Abstract
Background and Objectives:
Treatment of gastroesophageal reflux disease (GERD) with hiatal hernia in obese patients has proven difficult, as studies demonstrate poor symptom control and high failure rates in this patient population. Recent data have shown that incorporating weight loss procedures into the treatment of reflux may improve overall outcomes.
Methods:
We retrospectively reviewed 28 obese and morbidly obese patients who presented from December 2007 through July 2013 with large or recurrent type 3 or 4 paraesophageal hernia. All of the patients underwent combined paraesophageal hernia repair and partial longitudinal gastrectomy. Charts were retrospectively reviewed, and the patients were contacted to determine symptomatic relief.
Results:
Mean preoperative body mass index was 38.1 ± 4.9 kg/m2. Anatomic failure of prior fundoplication occurred in 7 patients (25%). The remaining 21 had primary paraesophageal hernia, 3 of which were type 4. Postoperative complications included pulmonary embolism (n = 1), pulmonary decompensation (n = 2), and wound infection (n = 1). Mean hospital stay was 5 ± 3 days. Upper gastrointestinal esophagogram was performed in 21 patients with no immediate recurrence or staple line dehiscence. Mean excess weight loss was 44 ± 25%. All of the patients surveyed experienced near to total resolution of their preoperative symptoms within the first month. At 1 year, symptom scores decreased significantly. At 27 months, however, there was a mild increase in the scores. Return of severe symptoms occurred in 2 patients, both of whom underwent conversion to gastric bypass.
Conclusions:
Combined laparoscopic paraesophageal hernia repair with longitudinal partial gastrectomy offers a safe, feasible approach to the management of large or recurrent paraesophageal hernia in well-selected obese and morbidly obese patients. Short-term results were promising; however, intermediate results showed increasing rates of reflux symptoms that required medical therapy or conversion to gastric bypass.
Keywords: GERD, Obesity, Paraesophageal hernia, Sleeve gastrectomy
INTRODUCTION
Obesity is an ever-growing public health concern. The increasing rate is accentuated in the United States, where more than one-third of the population is categorized as obese, having a body mass index (BMI) greater than 30 kg/m2. A recent review of the economic burden of obesity reported that, over the past 20 years, healthcare costs have increased 21–54% for class I obesity (BMI, 30–34.9), 43–57% for class II (BMI, 35–39.9), and 78–111% for class III (BMI, ≥40).1 These costs were calculated on the a basis of the increases attributable to comorbidities associated with obesity and not simply to the treatment of obesity itself. Included were outcomes, such as increased length of stay for all admissions and increased medical resource usage for nonbariatric surgery.
Obesity is a multifactorial condition that has been linked to the development of many other comorbidities and recently has been classified as a unique disease by the American Medical Association.2 One such comorbidity is gastroesophageal reflux disease (GERD), and another is hiatal hernia. The connection between obesity, hiatal hernia, and GERD is well established. El-Serag et al3 surveyed more than 400 Veterans Affair volunteers and found that 34.6% of individuals with a BMI exceeding 30 kg/m2 had GERD symptoms, compared with 17.6% of those with a BMI lower than 25 kg/m2.
The Nurse's Health Study has also been used to investigate the relationship between BMI and GERD. In 2000, a supplemental questionnaire regarding GERD symptoms was sent to more than 10,000 participants. Both univariate and multivariate analyses in that study found a strong dose-dependent relationship between BMI and frequency of reflux symptoms. The findings showed a 2- to 3-fold increase in the frequency of reflux symptoms in overweight (BMI, 25–30 kg/m2) and obese (BMI, >30 kg/m2) women.4
There is a similar relationship between hiatal hernia and obesity. In 1999, Wilson et al5 found that individuals with a BMI exceeding 30 kg/m2 were 4.2 times more likely to have hiatal hernia than those with a BMI lower than 25 kg/m2. Hiatal hernia also has been found in up to 40% of patients who undergo preoperative esophagogastroduodenoscopy (EGD) for bariatric surgery.6
Compared to medical management, antireflux operations for GERD have been found to achieve better symptom control, better quality of life, greater reduction of acid reflux, and fewer treatment failures.7–10 As well, in patients presenting with uncontrolled reflux, the symptoms have often already failed to improve with several over-the-counter medications.
Few publications have documented the natural history of nonsurgical management of hiatal hernia. Regarding operative repair, however, several studies have shown that surgery is safe and feasible and provides short-term resolution of symptoms, especially in the symptomatic population.11–13 Among these studies, a recent 10-year retrospective review of laparoscopic hiatal hernia repair in giant paraesophageal hernia identified obesity as a risk factor for long-term adverse outcomes.14
In the nonobese population, therefore, surgical options are readily considered for definitive treatment of GERD and hiatal hernia. However, concomitant obesity complicates this decision, as obesity has also been shown to increase the failure rate of antireflux surgery.7 Because of the increased risk of surgical failure in this challenging population, some groups now recommend consideration of weight loss surgery as a treatment for GERD. In fact, the most recent Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) guidelines on antireflux surgery recommended that gastric bypass be the operation of choice in the morbidly obese population (BMI, >35 kg/m2) (grade B evidence). The alternative, fundoplication, was thought less desirable because of the higher failure rate in this patient group and because it does not correct the underlying problem of obesity.15
Although there has been increased acceptance of bariatric surgery for GERD and hiatal hernia in the obese population, there are several obstacles, such as patient preference, lack of insurance coverage, and delay in treatment of GERD. Many patients with severe reflux or hiatal hernia do not meet Medicare requirements for bariatric surgery (BMI >40 kg/m2, alone, or 35–40 kg/m2, with significant comorbidities). Other patients may meet these requirements, but may prefer not to undergo gastric bypass or are unable to comply with postoperative lifestyle modifications.
The objective of the current study was to analyze outcomes of obese and morbidly obese patients with symptomatic hiatal hernia who underwent surgical repair with the addition of a partial longitudinal gastrectomy during the same operation. One recent case series showed that this combined approach is both safe and feasible.16 A second series reported positive symptom relief, with no recurrences at a mean of 20 months of postoperative follow-up.17 In our series, a Roux-en-Y gastric bypass (RYGB) was not performed because of insurance denial or patient preference. We hypothesized that the addition of a weight loss component and a tailored esophageal-lengthening concept to the standard structural repair of a hiatal hernia would result in decreased failure rates and improved symptoms in this patient population.
METHODS
Study Population
This study was a retrospective analysis of a prospectively maintained database of patients with a BMI greater than 30 kg/m2 and symptomatic type III or IV paraesophageal hernia. The study is a follow-up of a short-term series18 involving 19 patients. These patients were contacted for updated data, and 9 new patients were added. Operations were performed from December 2007 though July 2013. None of the patients presented with the sole intention of undergoing weight loss surgery.
A review of the patient's charts was conducted for collection of preoperative data including demographics, BMI, symptoms, use of prescription and nonprescription acid-reducing medication, operative indications, perioperative data (operative time, technique of crural closure, hospital length of stay, and complications), and follow up data (resolution of symptoms, use of prescription and nonprescription acid-reducing medication, BMI, and further intervention or need for revision).
Preoperative Workup
Imaging studies, including upper gastrointestinal (GI) series, EGD, or computed tomographic scan were performed or reviewed if already completed. Patients were also evaluated by a preoperative clearance team consisting of internal medicine and anesthesia practitioners for preoperative risk stratification based on history and medical comorbidities.
Surgical Technique and Postoperative Management
The technical aspects of the operation revolved around 3 therapeutic goals:
Complete reduction of the hernia contents into the abdominal cavity and excision of the hernia sac.
Enteric mediastinal mobilization for >3 cm intra-abdominal esophageal length.
Primary crural closure, with or without mesh reinforcement with acellular porcine dermis—Permacol (Covidien, Dublin, Ireland) or Strattice (LifeCell, Bridgewater, New Jersey)—or copolymer polyglycolic acid/trimethylene carbonate composite matrix, Bio-A (W. L. Gore and Associates, Flagstaff, Arizona).
Partial longitudinal gastrectomy extending from the incisura angularis to the angle of His with sutured staple line reinforcement.
Access to the peritoneal cavity was obtained with an optical trocar, and 4 to 5 working trocars were introduced into the upper abdomen. A liver retractor was used to expose the hiatus. The hernia sac was identified and carefully dissected from the crura. The hernia was completely reduced, and the sac was excised to the level of the angle of His. The esophagus was circumferentially dissected proximally into the mediastinum, to achieve an intra-abdominal esophageal length of 4–5 cm. The hiatus was closed primarily with nonabsorbable suture. Based on the presumed tension and surgeon's discretion, mesh reinforcement was performed with biologic or bioabsorbable material.
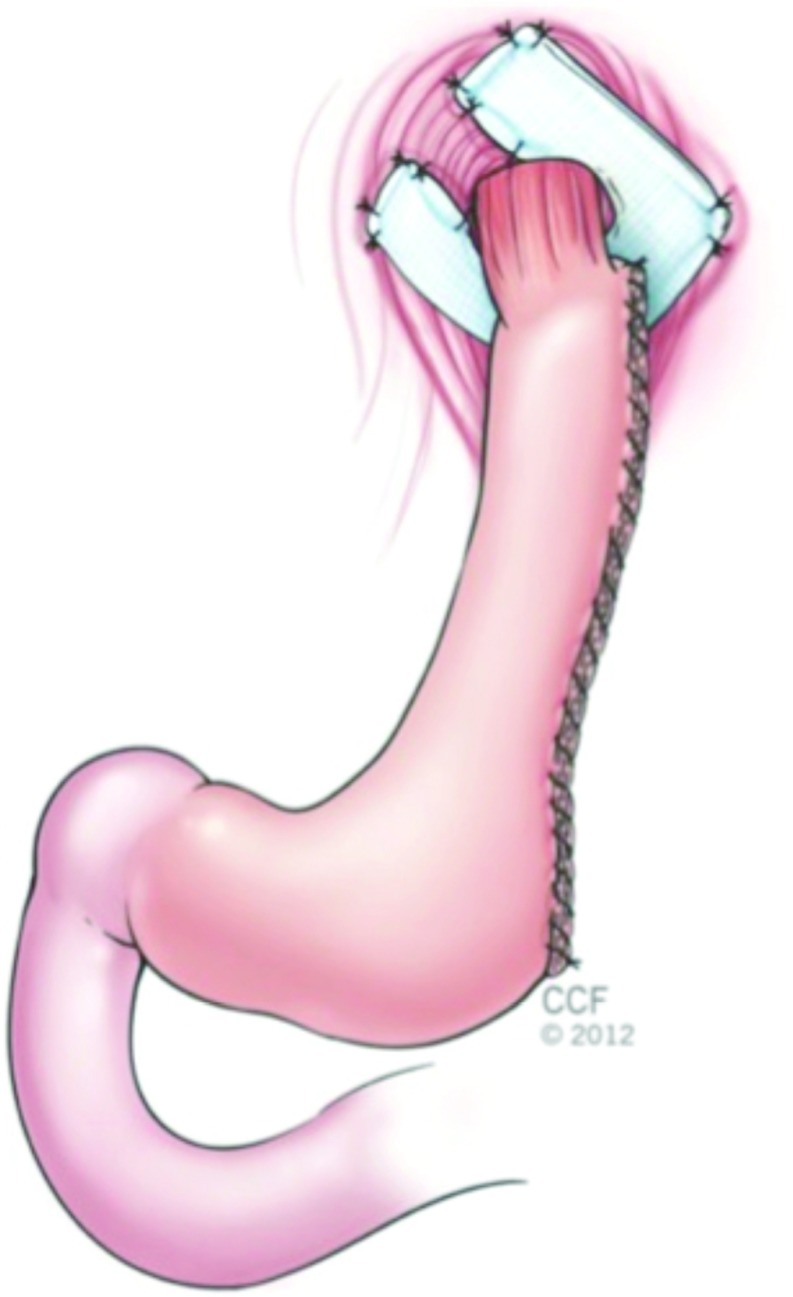
The stomach was mobilized, and the short gastric vessels were divided with ultrasonic shears along the greater curvature. A longitudinal gastrectomy was performed with sequential firings of the endoscopic linear stapler (Echelon Flex Endopath, Ethicon, Cincinnati, Ohio) over a bougie dilator or a therapeutic upper endoscope to calibrate the gastrectomy. The line of transection extended from just proximal to the incisura angularis to the angle of His. The staple line then was reinforced with a running absorbable suture or staple line reinforcement product (Figure 1). An intraoperative endoscopy was performed to identify potential leaks and to confirm adequate width of the gastric lumen. A closed suction drain then was placed along the line of resection in selected cases.
Figure 1.
Combined laparoscopic paraesophageal hernia repair with mesh reinforcement and longitudinal gastrectomy.
After surgery, most patients (24/28) underwent an upper gastrointestinal (UGI) study. After a normal contrast study, a liquid diet was initiated and continued throughout the hospital stay. Patients then were seen at a follow-up visit within 4 weeks of discharge and at 3-month intervals as needed.
Patient Questionnaire and Evaluation of Outcomes
A patient questionnaire (Figure 2) was designed to evaluate the main objectives of our study as follows:
Resolution of symptoms: questions 2 to 8 focused on the presence of upper GI symptoms commonly related to GERD and hiatal hernias. To evaluate the severity of symptoms objectively, a severity index score was calculated by assigning 0 to 3 points to each question based on frequency: 0, absence of symptoms; 1, symptoms once a week; 2, symptoms 2–3 days/week; and 3, symptoms more than 4 days/week. The maximum possible score was 21 (more severe), and the minimum score was 0 (less severe). The pre- and postoperative score for each patient was compared, to assess improvement of symptoms. Patients were also interrogated on the use of prescribed antisecretory medication (proton pump inhibitor [PPI]/histamine type 2 receptor [H2]-blocker) and over-the-counter medications for related GI symptoms before and after surgery.
Weight loss: question 1 was used to calculate BMI at the time the questionnaire was conducted.
Patient satisfaction: questions 11–13 evaluated the patient's perception of improvement and satisfaction with the results.
Figure 2.

Patient questionnaire.
Statistical Analysis
BMI and scores were analyzed on a log scale, using additive mixed-modeling techniques, as implemented in the mgcv19 package in R (www.r-project.org). The model for BMI includes a random effect for each subject and assumes a first-order autoregressive correlation structure within subjects. The model for scores includes a random effect for each subject, a random effect for follow-up within each subject, and a first-order autoregressive correlation structure. The additional complexity of the model was necessitated by the structure of the questionnaire. Changes in PPI use as a function of time were analyzed by using generalized additive modeling techniques and incorporated subject-specific random effects. Comparisons were performed with the multcomp20 package in R and control the overall error rate at the stated level. All analyses were achieved with the R software (ver. 3.0.2; Vienna, Austria). P < 0.05 denoted significance for all testing.
RESULTS
The study enrolled 28 patients. The patient demographics are listed in Table 1. Of the 28 patients, 27 (96%) were women. The mean age was 59 ± 11 years. The mean preoperative BMI was 38.1 ± 4.9 kg/m2 (range, 30–48), with 13 patients (46.4%) meeting the criteria for class 2 obesity (35–40 kg/m2) and 8 (28.6%) meeting the criteria for class 3 obesity (>40 kg/m2). Seven patients (25.0%) had BMIs of 30 to 35 kg/m2. Symptoms at presentation included epigastric pain (n = 10), reflux/regurgitation (n = 18), dysphagia (n = 10), and nausea/emesis (n = 4).
Table 1.
Patient Demographics and Operative Data
| Age | Sex | BMI | Symptoms | PPI/H2B | Previous Surgery | Crural Closure | Operative Time | LOS (days) |
|---|---|---|---|---|---|---|---|---|
| 47 | F | 39 | Dysphagia, heartburn | Yes | primary | 205 | 4 | |
| 55 | F | 35 | Regurgitation, heartburn | Yes | Primary+Bio-A | 102 | 4 | |
| 49 | F | 40 | Epigastric pain, regurgitation | Yes | Primary+Permacol | 4 | ||
| 63 | F | 38 | Heartburn, SOB, regurgitation | No | PEHR-Nissen (partial herniation) | Primary+Permacol | 300 | 3 |
| 54 | F | 34 | Epigastric pain | Yes | PEHR-Nissen (partial herniation) | Primary | 196 | 8 |
| 66 | F | 47 | epigastric pain, regurgitation | Yes | Primary+Permacol | 4 | ||
| 48 | F | 41 | epigastric pain | Yes | Primary+Permacol | 4 | ||
| 50 | M | 33 | Regurgitation, epigastric pain, dysphagia | Yes | Primary+Permacol | 236 | 10 | |
| 51 | F | 40 | Regurgitation, epigastric pain | Yes | PEHR-Nissen | Primary+Bio-A | 157 | 3 |
| 58 | F | 39 | Regurgitation, nausea | Yes | Primary+Strattice | 325 | 7 | |
| 73 | F | 35 | Dysphagia, heartburn | Yes | Primary+Permacol | 227 | 4 | |
| 66 | F | 35 | Regurgitation, nausea, vomiting, epigastric pain | Yes | Primary+Permacol | 147 | 5 | |
| 70 | F | 35 | Recurrent aspiration, reflux | Yes | open PEHR-Nissen | Primary+Bio-A | 373 | 15 |
| 77 | F | 42 | Regurgitation, nausea, vomiting, epigastric pain | Yes | Primary+Bio-A | 269 | 4 | |
| 66 | F | 36 | Epigastric pain, regurgitation, vomiting | Yes | Primary | 258 | 4 | |
| 55 | F | 30 | Dysphagia, regurgitation, heartburn | Yes | Primary | 148 | 3 | |
| 79 | F | 33 | Dysphagia, epigastric pain | Yes | Primary+Permacol | 228 | 5 | |
| 50 | F | 39 | Dysphagia | Yes | PEHR-Nissen X 2 (early re-herniation) | Primary+Bio-A | 361 | 4 |
| 65 | F | 48 | Dysphagia, reflux | Yes | Primary+Permacol | 289 | 5 | |
| 53 | F | 33.6 | Twenty years of reflux, recent nausea/emesis | Yes | Primary | 163 | 2 | |
| 50 | F | 41.5 | Reflux, dysphagia, chest pain | Yes | Primary | 207 | 5 | |
| 52 | F | 42.6 | Dysphasia, upper abd pain | No | Hand assisted Nissen | Primary | 223 | 5 |
| 45 | F | 35.6 | Dysphagia, post-prandial pain | No | Lap Nissen | Primary+Permacol | 339 | 4 |
| 37 | F | 38.2 | Refractory reflux | Yes | Primary | 125 | 2 | |
| 64 | F | 47.8 | Severe reflux | Yes | Primary | 132 | 3 | |
| 73 | F | 30.9 | None | No | Sutures with Bio-A mesh posteriorly | 213 | 2 | |
| 71 | F | 33.3 | None | No | Sutures with Bio-A mesh posteriorly | 259 | 5 | |
| 63 | F | 44.0 | Bloating, chest pain, regurgitation and emesis. | Yes | Primary | 190 | 5 |
H2B, H2-blocker; LOS, length of stay; PEHR, paraesophageal hernia repair.
All patients underwent preoperative evaluation with a UGI contrast study, EGD (those studies performed outside of our institution were reviewed), or both. Selected patients had a gastric-emptying study or computed tomography as part of their workup, as indicated. Seven patients (25%) had a history of prior hiatal hernia repair with Nissen fundoplication, which was performed laparoscopically (n = 5), laparoscopically with a hand-assisted technique (n = 1), or via open laparotomy (n = 1). All 7 of these patients experienced anatomic failures identified during the preoperative workup as defined by transhiatal migration (n = 6), wrap disruption (n = 2), and failure of the diaphragmatic closure (n = 1). Of the remaining 21 patients, 16 had type III and 3 had type IV hernia. Hernias in 2 patients were not classified because of lack of available preoperative imaging.
All cases (100%) were completed laparoscopically. The mean operative time was 227 ± 75 minutes (range, 102–373). Primary hiatal closure was performed in all cases after complete reduction of the hernia and excision of the sac. Mesh overlay reinforcement was performed in 18 (64%) of the cases. Permacol mesh was used was in 10 cases (56%), Bio-A in 7 (39%), and Strattice in 1 (5%). A longitudinal gastrectomy was performed in all of the patients. The procedure was calibrated by using a 36, 40, or 50 French Maloney dilator. The gastrectomy staple line was reinforced by imbricated suturing in 27 cases (96%).
Four cases had slight variations in the surgical technique. One patient had a Nissen fundoplication created with the proximal fundus after longitudinal gastroplasty, and 2 patients had an anterior gastropexy. In 2 patients, additional procedures were necessary because of intraoperative complications. In 1 case, a tube thoracostomy was placed for suspected pneumothorax. The other patient, with a type IV paraesophageal hernia containing transverse colon and spleen, underwent splenectomy for subcapsular bleeding. Two other patients underwent adjunct procedures during their operations. One had a liver biopsy performed to evaluate the patient's hepatomegaly for nonalcoholic steatohepatitis (NASH) and the second had a left lateral liver resection for a suspect liver lesion.
The overall morbidity rate was 14.3% (4/28). One patient returned to the operating room for diagnostic laparoscopy and EGD on postoperative day 3 for suspected staple line leakage noted during the contrast study. No abnormalities were detected during the exploration. This patient was later found to have a pulmonary embolism, despite receiving appropriate prophylaxis. Other complications included respiratory failure in 2 patients that necessitated admission to the surgical intensive care unit for respiratory support. The cause in both cases was decompensation of underlying chronic obstructive pulmonary disease (COPD), resulting in a prolonged hospital stay (10 and 15 days). Finally, there was 1 case of wound infection, which required operative incision and drainage.
The mean length of hospital stay was 5 ± 3 days (range, 2–15). Within 4 weeks after discharge, 23 patients (82%) returned for a follow-up visit. Of the 23 patients, 19 (82.6%) had complete resolution of gastrointestinal symptoms, 2 reported occasional nausea, 1 reported dysphagia, and 1 had reflux symptoms controlled with antisecretory medication.
Follow-up data were available for 26 of the 28 patients, as 2 had only recently undergone surgery at the time of collection of data. Of these patients, 21 (75%) completed the telephone survey during a mean follow-up period of 27 months. One patient was lost to follow-up evaluation, and another died. The remaining 3 patients could not be reached for the survey.
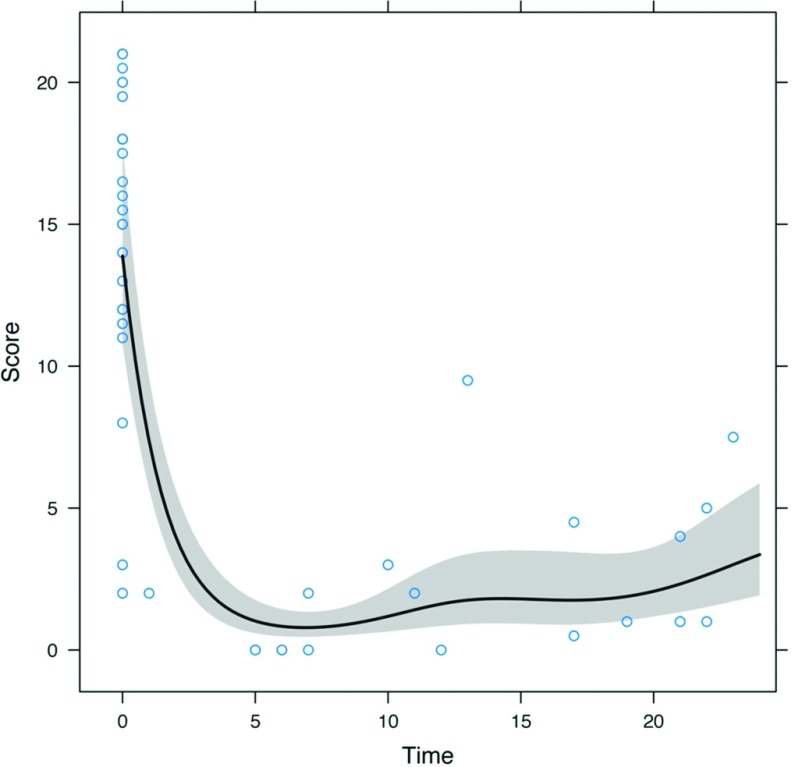
The results after the mean follow-up period of 27 months (range, 5–46) are summarized in Table 2. The symptom severity index (0–21) was calculated for all 21 patients. Before surgery, the patients had a mean score of 15 ± 5 (range, 2–21). The mean postoperative symptom severity index was 6 ± 5 (range, 0–20). Surgery also was effective in reducing the use of daily antisecretory medications (PPIs/H2-blockers), from 96% (before surgery) to 62% (at the time of the survey). The average score at 12 months was about one-tenth of the average score before surgery, and the average score at 24 months was approximately one-fifth of the average score before surgery (both P < .001). The average score at 24 months was estimated to be about twice the average score at 12 months, but the difference was not statistically significant (P = .23). Figure 3 gives the average score as estimated by the model for a 2-year follow-up time. After a fairly precipitous drop during the ∼6 months after surgery, the average score did not seem to change appreciably or increased slightly.
Table 2.
Pre- and Postoperative Data
| Time of Postop Survey (mo) | Preop BMI | BMI at Survey | BMI Diff | Preop Score | Postop Score | Score Change | Preop PPI/H2B | Post op PPI/H2B | GI Meds |
|---|---|---|---|---|---|---|---|---|---|
| 37 | 39 | 31.6 | 7.4 | 18 | 11 | 7 | Yes | No | |
| 21 | 35 | 28.5 | 6.5 | 17.5 | 4 | 13.5 | Yes | No | No |
| 46 | 40 | 28 | 12 | 18 | 6 | 12 | Yes | No | Yes |
| 38 | No | ||||||||
| 34 | Yes | ||||||||
| 46 | 47 | 40.2 | 6.8 | 21 | 2 | 19 | Yes | Yes | No |
| 63 | 41 | 38.7 | 2.3 | 18 | 20 | −2 | Yes | Yes | No |
| 27 | 33 | 24.7 | 8.3 | 21 | 12 | 9 | Yes | Yes | |
| 40 | Yes | ||||||||
| 23 | 39 | 29.3 | 9.7 | 15.5 | 7.5 | 8 | Yes | Yes | |
| 22 | 35 | 27.7 | 7.3 | 11 | 1 | 10 | Yes | Yes | No |
| 27 | 35 | 30.8 | 4.2 | 12 | 5 | 7 | Yes | No | |
| 35 | Yes | ||||||||
| 34 | 42 | 42.5 | −0.5 | 12 | 11.5 | 0.5 | Yes | Yes | No |
| 17 | 36 | 28.2 | 7.8 | 18 | 4.5 | 13.5 | Yes | Yes | No |
| 26 | 30 | 24.2 | 5.8 | 16.5 | 3.5 | 13 | Yes | Yes | Yes |
| 22 | 33 | 30.6 | 2.4 | 19.5 | 5 | 14.5 | Yes | No | No |
| 39 | Yes | ||||||||
| 19 | 48 | 37.6 | 10.4 | 3 | 1 | 2 | Yes | No | No |
| 29 | 35.6 | 25.3 | 10.3 | 11.5 | 13 | −1.5 | Yes | Yes | Yes |
| 28 | 47.8 | 36.2 | 11.6 | 19.5 | 8 | 11.5 | Yes | Yes | Yes |
| 28 | 42.6 | 34.9 | 7.7 | 20.5 | 1 | 19.5 | Yes | Yes | No |
| 17 | 41.5 | 31.3 | 10.2 | 11.5 | 0.5 | 11 | Yes | No | No |
| 12 | 38.2 | 26.8 | 11.4 | 15 | 0 | 15 | Yes | No | No |
| 5 | 30.9 | 28.9 | 2.0 | 2 | 0 | 2 | Yes | Yes | No |
| 13 | 33.6 | 25.2 | 8.4 | 18 | 9.5 | 8.5 | Yes | Yes | No |
| 33.3 | No | ||||||||
| 44.0 | Yes |
Figure 3.
Average symptom score over the 2-year follow-up.
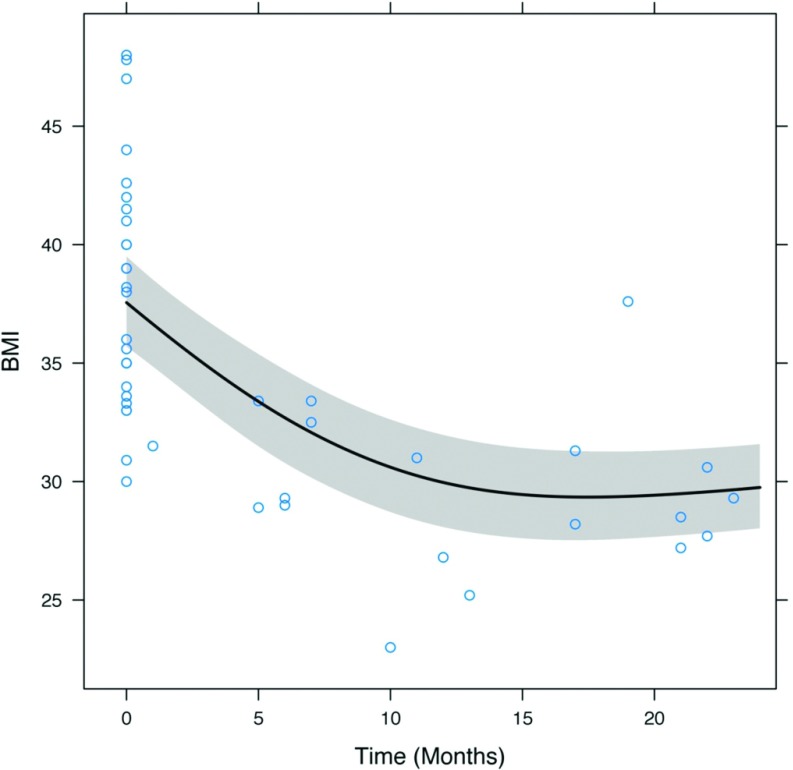
Of the 21 patients surveyed, 20 (95.2%) experienced weight loss. The mean preoperative excess body weight (EBW) was 45.3 ± 13.6 kg (range, 27.3–76.8). The mean EBW loss was 19.7 kg (or 44% of EBW) ± 10.3 kg (range, 0.4–38.7 kg, or 1–115%). Five patients (23.8%) lost less than 10 kg of EBW. Mean preoperative BMI was 38.1 ± 4.9 kg/m2 (range, 30–48), compared to 31.0 ± 5.4 kg/m2 after surgery (range, 24–43). BMIs at 12 and 24 months represent reductions of ∼20% from preoperative levels (both P < .001). BMI change over time is shown in Figure 4.
Figure 4.
Change in BMI before and after surgery.
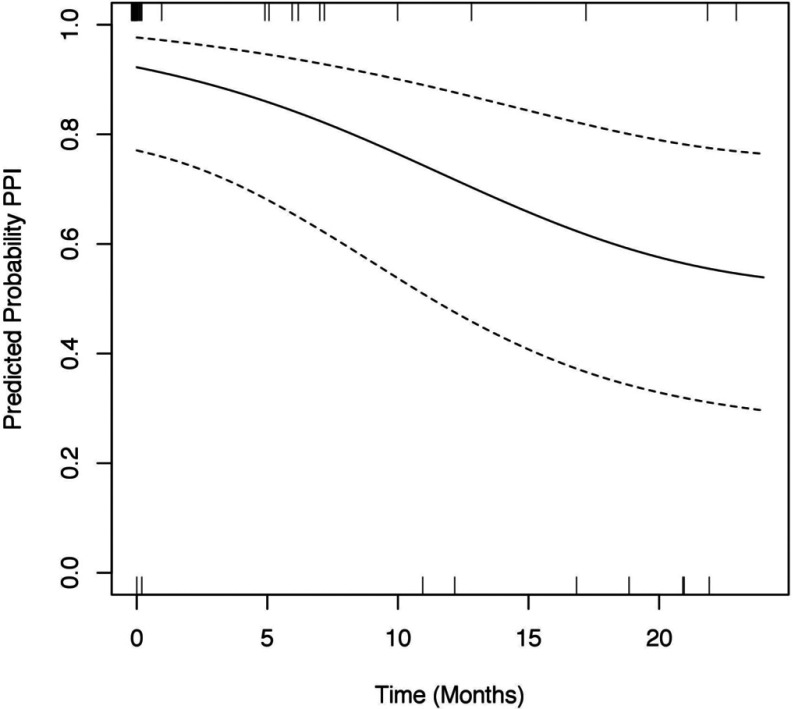
Regarding PPI use, Figure 5 shows the changes in the estimated average probability of PPI use during the 2 years after surgery. Before surgery, slightly more than 90% of the patients were on PPIs. The rate of usage decreased in the 2 years after surgery, at which time PPI use was roughly 60%. The odds of PPI use at 12 months were about one-fifth of those before surgery (P = .026). The odds of PPI use at 24 months were about one-tenth of those before surgery (P = .007). Finally, the odds of PPI use at 24 months were about half of those at 12 months, although the difference was not statistically significant (P = .15).
Figure 5.
Predicted probability of PPI use.
The majority (20/28; 71%) of patients surveyed perceived a complete to near complete resolution of symptoms, and all of the patients stated that they would recommend this operation to patients with similar conditions.
Treatment failure, defined as recurrence of symptoms at or near the preoperative level, occurred in 2 patients. One of these patients has since returned to the clinic and reported occasional spasms, persistent reflux including nocturnal cough, and dumping-like symptoms. This patient is currently undergoing evaluation for conversion to RYGB. The second patient has not yet presented for follow-up evaluation.
An upper GI series was scheduled for the 6-month postoperative visit in all patients. This study was completed in 9 of the patients (32%). Some patients did not present for their follow-up appointment, whereas others attended their visit, but did not complete the study. In those studies that were completed, only 1 anatomic failure was observed; however, it was deemed to be clinically insignificant, and the patient was found to be asymptomatic.
One patient was lost to follow-up. Another died at home of an unknown cause during the 30-day perioperative period.
DISCUSSION
Failure of antireflux surgery and paraesophageal hernia repair in the obese population has been reported in significantly higher rates than in the population with a normal BMI.21,22 Unfortunately, the complex nature of obesity suggests that there are many factors that contribute to the failure of treatment. Lower success rates of operations are seen in the obese population,23 even in those who do not have symptoms caused by being overweight. In the current study group, this record is compounded by the fact that GERD is caused in part by obesity, and any treatment will be less successful as long as the extra weight is present.
Symptom relief was reported by most of our patients in the follow-up survey. Outcomes from the initial short-term study by Rodriguez et al18 showed a significant symptomatic response, in addition to a considerable weight loss. Upon follow-up analysis, we observed a plateau and possible shift toward symptom recurrence, as well as a softening of the weight loss curve. Beyond the 1-year mark of the previous study, some patients began experiencing symptom recurrence, which may be interpreted as the “life span” of the antireflux aspect of this approach.
The return of symptoms in our study group was observed to occur only after the initial period of rapid weight loss. Once the rapidity of weight loss decreased, the patients began to experience the discomfort associated with the recurrent reflux. This observation highlights the contribution of weight to GERD symptomatology in the obese population. It follows that a more definitive bariatric approach, providing greater initial weight loss and long-term reinforcement of weight maintenance may result in a more sustained resolution of reflux in this patient group, perhaps in part because of modifications of this operation compared with standard sleeve gastrectomy, including larger bougie size and more proximal stomach transection.
We observed an EBW loss of 44% at a mean follow-up of 26.8 months. A review of the solitary sleeve gastrectomies reported an EBW loss of 64.7% within a similar time frame.24 It should be noted, however, that our patient group differs from the traditional group that undergoes laparoscopic sleeve gastrostomy (LSG) in several ways. First, our patients' BMI distribution did not exceed 48, thus excluding the superobese population. Second, the primary end point of our operation was resolution of reflux, not maximal weight loss, which may have affected patients' postoperative habits regarding diet and lifestyle modification. Finally, the longitudinal gastrectomy performed in our patient group was tailored to be wider than traditional LSG, specifically by using a bougie up to 50 French, as opposed to the 36 to 40 French dilators used in traditional LSG. This widening of the gastrectomy was performed with the intention of preventing the increased reflux disease that occurs with standard LSG.
The softening of the weight loss curve after the initial decrease in weight of the first postoperative year was similar to that observed in both LSG and RYGB.25 This outcome indicates that, although their initial weight loss was not as significant as that found in a purely bariatric setting, our patients tended to initiate some form of lifestyle modification to maintain their slimmer state. This concept is important, as regression toward weight gain may complicate the antireflux aspect of this operation and could confound the reports of symptom recurrence.
Evaluating symptom resolution in a patient population is difficult, as each patient reports varying degrees of a variety of symptoms for the same condition. In the current group, several patients reported minimal initial symptoms, either because of incidental detection of the hernia or because of current pharmacological therapy. The standardized survey provided an objective set of criteria for patients to report, and, with the aid of frequency options (days per week), patients were also able to describe the degree of symptom resolution or recurrence. With these tools, we found that all of the patients surveyed reported an improvement of symptoms and most (95%) who underwent this operation had documented weight loss.
The present study, although novel in its approach to combined GERD and obesity treatment, is limited by its small patient group and retrospective design. As a follow-up to the initial short-term study, we observed a change in trajectory in symptom score that tended toward a life span for this procedure. However, this scoring system is a subjective interpretation by the patient without any correlation with diagnostic imaging or objective follow-up testing. Greater proof of the efficacy of our procedure may be that only 1 patient experienced symptom recurrence severe enough to return for re-evaluation.
A limitation specific to our accumulation of data via survey is the inability to account for outside factors that may have contributed to return of symptoms. The surveys did not include data on adherence to diet and lifestyle modification, exercise involvement, or smoking status—all factors that may increase reflux, even after successful surgical repair. As well, patients were not included in our routine post–bariatric surgery study protocols and follow-up schedule.
CONCLUSION
Repair of paraesophageal hernia in the obese is a challenging clinical scenario, especially when the goal is to achieve reduction in symptoms of GERD. Thus, a new approach is needed that can allow the surgeon to repair the hernia while concomitantly fixing other confounding conditions. This combined approach, as seen with the addition of a modified bariatric component, has been shown to improve the resolution of symptoms of GERD. Partial longitudinal gastrectomy, as opposed to more definitive bariatric operations, is a viable option as a short-term means of decreasing the impact of obesity on reflux. Although more technically demanding than repair of an isolated hernia, the outcome of greater symptom regression and the possibility of avoiding failure of hernia repair make this approach reasonable to pursue in this population.
Immediate GERD symptom relief can be achieved through a combined antireflux and weight loss approach, as seen in this cohort. However, this beneficial effect may be a short-term gain. The surgical approach described herein may represent a new option for those obese patients who fail to qualify for gastric bypass or would initially prefer to undergo what they deem is a less complicated surgery.
Further analysis of larger groups of bariatric patients with reflux is warranted, to determine the efficacy of this combined approach. Longer-term follow-up will aid in observing the natural history of the antireflux aspect of this surgery and will provide a better understanding of the recurrence of reflux after repair.
Contributor Information
Matthew Davis, Department of Surgery, Digestive Disease Institute.
John Rodriguez, Department of Surgery, Digestive Disease Institute.
Kevin El-Hayek, Department of Surgery, Digestive Disease Institute.
Stacy Brethauer, Department of Surgery, Bariatric and Metabolic Institute.
Philip Schauer, Department of Surgery, Bariatric and Metabolic Institute.
Andrea Zelisko, Department of General Surgery, Akron General Medical Center, Akron, Ohio.
Bipan Chand, Division of GI/Minimally Invasive Surgery, Loyola University Medical Center, Stritch School of Medicine, Maywood, Illinois..
Colin O'Rourke, Digestive Disease Institute, Cleveland Clinic Foundation, Cleveland, Ohio.
Matthew Kroh, Department of Surgery, Digestive Disease Institute.
References:
- 1. Grieve E, Fenwick E, Yang HC, Lean M. The disproportionate economic burden associated with severe and complicated obesity: a systematic review. Obes Rev. 2013:14:883–894. [DOI] [PubMed] [Google Scholar]
- 2. AMA Adopts New Policies on Second Day of Voting at Annual Meeting. News release. Chicago: American Medical Association; June, 18, 2013. Available at http://www.ama-assn.org/ama/pub/news/news/2013/2013-06-18-new-ama-policies-annual-meeting.page. [Google Scholar]
- 3. El-Serag HB, Graham DY, Satia JA, Rabeneck L. Obesity is an independent risk factor for GERD symptoms and erosive esophagitis. Am J Gastroenterol. 2005;100:1243–1250. [DOI] [PubMed] [Google Scholar]
- 4. Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA. Body mass index and symptoms of gastroesophageal reflux in women. N Engl J Med. 2006;35:42340–42348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilson LJ, Ma W, Hirschowitz BI. Association of obesity with hiatal hernia and esophagitis. Am J Gastroenterol. 1999;94:2840–2844. [DOI] [PubMed] [Google Scholar]
- 6. Sharaf RN, Weinshel EH, Bini EJ, Rosenberg J, Sherman A, Ren CJ. Endoscopy plays an important preoperative role in bariatric surgery. Obes Surg. 2004;14:1367–1372. [DOI] [PubMed] [Google Scholar]
- 7. Anvari M, Allen C, Marshall J, et al. Randomized controlled trial of laparoscopic Nissen fundoplication versus proton pump inhibitors for treatment of patients with chronic gastroesophageal reflux disease: one-year follow-up. Surg Innov, 2006;13:238–249. [DOI] [PubMed] [Google Scholar]
- 8. Lundell L, Miettinen P, Myrvold HE, et al. Seven-year follow-up of a randomized clinical trial comparing proton-pump inhibition with surgical therapy for reflux oesophagitis. Br J Surg. 2007;94:198–203. [DOI] [PubMed] [Google Scholar]
- 9. Mahon D, Rhodes M, Decadt B, et al. Randomized clinical trial of laparoscopic Nissen fundoplication compared with proton pump inhibitors for treatment of chronic gastro-esophageal reflux. Br J Surg 2005;92:695–699. [DOI] [PubMed] [Google Scholar]
- 10. Mehta S, Bennett J, Mahon D, Rhodes M. Prospective trial of laparoscopic Nissen fundoplication versus proton pump inhibitor therapy for gastroesophageal reflux disease: seven-year follow-up. J Gastrointest Surg. 2006:10:1312–1316, discussion 1316–1317. [DOI] [PubMed] [Google Scholar]
- 11. Luketich JD, Raja S, Fernando HC, et al. Laparoscopic repair of giant paraesophageal hernia: 100 consecutive cases. Ann Surg. 2000;232:608–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pierre AF, Luketich JD, Fernando HC, et al. Results of laparoscopic repair of giant paraesophageal hernias: 200 consecutive patients. Ann Thorac Surg. 2002;74:1909–1916. [DOI] [PubMed] [Google Scholar]
- 13. Pitcher DE, Curet MJ, Martin DT, Vogt DM, Mason J, Zucker KA. Successful laparoscopic repair of paraesophageal hernia. Arch Surg. 1995;130:590–596. [DOI] [PubMed] [Google Scholar]
- 14. Luketich JD, Nason KS, Christie NA, et al. Outcomes after a decade of laparoscopic giant paraesophageal hernia repair. J Thorac Cardiovasc Surg. 2010;139:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guidelines for Surgical Treatment of Gastroesophageal Reflux Disease (GERD). Los Angeles: SAGES, February, 2010. http://www.sages.org/publications/guidelines/guidelines-for-surgical-treatment-of-gastroesophageal-reflux-disease-gerd/. [PubMed] [Google Scholar]
- 16. Clapp B. Prosthetic bioabsorbable mesh for hiatal hernia repair during sleeve gastrectomy. JSLS. 2013:17:641–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patel AD, Lin E, Lytle NW, et al. Combining laparoscopic giant paraesophageal hernia repair with sleeve gastrectomy in obese patients. Surg Endosc. 2015;29:1115–1122. [DOI] [PubMed] [Google Scholar]
- 18. Rodriguez JH, Kroh M, El-Hayek K, Timratana P, Chand B. Combined paraesophageal hernia repair and partial longitudinal gastrectomy in obese patients with symptomatic paraesophageal hernias. Surg Endosc. 2012;26:3382–3390. [DOI] [PubMed] [Google Scholar]
- 19. Wood SN. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Stat Assoc. 2004;99:673–686. [Google Scholar]
- 20. Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biomed J. 2008;50:346–363. [DOI] [PubMed] [Google Scholar]
- 21. Morgenthal CB, Lin E, Shane MD, Hunter JG, Smith CD. Who will fail laparoscopic Nissen fundoplication? Preoperative prediction of long-term outcomes. Surg Endosc. 2007;21:1978–1984. [DOI] [PubMed] [Google Scholar]
- 22. Perez AR, Moncure AC, Rattner DW. Obesity adversely affects the outcome of antireflux operations. Surg Endosc. 2001;15:986–989. [DOI] [PubMed] [Google Scholar]
- 23. Mathieu P. Abdominal obesity and the metabolic syndrome: a surgeon's perspective. Can J Cardiol. 2008:24(Suppl D):19D–23D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shi X, Karmali S, Sharma AM, Birch DW. A review of laparoscopic sleeve gastrectomy for morbid obesity. Obes Surg, 2010;20:1171–1177. [DOI] [PubMed] [Google Scholar]
- 25. Vidal P, Ramon JM, Goday A, et al. Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy as a definitive surgical procedure for morbid obesity: mid-term Results. Obes Surg. 2013;23:292–299. [DOI] [PubMed] [Google Scholar]