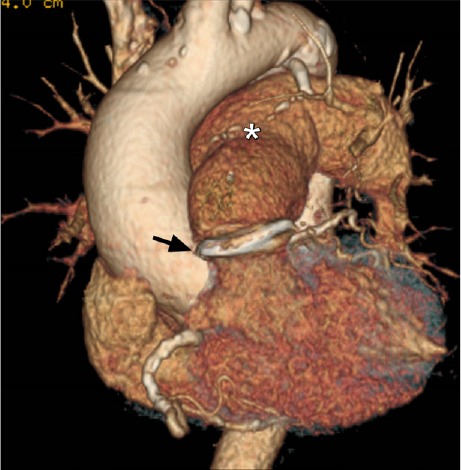
Postoperative computed tomographic angiogram (3-dimensional volume-rendering with electrocardiographic gating) shows a marked decrease in the size of the main pulmonary artery (asterisk), and an annuloplasty ring in place (arrow).
Fig. 6.
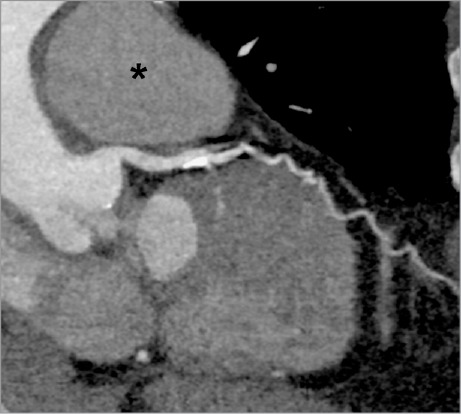
Postoperative computed tomographic angiogram (curved multiplanar reconstruction, along the course of the left main and proximal left anterior descending coronary arteries) shows complete relief of the extrinsic compression from the left main coronary artery. The pulmonary artery (asterisk) is no longer enlarged.
Discussion
Proximal LMCA compression by an enlarged PA was first described in 1957 in patients with severe pulmonary hypertension,5 both primary and secondary to pulmonary vascular obstruction or Eisenmenger syndrome. Radiographic risk factors for coronary insufficiency include the degree of coronary compression, a left main angle of takeoff from the left sinus of Valsalva of <30°, and a main PA/aorta diameter ratio >2.6 Some authors consider unprotected LMCA stenting7,8 to be a palliative alternative if surgical correction is not an option. Many case reports have described LMCA compression by an enlarged, severely hypertensive PA. Our report illustrates this rare and, to our knowledge, unique occurrence of LMCA compression caused by a massive PA aneurysm in the absence of PA hypertension. Heretofore, coronary insufficiency was not deemed to be a complication of dilated PAs without pulmonary hypertension. This important complication should be considered in patients who have valvular pulmonic stenosis-related pulmonary arteriopathy.
Footnotes
From: Departments of Medicine, Division of Cardiology (Drs. Bhatt, DeFaria Yeh, and Inglessis-Azuaje); Radiology, Cardiac Radiology Section (Dr. Ghoshhajra); Surgery, Cardiothoracic Surgery Section (Dr. MacGillivray); and Medicine and Pediatrics, Division of Cardiology (Dr. Liberthson); Massachusetts General Hospital, Boston, Massachusetts 02114
References
- 1.Miller RA, Lev M, Paul MH. Congenital absence of the pulmonary valve. The clinical syndrome of tetralogy of Fallot with pulmonary regurgitation. Circulation. 1962;26:266–78. doi: 10.1161/01.cir.26.2.266. [DOI] [PubMed] [Google Scholar]
- 2.Nagao GI, Daoud GI, McAdams AJ, Schwartz DC, Kaplan S. Cardiovascular anomalies associated with tetralogy of Fallot. Am J Cardiol. 1967;20(2):206–15. doi: 10.1016/0002-9149(67)90079-3. [DOI] [PubMed] [Google Scholar]
- 3.Lev M, Eckner FA. The pathologic anatomy of tetralogy of Fallot and its variations. Dis Chest. 1964;45:251–61. doi: 10.1378/chest.45.3.251. [DOI] [PubMed] [Google Scholar]
- 4.Bartter T, Irwin RS, Nash G. Aneurysms of the pulmonary arteries. Chest. 1988;94(5):1065–75. doi: 10.1378/chest.94.5.1065. [DOI] [PubMed] [Google Scholar]
- 5.Corday E, Gold H, Kaplan L. Coronary artery compression: an explanation for the cause of coronary insufficiency in pulmonary hypertension. Trans Am Coll Cardiol. 1957;7:93–103. [PubMed] [Google Scholar]
- 6.Dodd JD, Maree A, Palacios I, de Moor MM, Mooyaart EA, Shapiro MD et al. Images in cardiovascular medicine. Left main coronary artery compression syndrome: evaluation with 64-slice cardiac multidetector computed tomography. Circulation. 2007;115(1):e7–8. doi: 10.1161/CIRCULATIONAHA.106.645622. [DOI] [PubMed] [Google Scholar]
- 7.Rich S, McLaughlin VV, O'Neil W. Stenting to reverse left ventricular ischemia due to left main coronary artery compression in primary pulmonary hypertension. Chest. 2001;120(4):1412–5. doi: 10.1378/chest.120.4.1412. [DOI] [PubMed] [Google Scholar]
- 8.Caldera AE, Cruz-Gonzalez I, Bezerra HG, Cury RC, Palacios IF, Cockrill BA, Inglessis-Azuaje I. Endovascular therapy for left main compression syndrome. Case report and literature review. Chest. 2009;135(6):1648–50. doi: 10.1378/chest.08-2922. [DOI] [PubMed] [Google Scholar]


