Abstract
The timing of surgery for active infective endocarditis is challenging when patients exhibit mechanical dysfunction and hemodynamic compromise. Extracorporeal membrane oxygenation has been described in treating sepsis but not, insofar as we know, in treating the acute mechanical sequelae that arise from infective endocarditis. We report perhaps the first case that shows the usefulness of extracorporeal membrane oxygenation as a bridge to definitive treatment in a 35-year-old man who had infective endocarditis followed by aorto-atrial fistula and cardiopulmonary collapse.
Keywords: Aortic valve insufficiency/etiology/surgery; debridement; endocarditis, bacterial/complications/surgery; heart valve diseases/complications; extracorporeal membrane oxygenation/trends; infective endocarditis; shock, cardiogenic/therapy; shock, septic; substance abuse, intravenous/complications
Infective endocarditis (IE) remains a dangerous condition that carries substantial morbidity and a 1-year mortality rate approaching 30%.1 Surgery is potentially life-saving2 and is essential in 25% to 30% of patients during acute infection and in 20% to 40% during convalescence.3 Although early surgical intervention can improve survival prospects in patients who have severe complicated IE,4 hemodynamic failure is a strong predictor of operative death.5 Consequently, careful consideration is crucial in advance of high-risk surgery for patients with IE, and it is often provocative of controversy.3
Extracorporeal membrane oxygenation (ECMO) is a modified cardiopulmonary bypass technique that provides life support for patients with cardiac or respiratory failure by maintaining perfusion and oxygenation as a bridge to definitive therapy, or until native-organ function can be restored. Reports have described the use of ECMO for severe cardiogenic shock, for postcardiotomy shock, and (as an additional tool) for resuscitation from cardiac arrest.6 There is evidence for the use of ECMO in sepsis, but not in the presence of the mechanical sequelae of endocarditis.7 We report what we think is the first case of successful ECMO use to stabilize an adult patient who presented with cardiopulmonary collapse from aorto-atrial fistula (AAF) secondary to native-valve IE.
Case Report
In October 2013, a 35-year-old male intravenous-drug user presented with a one-week history of fatigue, chest pain, dyspnea, orthopnea, and right-foot pain. He needed urgent intubation, mechanical ventilation, and vasopressors for cardiopulmonary collapse. Physical examination revealed jugular venous distention, rales, a grade 4/6 systolic murmur, a loud diastolic murmur, and a painful erythematous lesion below the medial malleolus on the right foot consistent with an Osler's node (Fig. 1). Laboratory findings included leukocytosis and lactic acidosis. Urine toxicology results were positive for opiates, and blood cultures grew viridans streptococcus. The patient was started on ampicillin and ceftriaxone antibiotic agents on the basis of his blood culture and sensitivity results.
Fig. 1.
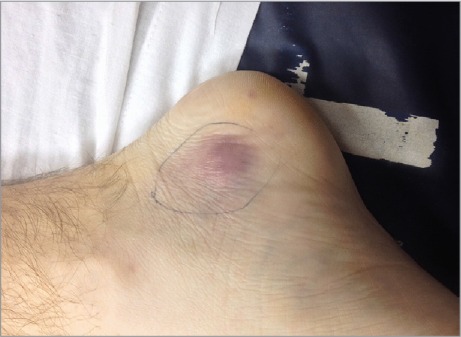
Photograph shows an erythematous lesion on the right foot suggestive of Osler's node.
A transthoracic echocardiogram (TTE) revealed a 2 × 1.5-cm vegetation on the anterior leaflet of the mitral valve (MV), together with moderate mitral regurgitation and aortic insufficiency (AI). Left ventricular systolic function was preserved. A subsequent transesophageal echocardiogram (TEE) showed a bicuspid aortic valve, the mobile echodensity of which was continuous with the echodensity of the MV (Fig. 2A); further, an aortic root abscess involved the left sinus of Valsalva, which had ruptured into the left atrium, as revealed by color-flow Doppler TEE (Fig. 2B). There was also evidence of a patent foramen ovale (PFO), with left-to-right flow. The patient was evaluated by a cardiothoracic surgeon (D.U.) for valve replacement and closure of the fistula.
Fig. 2.
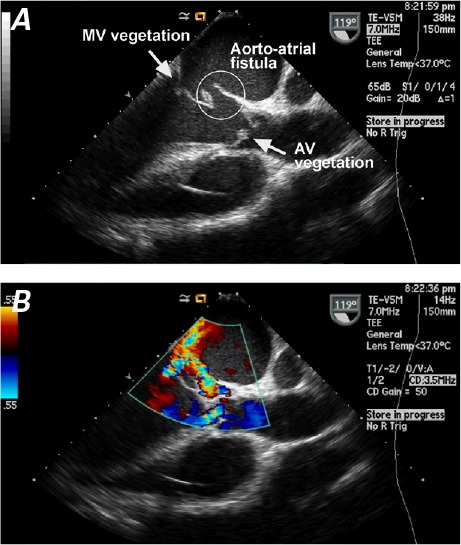
Two-dimensional transesophageal echocardiogram shows A) an aorto–left atrial fistula, together with vegetations on the mitral valve (MV) and aortic valve (AV). B) Color-flow Doppler mode shows the flow across the fistula, toward the left atrium.
On hospital day 2, the patient developed refractory hypoxemia due to worsening pulmonary edema. Emergency TTE revealed complete rupture of the aortic valve, with severe AI. Consequently, percutaneous venoarterial ECMO was initiated.
A 17F left femoral artery catheter and a 23F–25F right femoral vein cannula were placed percutaneously for venoarterial ECMO; an 8F catheter was passed into the left femoral artery for distal perfusion of the leg. The patient was placed on venoarterial ECMO, and flow was adjusted to achieve adequate perfusion, as evidenced by improved vital signs, oxygenation, and normalization of serum lactate levels.
Although this patient had improved hemodynamically, there was substantial concern, from a physiologic standpoint, regarding his pulmonary status while on ECMO. The pulmonary circuit was now being charged with both antegrade flow from the right ventricle and retrograde flow by means of venoarterial ECMO in the presence of severe AI and mitral regurgitation. Consequently, pulmonary edema was a major concern, and the patient was taken to the operating room immediately after the serum lactate cleared, 8 hours after ECMO stabilization.
The aorta was opened obliquely, revealing a bicuspid aortic valve with a 1 × 3-cm vegetation filling the noncoronary sinus, and a destroyed noncoronary leaflet. The sinus had perforated just below the commissure—between the left and noncoronary cusps—into the left atrium, just above the mitral annulus. The valve was excised, all vegetations were débrided, and the edges of the fistula were débrided back to clean tissue. A Carpentier-Edwards Perimount® Magna® 23-mm bovine pericardial aortic valve prosthesis (Edwards Life-sciences LLC; Irvine, Calif) was sutured in place near the noncoronary cusp by means of the ThermaFix® tissue process (Edwards Lifesciences). The A2 and A1 segments of the MV were also débrided. There was no evidence of ruptured chords or of any other pathologic condition extending onto any other facets of the valve. Once débridement was complete, a 2.5 × 3-cm bovine pericardial patch was sutured to the aorto-atrial defect. The defect in the anterior leaflet of the MV was also repaired, and this was followed by placement of a 27-mm Carpentier-Edwards Physio II® annuloplasty ring (Edwards Lifesciences). A 3 × 3-cm patch was then sutured in place to reinforce the area of the fistula and the mitral annulus, and the PFO was also surgically closed. The patient was weaned from cardiopulmonary bypass and rewarmed, and ECMO was withdrawn.
Our patient's postoperative course was uneventful: the cardiogenic shock resolved, and the sepsis cleared. Postoperative TEE revealed successful closure of the AAF with loss of color-flow Doppler communication between the aorta and the left atrium (Fig. 3). Two weeks later, the patient was discharged from the hospital with appropriate antibiotics for an additional 4 weeks. At follow-up evaluation, 4 and 8 months later, he remained asymptomatic and was doing well.
Fig. 3.
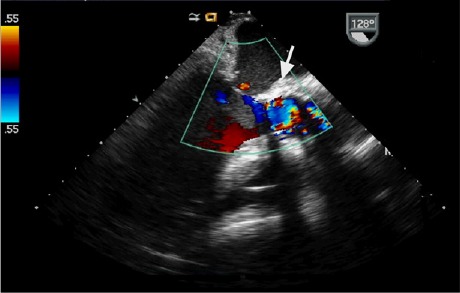
Transesophageal echocardiogram shows a surgically closed fistula (arrow) and the concomitant loss of color flow into the left atrium.
Discussion
Aorto-atrial fistula is a rare sequela of IE, reported in 1% to 1.6% of cases of IE.8 Such fistulae create intracardiac shunts, which can be hemodynamically unstable and are associated with increased morbidity and mortality rates. Prompt consideration of hemodynamic stabilization and surgery is indicated. According to a retrospective analysis,8 76 patients (1.6%), among 4,681 with episodes of IE, developed AAFs that were identified intraoperatively or on echocardiography. The AAFs occurred in the aortic position and were more frequently found in prosthetic- than in native-valve endocarditis (5.8% vs 3.6%, P=0.04). Sixty-six of these 76 patients (87%) underwent surgical treatment, with an in-hospital mortality rate of over 40%, despite surgery.
Extracorporeal membrane oxygenation is a valuable form of life support that should be considered in order to stabilize the patient until definitive surgical management can be achieved. There is scant evidence concerning the use of ECMO in complex cases of IE. In one reported case of Aerococcus urinae mitral and aortic valve endocarditis,9 the patient sustained refractory cardiogenic shock after valve replacement; ECMO was used successfully as rescue mechanical support. In another instance, recurrent prosthetic-valve IE in a heart-transplant patient presented as cardiogenic shock; that patient underwent hemodynamic stabilization with ECMO until repeat heart transplantation could be performed.10 To our knowledge, our case report is the first to describe the successful use of venoarterial ECMO in an adult patient who presented with profound cardiopulmonary failure due to native-valve IE, complicated by an AAF.
Our case illustrates the usefulness of ECMO as a bridge to definitive treatment in a patient with IE complicated by AAF, acute valvular insufficiency, and multifactorial shock that required both mechanical ventilation and circulatory support. Our patient was not expected to survive without the hemodynamic stabilization provided by ECMO. Short-term ECMO can be considered for the mechanical sequelae of IE in a select population.
Footnotes
From: Department of Medicine (Drs. Gluck and Noyes), University of Connecticut School of Medicine, Farmington, Connecticut 06032; and Division of Cardiology (Drs. Gluck, Parker, and Ramu) and Department of Cardiac Surgery (Dr. Underhill), Hartford Hospital, Hartford, Connecticut 06102
Presented at the American College of Cardiology 63rd Annual Scientific Session & Expo, Washington, DC, 29–31 March 2014.
References
- 1.Cabell CH, Jollis JG, Peterson GE, Corey GR, Anderson DJ, Sexton DJ et al. Changing patient characteristics and the effect on mortality in endocarditis. Arch Intern Med. 2002;162(1):90–4. doi: 10.1001/archinte.162.1.90. [DOI] [PubMed] [Google Scholar]
- 2.Olaison L, Pettersson G. Current best practices and guidelines: indications for surgical intervention in infective endocarditis. Infect Dis Clin North Am. 2002;16(2):453–75. xi. doi: 10.1016/s0891-5520(01)00006-x. [DOI] [PubMed] [Google Scholar]
- 3.Manne MB, Shrestha NK, Lytle BW, Nowicki ER, Blackstone E, Gordon SM et al. Outcomes after surgical treatment of native and prosthetic valve infective endocarditis. Ann Thorac Surg. 2012;93(2):489–93. doi: 10.1016/j.athoracsur.2011.10.063. [DOI] [PubMed] [Google Scholar]
- 4.Thuny F, Beurtheret S, Mancini J, Gariboldi V, Casalta JP, Riberi A et al. The timing of surgery influences mortality and morbidity in adults with severe complicated infective endocarditis: a propensity analysis. Eur Heart J. 2011;32(16):2027–33. doi: 10.1093/eurheartj/ehp089. [DOI] [PubMed] [Google Scholar]
- 5.Jault F, Gandjbakhch I, Rama A, Nectoux M, Bors V, Vaissier E et al. Active native valve endocarditis: determinants of operative death and late mortality. Ann Thorac Surg. 1997;63(6):1737–41. doi: 10.1016/s0003-4975(97)00117-3. [DOI] [PubMed] [Google Scholar]
- 6.Schuerer DJ, Kolovos NS, Boyd KV, Coopersmith CM. Extracorporeal membrane oxygenation: current clinical practice, coding, and reimbursement. Chest. 2008;134(1):179–84. doi: 10.1378/chest.07-2512. [DOI] [PubMed] [Google Scholar]
- 7.Maclaren G, Butt W. Extracorporeal membrane oxygenation and sepsis. Crit Care Resusc. 2007;9(1):76–80. [PubMed] [Google Scholar]
- 8.Anguera I, Miro JM, Vilacosta I, Almirante B, Anguita M, Munoz P et al. Aortocavitary fistulous tract formation in infective endocarditis: clinical and echocardiographic features of 76 cases and risk factors for mortality. Eur Heart J. 2005;26(3):288–97. doi: 10.1093/eurheartj/ehi034. [DOI] [PubMed] [Google Scholar]
- 9.Bruegger D, Beiras-Fernandez A, Weis F, Weis M, Kur F. Extracorporeal support in a patient with cardiogenic shock due to Aerococcus urinae endocarditis. J Heart Valve Dis. 2009;18(4):418–20. [PubMed] [Google Scholar]
- 10.Huang HH, Chuang YC, Lee KC, Sue SH, Chang CY, Wei J. Prosthetic endocarditis treated by repeated heart transplantation: report of a successful case. Transplant Proc. 2012;44(4):1171–3. doi: 10.1016/j.transproceed.2012.01.095. [DOI] [PubMed] [Google Scholar]


