Abstract
Spontaneous coronary artery dissection is a rare cause of acute coronary syndrome. Clinical presentation ranges from chest pain alone to ST-segment-elevation myocardial infarction, ventricular fibrillation, and sudden death. The treatment of patients with spontaneous coronary artery dissection is challenging because the disease pathophysiology is unclear, optimal treatment is unknown, and short- and long-term prognostic data are minimal.
We report the case of a 70-year-old woman who presented with an acute ST-segment-elevation myocardial infarction secondary to a spontaneous dissection of the left anterior descending coronary artery. She was treated conservatively. Cardiac tamponade developed 16 hours after presentation. Repeat coronary angiography revealed extension of the dissection. Medical therapy was continued after the hemopericardium was aspirated. The patient remained asymptomatic 3 years after hospital discharge. To our knowledge, this is the first reported case of spontaneous coronary artery dissection in association with cardiac tamponade that was treated conservatively and had a successful outcome.
Keywords: Acute coronary syndrome/therapy; aneurysm, dissecting/therapy; cardiac tamponade/etiology/therapy; disease management; elderly; female; myocardial infarction/etiology; rupture, spontaneous; thrombolytic therapy/contraindications; treatment outcome
Spontaneous coronary artery dissection (SCAD) is a rare cause of acute coronary syndrome.1,2 Pretty reported the first case of SCAD and hemopericardium in 1931.3 Subsequently, there have been 5 reported cases of SCAD with cardiac tamponade, which have had varying outcomes.4–8 We report a case of a 70-year-old woman who presented with spontaneous dissection of the left coronary artery and cardiac tamponade, which we managed conservatively with a successful outcome.
Case Report
A 70-year-old woman presented at the emergency department with severe upper-back and chest pain, after lifting her husband. She was hemodynamically stable. The results of cardiovascular examination were normal: there were no murmurs, gallops, or rubs, no jugular venous distention, and normal peripheral pulses. An electrocardiogram revealed Q waves in leads V2 through V6 and 3- to 4-mm ST-segment elevations in leads V2 through V5. A computed tomographic angiogram of the chest did not show aortic dissection. The patient was given aspirin, clopidogrel, and metoprolol before emergency cardiac catheterization.
Coronary angiography revealed a dissection or occlusion in the left anterior descending coronary artery (LAD) at the origin of a large 2nd diagonal branch (Fig. 1). The LAD was tortuous and a dissection flap extended 2 to 3 cm into the distal vessel, which was well opacified by Thrombolysis In Myocardial Infarction (TIMI)-3 flow. All other coronary arteries were normal. Left ventricular angiography showed anterior-wall hypokinesis and a left ventricular ejection fraction (LVEF) of 0.50. Percutaneous coronary intervention (PCI) was not attempted because of the tortuous course of the LAD, the risk of compromised flow to the large diagonal branch, and the presence of TIMI-3 flow. The patient was treated with enoxaparin, eptifibatide, aspirin, clopidogrel, and metoprolol.
Fig. 1.
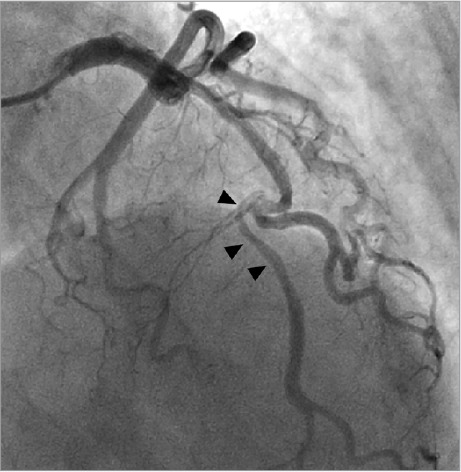
Coronary angiogram at initial presentation reveals dissection or occlusion (arrowheads) in the mid left anterior descending coronary artery at the origin of a large 2nd diagonal branch.
Supplemental motion image (3.8MB, mp4) is available for Figure 1.
Sixteen hours after her presentation, the patient developed recurrent chest pain that worsened with inspiration. She became hypotensive and developed jugular venous distention and a loud pericardial friction rub. A transthoracic echocardiogram (TTE) showed a 1-cm anterior pericardial effusion with fibrinous material, inversion of the right atrial wall, a small under-filled right ventricle, a dyskinetic left ventricular apex, an akinetic anterior wall and septum, and an LVEF of 0.35.
An emergency coronary angiogram showed extension of the LAD dissection toward the apex (Fig. 2), with TIMI-1 filling of the LAD and the major diagonal branch. The left main and left circumflex coronary arteries remained intact. Pericardiocentesis yielded 150 mL of blood and was accompanied by immediate improvement in blood pressure. When the patient developed cardiogenic shock, she needed intubation and mechanical ventilation, together with an intra-arterial balloon pump with inotropic and vasopressor support. She made a full recovery.
Fig. 2.
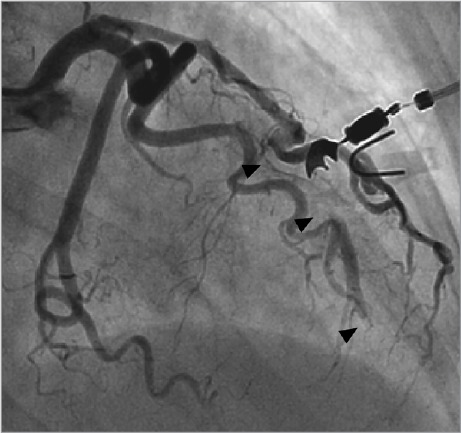
Coronary angiogram 16 hours after presentation shows extension of the dissection (arrowheads) of the left anterior descending coronary artery (LAD) toward the apex, with Thrombolysis In Myocardial Infarction-1 filling of the LAD and the major diagonal branch.
Supplemental motion image (3.1MB, mp4) is available for Figure 2.
Two weeks later, TTE showed a 2-cm apical thrombus, moderate left ventricular dysfunction, and resolution of the pericardial effusion. The patient was anticoagulated with warfarin and had no recurrence of cardiac tamponade.
Three months after her discharge from the hospital, the patient experienced similar upper-back and chest pain. Electrocardiography showed T-wave pseudo-normalization in the anterolateral leads, and her troponin level was 0.7 ng/mL. A coronary angiogram revealed normal anatomy in the LAD and diagonal branch (Fig. 3). She remained asymptomatic for the next 3 years.
Fig. 3.
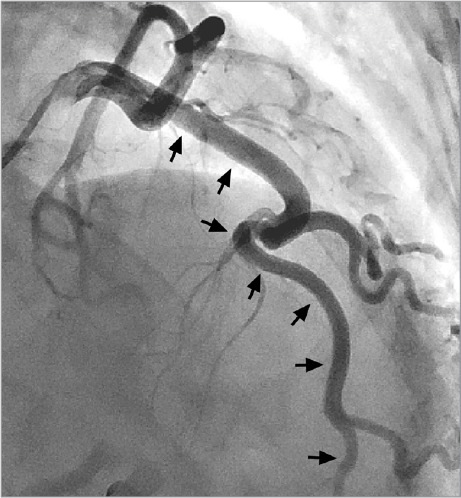
Coronary angiogram 3 months after presentation shows a normal left anterior descending coronary artery (arrows) and diagonal branch.
Supplemental motion image (4MB, mp4) is available for Figure 3.
Discussion
Spontaneous coronary artery dissection is an infrequent cause of acute coronary syndrome that predominantly affects young women without coronary risk factors, often in the peripartum period.1,2 Clinical presentation ranges from chest pain alone to ST-elevation myocardial infarction (STEMI), ventricular fibrillation, and sudden death.
The first reported case was that of a 42-year-old woman with a ruptured dissecting aneurysm of the right coronary artery and blood in the pericardial sac on autopsy.3 Subsequently, 5 cases of SCAD with cardiac tamponade have been reported4–8; 4 of those patients presented with chest pain and died within 48 hours.4–7 Autopsy confirmed coronary dissection and blood in the pericardial space in all 4, but only one had been diagnosed with SCAD before death, by coronary angiography.4 That patient received intracoronary urokinase for occlusion of the right coronary artery without successful thrombolysis; he died suddenly 2 days later. Another of these patients received systemic tenecteplase for STEMI and died during preparation for salvage angioplasty.5 The only patient reported to have survived underwent successful emergency coronary artery bypass grafting (CABG) of the LAD and left circumflex coronary arteries, the dissections of which had induced cardiac tamponade.8
The management of patients with SCAD is challenging for many reasons: the pathophysiology is not clear; there is a wide range of clinical presentations; coronary “lumenography” is unable to reveal the deeper structure of the vascular wall and possesses inadequate diagnostic accuracy; optimal treatment is unknown; and well-established short- and long-term prognostic data are not available.
Most patients with SCAD are at first assumed to have typical atherosclerotic acute coronary syndromes and are treated either with antithrombotic and antiplatelet agents for non-STEMI or unstable angina, or with thrombolytic agents or emergency PCI for STEMI. The potential for therapeutic misadventure in the treatment of SCAD is rife: thrombolytic agents and antithrombotic and antiplatelet agents might help to prevent coronary thrombosis and occlusion,9,10 but these might also expand the coronary intramural hematoma and worsen the dissection.11 Performing PCI for SCAD can mechanically propagate dissection, and stents can inadvertently buttress a false lumen.12 In addition, CABG is difficult because of the thinness and residual dissection of the coronary arteries at the anastomotic site. Even diagnostic coronary arteriography appears to be more hazardous in SCAD patients.13
The risk factors for cardiac tamponade in cases of SCAD are unknown. Thrombolytic agents were used in 2 of the 6 cases in the literature, and antithrombotic and antiplatelet agents were used in our patient. In 3 reported studies, 53% to 75% of the SCAD patients who were given thrombolytic agents deteriorated, necessitating rescue PCI or CABG.14–16 However, none of these studies reported cardiac tamponade as a sequela. Hemopericardium and tamponade could result from extension of medial dissection or intramural hematoma into the pericardial space, from ruptured vasa vasorum or myocardium in the infarcted area, or from postinfarction pericarditis. Our patient's prescription of aspirin, enoxaparin, and eptifibatide on presentation could have contributed to her tamponade.
There are no guidelines for the treatment of SCAD. The spectrum of treatment includes medical therapy, PCI, and CABG. We treated our patient conservatively because of the location and tortuosity of the involved vessel, and, despite her development of cardiac tamponade, she responded well to continued conservative treatment. Factors that favor revascularization are ongoing ischemia and hemodynamic instability, proximal location of dissection, and the extensive involvement and persistence of dissection after conservative treatment.2
The success rate of PCI in treating SCAD ranges from 65% to 92% among reported studies.1,2,15,16 Reasons for failure include inability to cross into the distal true lumen with a wire or balloon and reduction in flow caused by dissection or hematoma during PCI. Patients with left main disease or extensive involvement of coronary arteries treated with CABG tend to have favorable outcomes,1,2,15 although investigators in one study15 reported a high rate (73%) of late bypass-graft occlusion. A very good prognosis was reported in patients who were treated conservatively from the outset,1,15,17 with 54% showing spontaneous resolution at follow-up angiographic evaluation.17
Intravascular ultrasonography can play an important role in the diagnosis of SCAD, especially in cases that are angiographically unclear. Further, it can accurately direct wire placement in the true lumen, and it can ensure optimal sizing, positioning, and deployment of coronary stents.
To our knowledge, this is the first reported case of SCAD in association with cardiac tamponade that was treated conservatively and had a successful outcome.
Supplementary Material
Footnotes
From: Department of Cardiology, Kaiser Permanente Medical Center, San Francisco, California 94115
References
- 1.Vanzetto G, Berger-Coz E, Barone-Rochette G, Chavanon O, Bouvaist H, Hacini R et al. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg. 2009;35(2):250–4. doi: 10.1016/j.ejcts.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Mortensen KH, Thuesen L, Kristensen IB, Christiansen EH. Spontaneous coronary artery dissection: a Western Denmark Heart Registry study. Catheter Cardiovasc Interv. 2009;74(5):710–7. doi: 10.1002/ccd.22115. [DOI] [PubMed] [Google Scholar]
- 3.Pretty HC. Dissecting aneurysm of coronary artery in a woman aged 42: rupture. Br Med J. 1931;1(3667):667. [Google Scholar]
- 4.Ebe K, Nagai T, Wakiya Y, Igarashi T. Sudden death presenting cardiac tamponade in a case of coronary dissection [in Japanese] Kokyu To Junkan. 1992;40(4):403–6. [PubMed] [Google Scholar]
- 5.Hidalgo-Urbano RJ, Almendro-Delia M, Villar-Rodriguez JL. Haemopericardium in a fibrinolysis in acute myocardial infarction secondary to a spontaneous coronary artery dissection [in Spanish] Rev Esp Cardiol. 2011;64(6):539–40. doi: 10.1016/j.recesp.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Cheung A, Chan CW. Dissecting aneurysm of coronary artery presenting as cardiac tamponade. N Z Med J. 1990;103(886):129–30. [PubMed] [Google Scholar]
- 7.Hayes CR, Lewis D. Spontaneous coronary artery dissection of the left circumflex artery causing cardiac tamponade and presenting with atrial fibrillation: a case report and review of the literature. Angiology. 2007;58(5):630–5. doi: 10.1177/0003319707304532. [DOI] [PubMed] [Google Scholar]
- 8.Badmanaban B, McCarty D, Mole DJ, McKeown PP, Sarsam MA. Spontaneous coronary artery dissection presenting as cardiac tamponade. Ann Thorac Surg. 2002;73(4):1324–6. doi: 10.1016/s0003-4975(01)03240-4. [DOI] [PubMed] [Google Scholar]
- 9.Behnam R, Tillinghast S. Thrombolytic therapy in spontaneous coronary artery dissection. Clin Cardiol. 1991;14(7):611–4. doi: 10.1002/clc.4960140714. [DOI] [PubMed] [Google Scholar]
- 10.Leclercq F, Messner-Pellenc P, Carabasse D, Lucke N, Rivalland F, Grolleau R. Successful thrombolysis treatment of a spontaneous left main coronary artery dissection without subsequent surgery. Eur Heart J. 1996;17(2):320–1. doi: 10.1093/oxfordjournals.eurheartj.a014853. [DOI] [PubMed] [Google Scholar]
- 11.Buys EM, Suttorp MJ, Morshuis WJ, Plokker HW. Extension of a spontaneous coronary artery dissection due to thrombolytic therapy. Cathet Cardiovasc Diagn. 1994;33(2):157–60. doi: 10.1002/ccd.1810330216. [DOI] [PubMed] [Google Scholar]
- 12.Adlam D, Cuculi F, Lim C, Banning A. Management of spontaneous coronary artery dissection in the primary percutaneous coronary intervention era. J Invasive Cardiol. 2010;22(11):549–53. [PubMed] [Google Scholar]
- 13.Vrints CJ. Spontaneous coronary artery dissection. Heart. 2010;96(10):801–8. doi: 10.1136/hrt.2008.162073. [DOI] [PubMed] [Google Scholar]
- 14.Shamloo BK, Chintala RS, Nasur A, Ghazvini M, Shariat P, Diggs JA, Singh SN. Spontaneous coronary artery dissection: aggressive vs. conservative therapy. J Invasive Cardiol. 2010;22(5):222–8. [PubMed] [Google Scholar]
- 15.Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126(5):579–88. doi: 10.1161/CIRCULATIONAHA.112.105718. [DOI] [PubMed] [Google Scholar]
- 16.Moukarbel GV, Alam SE. Spontaneous coronary artery dissection: management options in the stent era. J Invasive Cardiol. 2004;16(6):333–5. [PubMed] [Google Scholar]
- 17.Alfonso F, Paulo M, Lennie V, Dutary J, Bernardo E, Jimenez-Quevedo P et al. Spontaneous coronary artery dissection: long-term follow-up of a large series of patients prospectively managed with a “conservative” therapeutic strategy. JACC Cardiovasc Interv. 2012;5(10):1062–70. doi: 10.1016/j.jcin.2012.06.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


