Abstract
The inflammatory response induced by cardiopulmonary bypass decreases vascular tone, which in turn can lead to vasoplegic syndrome. Indeed the hypotension consequent to on-pump cardiac surgery often necessitates vasopressor and intravenous fluid support. Methylene blue counteracts vasoplegic syndrome by inhibiting the formation of nitric oxide.
We report the use of methylene blue in a 75-year-old man who developed vasoplegic syndrome after cardiac surgery. After the administration of methylene blue, his hypotension improved to the extent that he could be weaned from vasopressors. The use of methylene blue should be considered in patients who develop hypotension refractory to standard treatment after cardiac surgery.
Keywords: Cardiopulmonary bypass/adverse effects; hemodynamics/drug effects; hypotension/drug therapy; methylene blue/administration & dosage/therapeutic use; nitric oxide/antagonists & inhibitors; shock, vasodilatory/physiopathology; vasoconstrictor agents/therapeutic use; vasodilation/physiology; vasoplegia/etiology/therapy; vasopressors/therapeutic use
Vasoplegic syndrome (VS) is a condition characterized by hypotension (mean arterial pressure, <50 mmHg), increased cardiac index (CI) (>2.5 L/min/m2), low systemic vascular resistance (<800 dynes·s/cm5), normal or increased filling pressures, and increased vasopressor and fluid requirements. This phenomenon occurs in approximately 5% of patients who undergo cardiac surgery.1 Although the mechanism of VS is largely unknown, study results suggest a multifactorial cause consisting of hemodilution, baroreceptor reflexes, complement activation, and an inflammatory response that results in the release of nitric oxide and in subsequent vasodilation.2
Methylene blue (MB), a nitric oxide synthase inhibitor, has been shown to be a safe and effective therapeutic option in patients with hypotension refractory to vasopressors and fluids after on-pump cardiac surgery.3,4 The following case report discusses the use of MB in a patient who remained hypotensive after aortic valve replacement and tricuspid valve repair, despite fluid resuscitation and standard vasopressor infusions.
Case Report
In September 2013, a 75-year-old black man with a history of coronary artery disease, congestive heart failure, end-stage renal disease, and hypertension presented at our hospital with shortness of breath and hypotension that had begun during dialysis. He was stabilized with dobutamine and fluids and was subsequently admitted for further evaluation.
A transthoracic echocardiogram revealed severe aortic stenosis with a valve area of 0.7 cm2, severe tricuspid regurgitation, moderate pulmonary hypertension with a peak right ventricular systolic pressure of 57 mmHg, and a left ventricular ejection fraction of 0.35 to 0.40. Coronary angiograms showed mild coronary artery disease. The patient's condition worsened, and he was transferred to the intensive care unit on high doses of inotropic agents. His pulmonary artery pressure was 60/30 mmHg, and his CI was 2.1 L/min/m2 on 25 μg/kg/min of dobutamine.
He was taken to the operating room for replacement of the aortic valve with a 23-mm Carpentier-Edwards Perimount® Magna® pericardial valve and for repair of the tricuspid valve with a 32-mm Carpentier-Edwards Physio II® annuloplasty ring (both Edwards Lifesciences LLC; Irvine, Calif). An intraoperative transesophageal echocardiogram showed normal left ventricular function. Total cross-clamp and cardiopulmonary bypass (CPB) times were 115 and 193 min, respectively. The patient's mean arterial pressure (MAP) postoperatively was 50 mmHg on 0.2 μg/kg/min of epinephrine, 0.25 μg/kg/min of norepinephrine, 0.04 U/min of vasopressin, and 0.3 μg/kg/min of milrinone. Hemodynamic measurements immediately after surgery revealed a CI of 3.47 L/min/m2 and a systemic vascular resistance (SVR) of 425 dynes·s/cm5. The pulmonary artery diastolic pressure (PAD) was 19 mmHg. Norepinephrine was increased gradually over the next 6 hours to 0.4 μg/kg/min, but the patient's MAP reached only 53 mmHg. His CI decreased to 2.9 L/min/m2 and his SVR increased to 625 dynes·s/cm5. The PAD remained 19 mmHg. In addition to administering the high dose of norepinephrine, we aggressively pursued and accomplished volume resuscitation, with no improvement in MAP.
Because the patient's blood pressure and SVR did not respond to high doses of vasopressors and fluids, we administered a 50-mg dose of MB. Over the next 3 hours, his MAP increased to 74 mmHg, SVR increased to 825 dynes·s/cm5, and CI decreased to 2.4 L/min/m2. The PAD was not affected by MB administration. Norepinephrine was tapered to 0.24 μg/kg/min, epinephrine was decreased to 0.08 μg/kg/min, and the patient remained normotensive. Figure 1 shows the postoperative variations in MAP and norepinephrine infusion. The cortisol level, which was checked to rule out adrenal insufficiency, was normal (35.6 μg/dL). About a month later, the patient died of noncardiac causes.
Fig. 1.
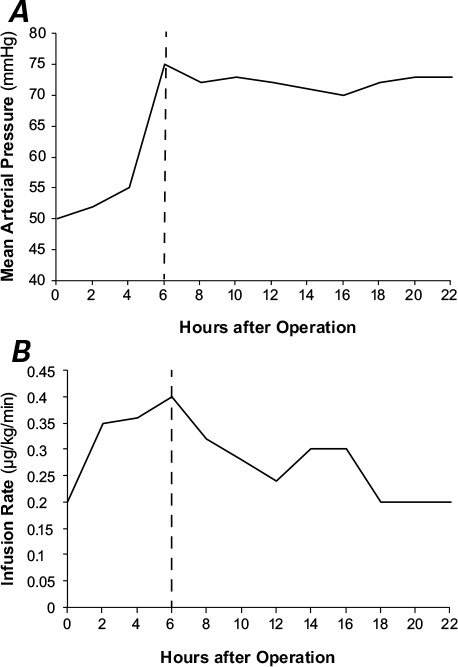
Graphs show A) mean arterial pressure variations and B) the norepinephrine infusion rate, versus time after cardiac surgery. Dashed lines indicate the time of methylene blue administration.
Discussion
Vasoplegic syndrome is a potentially fatal condition after cardiac surgery; Gomes and colleagues5 found an increased morbidity and mortality rate in patients who developed VS that persisted for 36 to 48 hours postoperatively. Cardiopulmonary bypass elicits a severe inflammatory response that releases such substances as histamine, cytokines, and interleukins. These substances initiate the conversion of arginine to nitric oxide, via nitric oxide synthase. Nitric oxide then up-regulates the guanylate cyclase enzyme, which in turn increases intracellular cyclic guanosine monophosphate (cGMP)—resulting in systemic vasodilation and hypotension.1,6,7 Methylene blue has been shown to counteract this vasodilation by inhibiting nitric oxide synthase3 (Fig. 2).
Fig. 2.
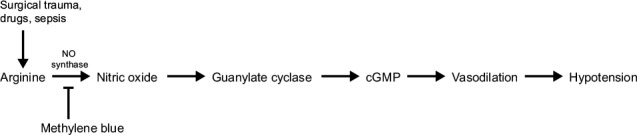
Diagram shows the mechanism of action of methylene blue in the nitric oxide pathway.
cGMP = cyclic guanosine monophosphate; NO = nitric oxide
Risk factors associated with the development of VS have been identified. Investigators have found an association between the development of VS after cardiac surgery and the preoperative use of heparin, angiotensin-converting enzyme (ACE) inhibitors, and calcium channel blockers.7,8 Our patient was not administered any of these medications preoperatively. The type of surgery being performed has also been shown to be an independent risk factor for the development of vasoplegia. Specifically, valve procedures increase the risk of developing VS, in comparison with coronary artery bypass grafting.1 In 2009, M.A. Levin and colleagues9 showed a correlation between a decrease in MAP immediately after CPB initiation and the development of VS. The decrease in MAP was quantified by calculating the area above the MAP curve. A clinically significant area above the curve was defined as a decrease in MAP of >20% within the first 5 minutes of cardiopulmonary bypass, beginning at baseline (before CPB initiation). Patients who experienced a significant decline in intraoperative MAP had a 23% chance of developing postoperative VS versus a 16.9% chance in those who did not have a significant decline in intraoperative MAP. Other risk factors for postoperative development of VS in Levin's study9 included the old additive EuroScore (the method used before 2011 to calculate predicted operative mortality rates in patients undergoing heart surgery), the procedure type, the length of CPB, the pre- and post-CPB hematocrits, the core temperature on CPB, and preoperative ACE-inhibitor or β-blocker use.
Because of its effect on the nitric oxide pathway, MB is able to improve hemodynamic status. In a study conducted by Leyh and colleagues,4 MB administration significantly increased SVR and MAP in 92.4% of patients within 12 hours after its infusion. Furthermore, norepinephrine requirements decreased from 0.5 to 0.2 μg/kg/m2 just 6 hours after the administration of MB. However, Leyh's study was limited by small sample size and lack of randomization. Ozal and coworkers10 validated the benefit of MB in their randomized-control trial. One hundred patients who were scheduled to undergo coronary artery bypass grafting and were at high risk of developing vasoplegic syndrome (because of their preoperative use of ACE inhibitors, calcium channel blockers, or heparin) were randomized to receive either MB or placebo preoperatively. The prevalence of vasoplegia in the treatment and control groups was 0 and 26%, respectively. Those who received MB had a significantly higher postoperative SVR and MAP, needed fewer norepinephrine and crystalloid infusions, and underwent a significantly shorter mean length of stay in intensive care units.
In 2004, 56 patients who developed VS after cardiac surgery were randomized to receive a single dose (1.5 mg/kg) of MB or placebo.3 The mortality rate in the placebo group was 21.4%, yet there were no deaths in the group that received MB. The rates of renal failure, respiratory failure, and sepsis in the placebo group were 14.3%, 14.3%, and 25%, respectively, whereas these particular comorbidities did not occur among the patients treated with MB.
Methylene blue is the primary treatment for methemoglobinemia and is sometimes used to treat cyanide poisoning. More recently it has been shown to be effective in the treatment of vasoplegia related to sepsis11 and vasoplegia after severe protamine reactions.12 In addition, MB has been shown to decrease hemodialysis-related hypotension13 and hypotension that results from chronic ACE-inhibitor use.7
Common adverse effects associated with MB are minimal and include greenish-blue–colored urine and decreased (misleadingly so) pulse oximetry readings. Other notable side effects include cardiac arrhythmias, decreased cardiac output, increased pulmonary vascular pressure, abdominal pain, nausea, and headache; however, these are less frequent.4,7
The dosing and delivery method of MB administration is debatable. Methylene blue is available as a solution (10 mg/mL), and is typically administered to patients who develop VS after cardiac surgery as a 1- to 2-mg/kg bolus.3,4,7 A continuous infusion may be used in patients who do not respond to a single bolus.7 Our patient's weight was 61 kg; a 50-mg dose of MB sufficiently improved hemodynamic levels, so we did not resort to a higher dose or continuous infusion. Because MB is not easily titratable and carries the risk of misleading pulse oximetry readings, we typically elect to use standard first-line treatments such as norepinephrine and vasopressin. If these medications fail, we use MB.
In conclusion, the goal of this case study is not to present novel findings; rather, it is to remind clinicians of the usefulness of MB in patients who develop vasoplegia. It is used in approximately 5% of our own surgical population and has proved to be a very successful tool in combating refractory hypotension. Vasoplegic syndrome seen after cardiac surgery is associated with high morbidity and mortality rates. Predictors of postoperative VS have been identified, and patients with those risk factors might well benefit from treatment with MB.
Footnotes
From: Departments of Cardiothoracic Surgery (Dr. Manghelli) and Anesthesiology (Dr. Tadros), Christian Hospital, St. Louis, Missouri 63136; A.T. Still University (Dr. Manghelli), Kirksville, Missouri 63501; and Division of Cardiothoracic Surgery (Drs. Brown and Munfakh), Washington University School of Medicine in St. Louis, St. Louis, Missouri 63110
Dr. Manghelli is now at the Indiana University School of Medicine, Indianapolis, Indiana 46202.
References
- 1.Fischer GW, Levin MA. Vasoplegia during cardiac surgery: current concepts and management. Semin Thorac Cardiovasc Surg. 2010;22(2):140–4. doi: 10.1053/j.semtcvs.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Carrel T, Englberger L, Mohacsi P, Neidhart P, Schmidli J. Low systemic vascular resistance after cardiopulmonary bypass: incidence, etiology, and clinical importance. J Card Surg. 2000;15(5):347–53. doi: 10.1111/j.1540-8191.2000.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 3.Levin RL, Degrange MA, Bruno GF, Del Mazo CD, Taborda DJ, Griotti JJ, Boullon FJ. Methylene blue reduces mortality and morbidity in vasoplegic patients after cardiac surgery. Ann Thorac Surg. 2004;77(2):496–9. doi: 10.1016/S0003-4975(03)01510-8. [DOI] [PubMed] [Google Scholar]
- 4.Leyh RG, Kofidis T, Struber M, Fischer S, Knobloch K, Wachsmann B et al. Methylene blue: the drug of choice for catecholamine-refractory vasoplegia after cardiopulmonary bypass? J Thorac Cardiovasc Surg. 2003;125(6):1426–32. doi: 10.1016/s0022-5223(02)73284-4. [DOI] [PubMed] [Google Scholar]
- 5.Gomes WJ, Carvalho AC, Palma JH, Teles CA, Branco JN, Silas MG, Buffolo E. Vasoplegic syndrome after open heart surgery. J Cardiovasc Surg (Torino) 1998;39(5):619–23. [PubMed] [Google Scholar]
- 6.Mayer B, Brunner F, Schmidt K. Inhibition of nitric oxide synthesis by methylene blue. Biochem Pharmacol. 1993;45(2):367–74. doi: 10.1016/0006-2952(93)90072-5. [DOI] [PubMed] [Google Scholar]
- 7.Shanmugam G. Vasoplegic syndrome--the role of methylene blue. Eur J Cardiothorac Surg. 2005;28(5):705–10. doi: 10.1016/j.ejcts.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Mekontso-Dessap A, Houel R, Soustelle C, Kirsch M, Thebert D, Loisance DY. Risk factors for post-cardiopulmonary bypass vasoplegia in patients with preserved left ventricular function. Ann Thorac Surg. 2001;71(5):1428–32. doi: 10.1016/s0003-4975(01)02486-9. [DOI] [PubMed] [Google Scholar]
- 9.Levin MA, Lin HM, Castillo JG, Adams DH, Reich DL, Fischer GW. Early on-cardiopulmonary bypass hypotension and other factors associated with vasoplegic syndrome. Circulation. 2009;120(17):1664–71. doi: 10.1161/CIRCULATIONAHA.108.814533. [DOI] [PubMed] [Google Scholar]
- 10.Ozal E, Kuralay E, Yildirim V, Kilic S, Bolcal C, Kucukarslan N et al. Preoperative methylene blue administration in patients at high risk for vasoplegic syndrome during cardiac surgery. Ann Thorac Surg. 2005;79(5):1615–9. doi: 10.1016/j.athoracsur.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 11.Kwok ES, Howes D. Use of methylene blue in sepsis: a systematic review. J Intensive Care Med. 2006;21(6):359–63. doi: 10.1177/0885066606290671. [DOI] [PubMed] [Google Scholar]
- 12.Del Duca D, Sheth SS, Clarke AE, Lachapelle KJ, Ergina PL. Use of methylene blue for catecholamine-refractory vasoplegia from protamine and aprotinin. Ann Thorac Surg. 2009;87(2):640–2. doi: 10.1016/j.athoracsur.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Peer G, Itzhakov E, Wollman Y, Chernihovsky T, Grosskopf I, Segev D et al. Methylene blue, a nitric oxide inhibitor, prevents haemodialysis hypotension. Nephrol Dial Transplant. 2001;16(7):1436–41. doi: 10.1093/ndt/16.7.1436. [DOI] [PubMed] [Google Scholar]


