Abstract
Platypnea-orthodeoxia syndrome is an uncommon condition of positional dyspnea and hypoxemia; symptoms occur when the patient is upright and resolve with recumbency. Causes can be broadly categorized into 4 groups: intracardiac shunting, pulmonary shunting, ventilation-perfusion mismatch, or a combination of these.
Platypnea-orthodeoxia syndrome should be suspected when normal arterial oxygen saturations are recorded while an individual is supine, followed by abrupt declines in those saturations when upright. Further investigations with use of imaging and cardiac catheterization aid in the evaluation. When platypnea-orthodeoxia syndrome is due to intracardiac shunting without pulmonary hypertension, intracardiac shunt closure can be curative.
In this article, we report a case of platypnea-orthodeoxia syndrome in an 83-year-old woman who was successfully treated by means of percutaneous transcatheter closure of an atrial septal defect.
Keywords: Aged, 80 and over; atrial septal defect; cardiac platypnea-orthodeoxia syndrome; dyspnea/etiology/physiopathology; foramen ovale, patent; heart septal defects, atrial/complications; oxygen/blood; posture/physiology; septal occluder device; supine position
Platypnea-orthodeoxia syndrome (POS) is an uncommon condition of positional dyspnea (platypnea) and hypoxemia (orthodeoxia). The symptoms occur when the patient is upright and resolve quickly with recumbency. These findings are the opposite of those typically seen in cases of advanced heart failure and can pose a diagnostic dilemma.
Even though POS was first described in the late 1940s,1 the pathophysiologic triggers of orthodeoxia and platypnea are still not completely understood. It appears that both a functional component and an anatomic component are required.2 The syndrome has been associated with cardiac, pulmonary, abdominal, and vascular disease (functional component) in conjunction with a shunt (anatomic component).2 The most frequently described cause is right-to-left interatrial shunting from a patent foramen ovale (PFO) or an atrial septal defect (ASD) in the presence of pulmonary hypertension.3 Positional variation in the degree of shunting in these situations remains incompletely understood. It is believed that the upright position changes or stretches the conformation of the interatrial communication, increasing the proportion of blood flow from the inferior vena cava through the defect and into the left atrium.3
We report our experience in treating a patient who presented with POS in the absence of substantial pulmonary hypertension.
Case Report
In September 2013, an 83-year-old woman with a history of paroxysmal atrial fibrillation was admitted to the hospital after several weeks of progressive positional dyspnea that worsened with standing and improved with lying supine. On admission, the patient's pulse oximetry on room air was 96% while supine and 81% while standing. Physical examination was noteworthy for marked kyphoscoliosis. Computed tomographic evaluation for lung disease revealed a tortuous thoracic aorta with otherwise normal lung parenchyma. The patient's arterial blood gases on room air showed a significant drop in arterial oxygen tension upon postural change (from lying down to standing), confirming our suspicion of POS (Table I).
TABLE I.
Analysis of Our Patient's Arterial Blood Gases

A transthoracic echocardiogram revealed normal pulmonary artery pressure, with an atrial bidirectional shunt. A transesophageal echocardiogram revealed an ASD of moderate size (area, 2.2 cm2) (Fig. 1), together with bidirectional shunting while the patient was supine and sedated.
Fig. 1.
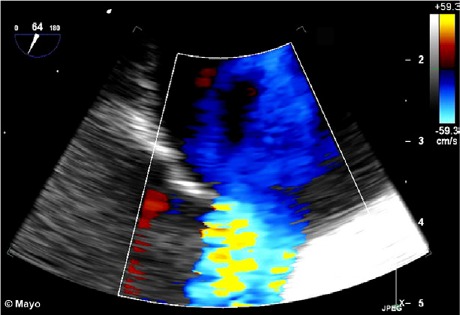
Transesophageal color-flow Doppler echocardiogram shows a secundum atrial septal defect of moderate size.
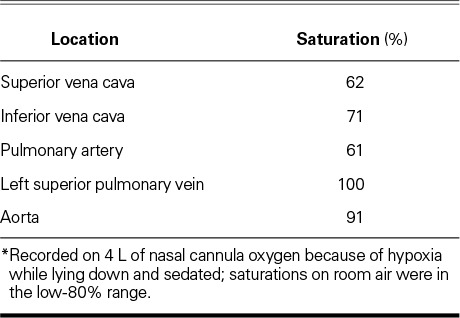
Results of right- and left-sided heart catheterization revealed a pulmonary flow (Qp) of 2.2 L/min, a systemic flow (Qs) of 3.4 L/min, a Qp/Qs ratio of 0.65, and a right-to-left flow of 1 L/min. Table II shows the oxygen saturations during catheterization. Evaluation by means of 3-dimensional intracardiac echocardiography revealed a single ASD of 10 to 11 mm in diameter, as well as compression of the tricuspid orifice by the aortic root. The ASD was successfully closed with a 25-mm Gore® Helex® atrial septal occluder (W.L. Gore & Associates, Inc.; Flagstaff, Ariz), without residual shunting on imaging. Postprocedural arterial blood gases showed immediate improvement of upright hypoxemia (Table I). Upon discharge from the hospital, the patient's positional shortness of breath resolved. At her 6-month follow-up examination, she reported that she had remained free of symptoms.
TABLE II.
Oxygen Saturations during Catheterization *
Discussion
Platypnea-orthodeoxia syndrome is a rare entity that apparently is induced by any of 4 pathologic processes: intracardiac shunting, intrapulmonary shunting, ventilation-perfusion mismatch, or a combination of these.4,5 The syndrome can occur when a left-to-right shunt reverses in response to increased right atrial pressure or decreased right ventricular compliance6,7—as in the presence of kyphoscoliosis,8 tortuous aortic root and ascending aorta,9 aortic elongation,10 or hemidiaphragmatic paralysis.9 Symptoms are exacerbated by an upright position because increased septal stretching causes preferential flow from the inferior vena cava through the shunt and a secondary reduction of pulmonary blood flow.3,11
The prevalence of PFO or ASD as a cause of physiologically significant POS is unknown. The PFO defect itself occurs in perhaps 20% to 34%12 of people, and ASDs are less common than that. Platypnea-orthodeoxia syndrome remains difficult to diagnose and frequently is considered only after nearly every other possible diagnosis is excluded.5 The taking of a thorough history, which elicits information on the improvement of symptoms when the patient is supine, is crucial. Platypnea-orthodeoxia syndrome should be suspected when an individual has normal oxygen saturations on room air while supine, yet experiences dyspnea and desaturations when upright. Correlation with blood-gas analysis that documents positional change in oxygen tension (Pao2) is certainly helpful. Nevertheless, the gold standard is cardiac catheterization, which shows a mismatch in oxygen saturation between the pulmonary vein and the aorta (or, as a surrogate, a mismatch in oxygen saturation between the pulmonary capillary wedge and the aorta). Concurrent measurement of pulmonary pressures is important in excluding pulmonary hypertension, which can worsen with PFO closure.5 Another confounder is that diagnostic studies are generally performed with the patient supine and sedated, a time when the shunt is generally inactive. When the suspicion of POS is high, it can be worthwhile to repeat the study with the patient standing, to better view the shunt and obtain accurate measurements.
It is important to calculate the hemodynamic characteristics of the shunt to determine the overall blood flow. This is done by calculating Qp and Qs. In normal individuals, Qp/Qs will be close to 1; in individuals with a left-to-right shunt, Qp/Qs >1; and in individuals with a right-to-left shunt, Qp/Qs <1. The Qp/Qs calculation has its basis in the Fick principle:
Qp = O2 consumption / (PV – PA) × 10,
in which PA is the pulmonary artery saturation and PV is the pulmonary vein saturation.
and
Qs = O2 consumption / (Ao – MV ) × 10,
wherein Ao is the aortic saturation and MV is the mixed venous saturation.
Whereas the Phlamm equation allows for chamber mixing:
MV = (3 × SVC + IVC ) / 4,
in which IVC is the inferior vena cava saturation and SVC is the superior vena cava saturation.
The ratio Qp/Qs can then be simplified to the following:
Qp/Qs = Ao – MV / PV – PA.
On the basis of the numbers presented in Table II, the ratio in our patient can be calculated as follows:
MV = (3 × 62 + 71) / 4 = 64.3,
and
Qp/Qs = (91 – 64.3) / (100 – 61) = 0.68.
The ratio of 0.68 suggests an overall right-to-left shunt.
Because of the rarity of POS, only a few case series that report patient outcomes have been published. One of the first of these described the cases of 7 patients from Mayo Clinic, 6 of whom had identifiable lung disease; all underwent surgical closure of an intracardiac shunt, with postoperative resolution of symptoms.13 A recent series by Takaya and colleagues10 described the cases of 3 patients, all with POS caused by compression of the right atrium by the ascending aorta; transcatheter closure of interatrial communication was 100% successful in achieving symptomatic relief. In the largest case series from France,14 76 of 78 patients with POS underwent successful PFO closure. In 5 patients, a small residual shunt was present, and in 1 of the 5 this necessitated the implantation of a 2nd closure device. The series with the longest follow-up duration (mean, 2.3 ± 2.2 yr) described the cases of 18 patients who underwent percutaneous closure; success in symptomatic resolution at discharge from the hospital was 100%, and only one of these patients (5.6%) experienced POS recurrence.15 Another case series of 17 patients with POS who underwent PFO closure reported that about one third of patients experienced complete resolution of symptoms, one third reported symptomatic relief but still required oxygen, and one third had no improvement of symptoms. Of note, those without symptomatic improvement had an elevated mean pulmonary artery pressure of 51.4 ± 16.8 mmHg, with pulmonary cause as a primary pathophysiologic component of their symptoms.16 Zavalloni and colleagues17 noted that high rates of residual shunts after primary PFO closure had necessitated the implantation of 2nd devices, most likely because 2-dimensional imaging limitations, during the initial procedures, had led to the choice of undersized prostheses. Recent advances in 3-dimensional imaging have improved our understanding of the anatomy and our subsequent procedural success.18 If accurate echocardiographic imaging is not available, analysis with balloon sizing is another approach.19
Our patient had POS from an ostium secundum ASD. Her right-to-left shunting was multifactorial—caused by aortic elongation and dilation (which has been shown to increase significantly with age),20 by compression of the tricuspid orifice, and by marked kyphosis. This combination caused the net effect of increased flow from the inferior vena cava to the left atrium, through the ASD. After closure, she did not have a residual shunt, and her symptoms of POS improved substantially. Although Pao2 while standing improved after the closure (Table I), the alveolar–arterial gradient did not fully normalize (expected gradient, 25 mmHg), most likely because of respiratory alkalosis in combination with chronically low carbon dioxide values (Table I).
We are optimistic that our patient will have an outcome of durable benefit, given her pathophysiology and our ability to offer definitive treatment by means of catheter-based device closure.
Footnotes
From: Department of Internal Medicine (Drs. Henkin and Negrotto) and Division of Cardiovascular Diseases (Drs. Cullen, Pollak, and Wright), Mayo Clinic, Rochester, Minnesota 55905; and Division of Cardiovascular Diseases (Dr. O'Cochlain), Mayo Clinic Health System, Eau Claire, Wisconsin 54703
References
- 1.Burchell HB, Helmholz HF, Jr, Wood EH. Reflex orthostatic dyspnea associated with pulmonary hypotension [abstract] Am J Physiol. 1949;159(3):563–4. [Google Scholar]
- 2.Cheng TO. Mechanisms of platypnea-orthodeoxia: what causes water to flow uphill? Circulation. 2002;105(6):e47. [PubMed] [Google Scholar]
- 3.Cheng TO. Platypnea-orthodeoxia syndrome: etiology, differential diagnosis, and management. Catheter Cardiovasc Interv. 1999;47(1):64–6. doi: 10.1002/(SICI)1522-726X(199905)47:1<64::AID-CCD15>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Chen GP, Goldberg SL, Gill EA., Jr. Patent foramen ovale and the platypnea-orthodeoxia syndrome. Cardiol Clin. 2005;23(1):85–9. doi: 10.1016/j.ccl.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Blanche C, Noble S, Roffi M, Testuz A, Muller H, Meyer P et al. Platypnea-orthodeoxia syndrome in the elderly treated by percutaneous patent foramen ovale closure: a case series and literature review. Eur J Intern Med. 2013;24(8):813–7. doi: 10.1016/j.ejim.2013.08.698. [DOI] [PubMed] [Google Scholar]
- 6.Sorrentino M, Resnekov L. Patent foramen ovale associated with platypnea and orthodeoxia. Chest. 1991;100(4):1157–8. doi: 10.1378/chest.100.4.1157. [DOI] [PubMed] [Google Scholar]
- 7.Strunk BL, Cheitlin MD, Stulbarg MS, Schiller NB. Right-to-left interatrial shunting through a patent foramen ovale despite normal intracardiac pressures. Am J Cardiol. 1987;60(4):413–5. doi: 10.1016/0002-9149(87)90271-2. [DOI] [PubMed] [Google Scholar]
- 8.Knapper JT, Schultz J, Das G, Sperling LS. Cardiac platypnea-orthodeoxia syndrome: an often unrecognized malady. Clin Cardiol. 2014;37(10):645–9. doi: 10.1002/clc.22301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanikommu V, Lasorda D, Poornima I. Anatomical factors triggering platypnea-orthodeoxia in adults. Clin Cardiol. 2009;32(11):E55–7. doi: 10.1002/clc.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takaya Y, Akagi T, Kijima Y, Nakagawa K, Taniguchi M, Ohtani H et al. Transcatheter closure of right-to-left atrial shunt in patients with platypnea-orthodeoxia syndrome associated with aortic elongation. Cardiovasc Interv Ther. 2014;29(3):221–5. doi: 10.1007/s12928-014-0244-x. [DOI] [PubMed] [Google Scholar]
- 11.Toffart AC, Bouvaist H, Feral V, Blin D, Pison C. Hypoxemia-orthodeoxia related to patent foramen ovale without pulmonary hypertension. Heart Lung. 2008;37(5):385–9. doi: 10.1016/j.hrtlng.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Calvert PA, Rana BS, Kydd AC, Shapiro LM. Patent foramen ovale: anatomy, outcomes, and closure. Nat Rev Cardiol. 2011;8(3):148–60. doi: 10.1038/nrcardio.2010.224. [DOI] [PubMed] [Google Scholar]
- 13.Seward JB, Hayes DL, Smith HC, Williams DE, Rosenow EC, 3rd, Reeder GS et al. Platypnea-orthodeoxia: clinical profile, diagnostic workup, management, and report of seven cases. Mayo Clin Proc. 1984;59(4):221–31. doi: 10.1016/s0025-6196(12)61253-1. [DOI] [PubMed] [Google Scholar]
- 14.Guerin P, Lambert V, Godart F, Legendre A, Petit J, Bourlon F et al. Transcatheter closure of patent foramen ovale in patients with platypnea-orthodeoxia: results of a multicentric French registry. Cardiovasc Intervent Radiol. 2005;28(2):164–8. doi: 10.1007/s00270-004-0035-3. [DOI] [PubMed] [Google Scholar]
- 15.Delgado G, Inglessis I, Martin-Herrero F, Yoerger D, Liberthson R, Buoanno F, Palacios I. Management of platypnea-orthodeoxia syndrome by transcatheter closure of atrial communication: hemodynamic characteristics, clinical and echocardiographic outcome. J Invasive Cardiol. 2004;16(10):578–82. [PubMed] [Google Scholar]
- 16.Mojadidi MK, Gevorgyan R, Noureddin N, Tobis JM. The effect of patent foramen ovale closure in patients with platypnea-orthodeoxia syndrome. Catheter Cardiovasc Interv. 2015 Jun 9 doi: 10.1002/ccd.25953. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Zavalloni D, Lisignoli V, Barbaro C, Mennuni M, Tosi P, Marcheselli S, Presbitero P. Platypnoea-orthodeoxia syndrome secondary to patent foramen ovale (PFO): a challenging subset for PFO percutaneous closure. Heart Lung Circ. 2013;22(8):642–6. doi: 10.1016/j.hlc.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Rana BS, Shapiro LM, McCarthy KP, Ho SY. Three-dimensional imaging of the atrial septum and patent foramen ovale anatomy: defining the morphological phenotypes of patent foramen ovale. Eur J Echocardiogr. 2010;11(10):i19–25. doi: 10.1093/ejechocard/jeq122. [DOI] [PubMed] [Google Scholar]
- 19.Alibegovic J, Bonvini R, Sigwart U, Dorsaz P, Camenzind E, Verin V. The role of the sizing balloon in selection of the patent foramen ovale closure device size. Exp Clin Cardiol. 2008;13(1):42–6. [PMC free article] [PubMed] [Google Scholar]
- 20.Redheuil A, Yu WC, Mousseaux E, Harouni AA, Kachenoura N, Wu CO et al. Age-related changes in aortic arch geometry: relationship with proximal aortic function and left ventricular mass and remodeling. J Am Coll Cardiol. 2011;58(12):1262–70. doi: 10.1016/j.jacc.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


