Abstract
Biological systems, ranging from bacteria and fungi to humans, can methylate arsenic. Recent studies have suggested that the AsIII S-adenosylmethionine methyltransferase (arsM) gene in bacteria was responsible for the removal of arsenic as the volatile arsines from the bacteria. However, there has been no direct measure of the arsines released from bacteria cultures. We describe here an integrated system incorporating the bacterial incubation and volatile arsenic species analysis, and we demonstrate its application to the identification of the volatile arsines produced in bacterial cultures. The headspace of the bacterial cultures was purged with helium, and the volatile arsenic species were trapped in a chromatographic column immersed in liquid nitrogen. The cryogenically trapped arsines [AsH3, (CH3)AsH2, (CH3)2AsH, and (CH3)3As] were separated by gas chromatography and were detected by inductively coupled plasma mass spectrometry. A hydride generation system was coupled to the bacterial culture system, allowing for spiking standards and for generating calibration arsines necessary for quantitative analysis. Both bacteria containing the arsM gene or its variant arsMC2 gene were able to produce 400–500 ng of trimethylarsine. No trimethylarsine was detectable in bacteria lacking the arsM gene (containing the vector plasmid as negative control). These results confirm that arsM is responsible for releasing arsenic as volatile species from the arsenic-resistant bacteria. Our results also show traces of AsH3, CH3AsH2, and (CH3)2AsH in cultures of bacteria expressing arsM. The method detection limits for AsH3, CH3AsH2, (CH3)2AsH, and (CH3)3As were 0.5, 0.5, 0.7, and 0.6 pg, respectively. The ability to quantify trace levels of these volatile arsenic species makes it possible to study the biotransformation and biochemical roles of the evolution of these volatile arsenic species by biological systems.
Introduction
Microbial activities play important roles in the biogeochemical cycling and the speciation of arsenic (1, 2), often through the oxidation of organic carbon or sulfide coupled with the reduction of arsenate to arsenite (3–5). Several arsenic resistance mechanisms have been discovered (6, 7), and the corresponding genes encoding arsenic reductases (or oxidases) have been isolated from several bacteria strains (5). An interesting mechanism of bacterial resistance to (and detoxification of) arsenic is through the release of volatile arsenic species, including arsine and mono-, di- and trimethylarsine (4, 5). Rosen and co-workers (8) recently showed that bacteria containing the AsIII S-adenosylmethionine methyltransferase (arsM) gene were able to methylate inorganic arsenic to volatile trimethylarsine. They trapped the volatile species on H2O2-impregnanted paper and detected trimethylarsine oxide (TMAO) that was eluted from the paper. The finding of TMAO was used as an indirect evidence for the production of trimethylarsine ((CH3)3As) by the bacteria because it was reasoned that the TMAO was due to the oxidation of trimethylarsine by H2O2. Although this is chemically sound, a direct measure of trimethylarsine would provide stronger direct evidence for the bacterial release of volatile trimethylarsine. In addition, it is not known whether other volatile arsenic species may be produced in the bacterial culture. The objectives of this research were to develop a sensitive method enabling direct speciation analysis of volatile arsenic species in the headspace of the bacterial culture and to confirm the role of ArsM in generating the volatile arsenic species.
An analytical challenge for the quantitative analysis of arsenic species in the headspace of bacterial cultures involves the method of calibration. Because the quantitative standards for volatile arsines are not available, it is necessary to generate quantitative amounts of volatile arsines for calibration purpose. Previous studies have shown that inorganic arsenic and methylated arsenic species in solution could be derivatized to volatile arsine species, AsH3, CH3AsH2, and (CH3)2AsH using a hydride generation process (9–11, 19–22). These compounds can be trapped in liquid N2 and separated according to their differences in boiling points. Therefore, many methods based on cold trapping (CT) and gas chromatography (GC) have been used for the analysis of arsenic species in solutions (12–18). Although these methods have not been used for direct analysis of volatile arsenic species in the headspace of a cell culture, we hypothesized that the principle could be adapted for generating arsine standards for calibration. Therefore, we have developed a HG-CT-GC-inductively coupled plasma mass spectrometry (ICPMS) system that is suitable for analysis of volatile arsenic species in headspace and arsenic species in solution amenable for hydride generation. We describe here the development, optimization, and application of this technique for bacterial culture analysis. Using a specially designed device for cell incubation, the bacterial culture can be directly analyzed. With this new analytical capability, we further confirm the production of trimethylarsine by Escherichia coli expressing the arsM arsenic-resistance gene after the bacterial incubation with inorganic arsenite.
Experimental
Reagent
Sodium hydroxide (Fisher), sodium borohydride (Aldrich), and oxalic acid (Fisher) were used for hydride generation reactions. Isopropyl β-D-1-thiogalactopyranoside (IPTG) (Fisher), Luria-Bertani (LB) medium (BD Biosciences), Kanamycin (Sigma), and chloramphenicol (Aldrich) were used for bacteria incubation. Solutions of arsenite (AsIII), monomethylarsonic acid (MMA), dimethylarsinic acid (DMA), and trimethylarsine oxide (TMAO) standards were prepared as previously described (23–25). Volatile arsine standards were generated from these arsenic species. (Caution: These volatile arsenic species are toxic and should be handled with care).
Instrumentation
An Agilent 7500cs octopole reaction system ICPMS (Agilent Technologies, Japan) was operated with the helium mode. The radio frequency power for the ICP was 1550 W. The flow rate of argon carrier gas to the ICP was 800 mL/min. ICPMS ChemStation (Agilent Technologies, Santa Clara, California) was used to record the chromatogram from CT-GC separation. The octopole was operated with a helium flow rate of 3.6 mL/min. The QP voltage was −20 V, and the cell exit voltage was −66 V. We tested the effectiveness of the helium collision cell technology in removing ArCl+ interference from the arsenic measurement and found that the presence of 40 mg/mL of Cl− in the sample did not interfere with arsenic analysis when a 20 μL sample was injected.
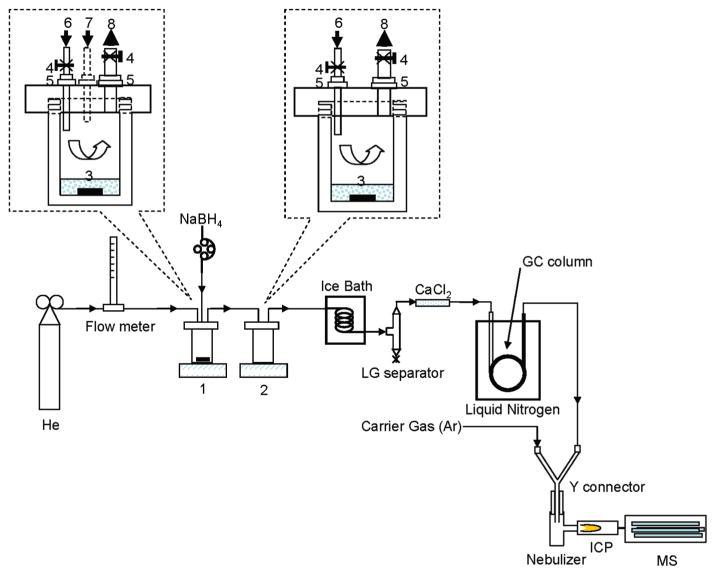
A bacterial incubation vessel (chamber 2 in Figure 1) was constructed to allow for both cell incubation and direct analysis of the headspace for volatile arsenic species. It was made of Teflon and had a total volume of 29.4 mL, with an internal diameter of 2.5 cm and inside height of 6 cm. The outer diameter was 4.5 cm, and outside height was 7 cm. The vessel was leak-proof with the use of a rubber O-ring along with the Teflon lid. A gas inlet and an outlet, each fit with a switch, were inserted through the lid. Both the gas inlet and outlet were turned off during bacteria incubation. They were switched on during the purge and trap step.
FIGURE 1.
Schematic diagram of the HG-CT-GC-ICPMS system. Chamber 1 is the hydride generation reaction vessel. Chamber 2 is the culture incubation vessel. 3, HG reaction medium; 4, clamps; 5, Teflon fittings; 6, carrier gas (He) inlet; 7, NaBH4 introduction port; 8, gas outlet.
To generate arsine standards online, a laboratory-built hydride generation vessel (chamber 1 in Figure 1) was connected to the bacterial incubation vessel. The hydride generation vessel (also made of Teflon) had a gas inlet (tubing 6, 1/16″ i.d.), a gas outlet (tubing 8, 1/8″ i.d.), and a port (tubing 7, 1/16″ i.d.) for introducing NaBH4 solution. The gaseous arsines generated by hydride generation or by bacterial culture in the headspace were purged with helium to a GC column immersed in liquid nitrogen. The boiling points for arsine, methylarsine, dimethylarsine, and trimethylarsine are −55, 2, 36, and 52 °C, respectively (17); and therefore, these arsenic species can be cryogenically trapped on the GC column. To prevent the moisture from being carried to the GC column, an ice–water bath, a liquid–gas separator, and a water-absorbent (CaCl2) cartridge were used. The use of the ice bath condensed most of the moisture in the gas flow, which was removed by the liquid–gas (LG) separator. The residual moisture was removed by the CaCl2 cartridge.
The GC column was made of a 100 cm PTFE tubing (3.2 mm i.d. and 4.0 mm o.d.), with the central 60 cm packed with 10% OV 101 (80/100 mesh Supelcoport, Supelco). This column was immersed in liquid nitrogen to trap the volatile arsenic species. A “Y” connector was used to introduce the arsine and carrier gas from the GC column (80 mL/min) while maintaining the usual carrier gas (Ar) flow at 800 mL/min to the ICP (Figure 1).
Bacteria Incubation
Cells of E. coli strain AW3110 (Δars), expressing the arsM and arsMC2 genes from Rhodopseudomonas palustris, and homologues were used in this study (8). E. coli with the pET-28a vector plasmid was used as the negative control. The bacteria were first cultured on a LB plate overnight and then kept in a fridge at 4 °C until use on the following day. The bacteria were precultured for 12 h at 37 °C in LB medium with 25 μg/mL kanamycin and 25 μg/mL chloramphenicol. The bacteria were then transferred to a Teflon chamber for further incubation in 5 mL LB medium supplemented with 10 μM AsIII, 20 μM IPTG, and 25 μg/mL kanamycin. The chamber was then tightly capped, and both the inlet and outlet switches were turned off. Multiple chambers were placed in an incubator (37 °C) for triplicate bacterial incubation experiments.
Sample Analysis
To determine the volatile arsenic species produced in bacterial cultures that were incubated with soluble arsenite in solution, the chambers were taken out from the incubator and were connected to the CT-GC-ICPMS system (Figure 1). The inlet and outlet switches were turned on. The headspace was purged with helium for 6 min, and the volatile species were trapped on the GC column that was immersed in liquid nitrogen (−196 °C). Following the complete trapping, liquid nitrogen was removed, and the gaseous arsines trapped on the GC column were separated on the basis of their boiling points (−55, 2, 36, and 52 °C). The arsenic species released from the GC column were carried by helium to the ICPMS for analysis.
Arsine Standards Generation
The arsine standards, including arsine (AsH3), monomethylarsine (CH3AsH2), dimethylarsine ((CH3)2AsH), and trimethylarsine ((CH3)3As), were generated from AsIII, MMAV, DMAV, and TMAO using a hydride generation procedure. A solution of 5% sodium borohydride in 0.1% sodium hydroxide and 4% oxalic acid was used as the hydride generation reagents. A 0.5 mL portion of AsIII, MMAV, DMAV, and TMAO solution (concentrations range from 10 ng/L to 10 μg/L) and 5 mL of 4% oxalic acid were added to the hydride generation chamber. A solution of 5% sodium borohydride in 0.1% sodium hydroxide was then introduced to the reaction chamber at 1 mL/min using a peristaltic pump (Figure 1). The reaction solution was continually stirred until the end of the reaction and trapping processes (typically 6 min).
Results and Discussion
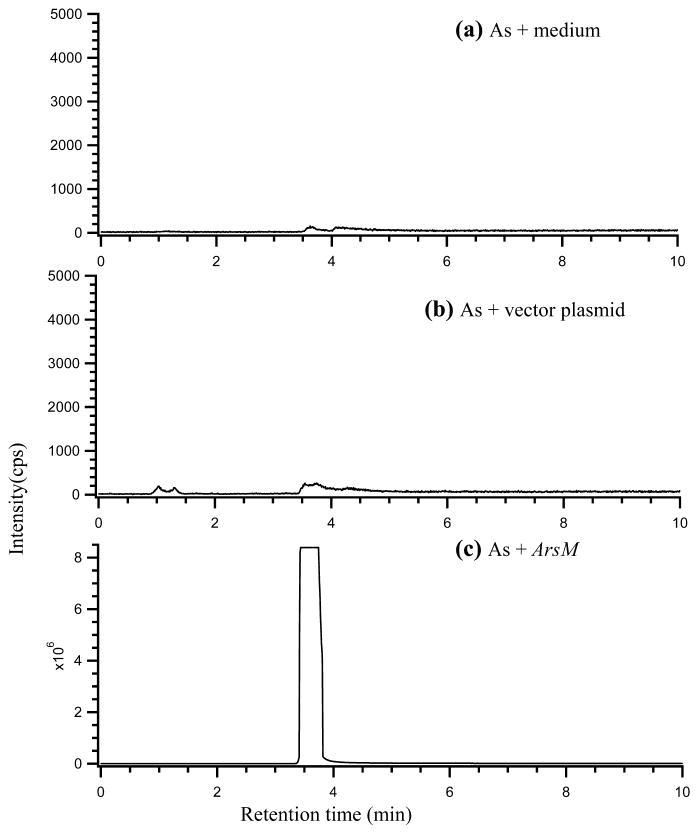
Figure 2 shows chromatograms from the analyses of head-space of control (no bacteria) (a) and bacteria expressed with either the cloning vector (b) or the arsM gene (c). As expected, there was no detectable volatile arsenic species when arsenite was incubated in culture medium only (without bacteria) (Figure 2a). A major volatile arsenic species, with the retention time corresponding to trimethylarsine [(CH3)3As], was detected in the culture of E. coli with the arsM gene expressed (Figure 2c). There was no appreciable trimethylarsine in the culture of E. coli expressing the vector plasmid pET-28a (without the arsM gene). These results support the conclusion that ArsM is responsible for the generation of trimethylarsine from arsenite in the bacterial culture (8).
FIGURE 2.
Chromatograms from the analyses of arsenic in the headspace of bacterial cultures and control. (a) arsenite (10 μM) incubated in culture medium without E. coli cells; (b) E. coli expressing the pET-28a vector plasmid, incubated with 10 μM arsenite; (c) E. coli expressing the arsM gene, incubated with 10 μM arsenite. The major peak in (c) corresponds to (CH3)3As.
The results of trimethylarsine generation from inorganic arsenite were further confirmed by using E. coli expressing arsMC2, a variant of the arsM gene. We found that E. coli cells expressing arsMC2 were also able to generate trimethylarsine from arsenite (Table 1).
TABLE 1.
Quantitative Analysis of Volatile Arsenic Species (ng As) Generated from E. coli Expressing Either Vector Plasmid pET28a or arsM Gene
| expression | AsH3 | CH3AsH2 | (CH3)2AsH | (CH3)3As |
|---|---|---|---|---|
| pET28a | 0.0051 ± 0.0012 | NDa | ND | ND |
| pET28/arsMC2 | 0.17 ± 0.072 | 0.049 ± 0.019 | 0.059 ± 0.043 | 414 ± 108 |
| pET28/arsM | 0.014 ± 0.006 | ND | ND | 483 ± 62.5 |
Below detection limit of CH3AsH2 (0.5 pg), (CH3)2AsH (0.7 pg), and (CH3)3As (0.6 pg).
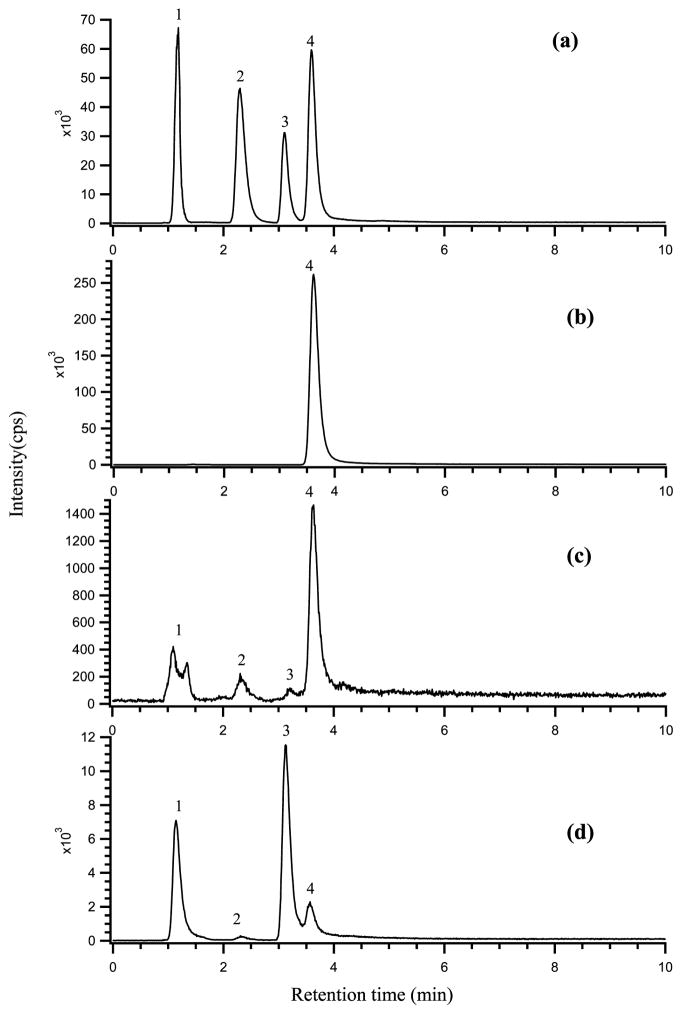
In addition to the trimethylarsine as the major volatile arsenic species, we have also observed trace amounts of AsH3, CH3AsH2, and (CH3)2AsH in bacterial cultures incubated with inorganic arsenite (Figure 3c). These volatile arsenic species were typically observed during a shorter period of incubation, for example, 2 h. This is the first direct observation of these arsines produced by bacteria. Prolonged incubation (e.g., 12 h) resulted in the further methylation of arsenic to trimethylarsine (Figure 3b).
FIGURE 3.
Chromatograms of volatile arsenic species from standards and bacterial cultures showing that the volatile species can be separated in four minutes, and that AsH3 (peak 1), CH3AsH2 (peak 2), (CH3)2AsH (peak 3), and (CH3)3As (peak 4), are present in the samples. (a) standards of volatile arsenic species (AsH3, CH3AsH2, (CH3)2AsH, and (CH3)3As) generated from AsIII, MMAV, DMAV, and TMAO, respectively; (b) sample of E. coli expressing ArsM7 incubated with 10 μM arsenite for 12 h; (c) sample of E. coli expressing ArsM7 incubated for two hours; (d) sample (c) spiked with AsH3 and (CH3)2AsH standards. The amounts of arsenic in chromatogram (a) were 1 ng each for AsH3, CH3AsH2, and (CH3)2AsH, and 1.5 ng for (CH3)3As.
To quantify the amounts of volatile arsines produced by the bacteria, we further developed an integrated hydride generation system that allowed for the standard addition and calibration. This new hydride generation system is necessary because there are no volatile arsine standards available. Figure 3a shows that the four arsines, AsH3 (bp −55 °C), CH3AsH2 (2 °C), (CH3)2AsH (36 °C), and (CH3)3As, (52 °C), are well separated according to their boiling points. These arsine standards were generated from inorganic arsenite, monomethylarsonic acid (MMAV), dimethylarsinic acid (DMAV), and trimethylarsine oxide (TMAO), respectively, by reacting these arsenic species with NaBH4 in the laboratory-built hydride generation vessel that was connected to the culture incubation vessel (Figure 1). The volatile arsenic species produced by the hydride generation process and by the bacteria were trapped together and detected by ICPMS. Therefore, the addition of standards could be performed to confirm the identity of the volatile arsenic species in the culture sample, based on the chromatographic retention time match between the sample and the standards. Retention times of chromatographic peaks from the analysis of bacterial culture after 2 h incubation (Figure 3c) match those of AsH3, CH3AsH2, (CH3)2AsH, and (CH3)3As standards (Figure 3a). Standard addition of AsH3 (peak 1) and (CH3)2AsH (peak 3 in Figure 3d) or CH3AsH2 and (CH3)3As (data not shown) further supports the identity of these volatile arsines.
To achieve quantitative determination of the volatile arsines, we optimized the hydride generation and trapping conditions. We optimized both the concentrations of the reaction reagents (acid and NaBH4) and the gas flow for purge and trap, to achieve quantitative and reproducible amounts of arsine standards for calibration.
We initially attempted the use of hydrochloric acid (2–10%), as this has commonly been used for hydride generation. However, the signals were noisy and not reproducible, probably because of possible interferences from the excess Cl− and from the rapid formation of H2 gas due to the reaction between strong hydrochloric acid and NaBH4. We subsequently found that oxalic acid was suitable for hydride generation. Supporting Information Figure S1 shows that consistent signals from the four arsenic species were obtained when oxalic acid was 2–10%. We choose 4% oxalic acid for the rest of the study.
Supporting Information Figure S2 shows the effect of NaBH4 on the signal intensity from the four arsenic species. NaBH4 is necessary for the reduction and conversion of inorganic arsenic, MMAV, DMAV, and TMAO to their corresponding arsines, AsH3, CH3AsH2, (CH3)2AsH, and (CH3)3As, respectively. Although 3% NaBH4 was sufficient to convert all arsenite to AsH3, 5% NaBH4 was used to achieve the optimum generation of all four arsines.
Supporting Information Figure S3 shows the effect of carrier gas flow rate on the signal intensity from the four arsenic species. With a too low gas flow (<40 mL/min), the dispersion resulted in broad peaks and low signal intensity. With a too high flow rate (>120 mL/min), the arsines were not completely trapped in the GC column. A flow rate of 80 mL/min was suitable for efficient trapping of all four arsines, resulting in optimum signal intensity.
We determined the efficiency of generating volatile arsines from the corresponding arsenic species in solution by comparing the amounts of arsenic species in solution before and after the hydride generation reactions. Under the conditions that we have established, ~100% arsenite, arsenate, and MMAV in solution were converted to volatile arsines, and 83 ± 2% DMAV and 89 ± 3% TMAO were converted to (CH3)2AsH and (CH3)3As, respectively.
The detection limits were 0.5 pg for AsH3 and CH3AsH2, 0.7 pg for (CH3)2AsH, and 0.6 pg for (CH3)3As. The detection limit was defined as the amount of arsenic that produced a chromatographic peak having an intensity equal to three times the standard deviation of the baseline noise (26). The calibration curves were linear (Supporting Information Figure S4) for a dynamic range over 2–3 orders of magnitude [5 pg to 5 ng for AsH3 and CH3AsH2; 25 pg to 5 ng for (CH3)2AsH and (CH3)3As].
Having established the method for calibration and standard addition, we further determined quantitative amounts of volatile arsines produced in bacterial cultures. Table 1 shows results from quantitative analysis of volatile arsenic species generated from bacterial cultures. Cultures of E. coli cells expressing arsM or its variant arsMC2 show the production of substantial amounts of trimethylarsine (414 ng and 483 ng). These amounts account for 11% and 13% of the total arsenic initially incubated in the culture. Trimethylarsine is not detectable in E. coli expressing only vector plasmid pET28a (negative control). These results confirm the previous finding (8) that ArsM is responsible for the release of trimethylarsine gas from E. coli incubated with arsenite. Interestingly, trace amounts of monomethylarsine (0.049 ng) and dimethylarsine (0.059 ng) are also produced by E. coli expressing arsMC2. Trace amounts of arsine gas (0.0051–0.17 ng) are present in cultures of E. coli expressing either the vector plasmid or arsM gene. Although these amounts are very small (<0.005% of the total arsenic in the culture), further research will be useful to understand what is responsible for the formation of these volatile arsines.
Previous research has shown that the expression of arsM or arsMC2 in E. coli conferred resistance of this bacterium to arsenite (8). The wild-type strain AW3110 of E. coli, in which all arsenic resistance genes had been deleted, is hypersensitive. The direct measurements of volatile trimethylarsine in cell cultures of E. coli expressing arsM or its variants supports the role of ArsM in the volatilization of trimethylarsine gas from the bacteria. Release of volatile arsines from the arsenic-resistant bacteria may be a mechanism of detoxification (4–8).
Direct determination of volatile arsenic species in the headspace of bacterial cultures was made possible by the newly developed method that was described here. The cryogenic trapping enhanced the sensitivity for detecting trace levels of arsines. The tandem hydride generation and bacterial incubation apparatus enabled the standard addition and calibration necessary for quantitative analysis of the gaseous arsenic species in the headspace. This method and the similar strategy can be applied to studying the biotransformation of other metalloids.
Supplementary Material
Acknowledgments
This work was supported by the Canadian Water Network, the Metals in the Human Environment Strategic Network, the National Cancer Institute of Canada, and the Innovative Persistent Toxic Substances to XCL and United States Public Health Service Grant AI45428 to BPR. C. Y. acknowledges the support from North China Electric Power University.
ABBREVIATION
- AsIII
arsenite
- AsV
arsenate
- AsH3
arsine
- CT
cold trap
- DMA
dimethylarsinic acid
- (CH3)2AsH
dimethylarsine
- GC
gas chromatography
- HG
hydride generation
- ICPMS
inductively coupled plasma mass spectrometry
- IPTG
Isopropyl β-D-1-thiogalactopyranoside
- LC
liquid chromatography
- MMA
monomethylarsonic acid
- CH3AsH2
monomethylarsine
- (CH3)3As
trimethylarsine
- TMAO
trimethylarsine oxide
Footnotes
Supporting Information Available
Supplemental Figures S1, S2, S3, and S4. This material is available free of charge via the Internet at http://pubs.acs.org.
Literature Cited
- 1.Oremland RS, Kulp TR, Blum JS, Hoeft SE, Baesman S, Miller LG, Stolz JF. A microbial arsenic cycle in a salt-saturated, extreme environment. Science. 2005;308:1305–8. doi: 10.1126/science.1110832. [DOI] [PubMed] [Google Scholar]
- 2.Malasarn D, Saltikov CW, Campbell KM, Santini JM, Hering JG, Newman DK. arrA is a reliable marker for As(V) respiration. Science. 2004;306:455. doi: 10.1126/science.1102374. [DOI] [PubMed] [Google Scholar]
- 3.Bentley R, Chasteen TG. Microbial methylation of metalloids: Arsenic, antimony, and bismuth. Microbiol Mol Biol Rev. 2002;66:250–71. doi: 10.1128/MMBR.66.2.250-271.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oremland RS, Stolz JF. The ecology of arsenic. Science. 2003;300:939–44. doi: 10.1126/science.1081903. [DOI] [PubMed] [Google Scholar]
- 5.Stolz JF, Basu P, Santini JM, Oremland RS. Arsenic and selenium in microbial metabolism. Annu Rev Microbiol. 2006;60:107–30. doi: 10.1146/annurev.micro.60.080805.142053. [DOI] [PubMed] [Google Scholar]
- 6.Rosen BP. Bacterial resistance to heavy metals and metalloids. J Biol Inorg Chem. 1996;1:273–7. [Google Scholar]
- 7.Silver S, Phung LT. Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol. 1996;50:753–89. doi: 10.1146/annurev.micro.50.1.753. [DOI] [PubMed] [Google Scholar]
- 8.Qin J, Rosen BP, Zhang Y, Wang G, Franke S, Rensing C. Arsenic detoxification and evolution of trimethylarsine gas by a microbial arsenite S-adenosylmethionine methyltransferase. Proc Natl Acad Sci USA. 2006;103:2075–80. doi: 10.1073/pnas.0506836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le XC, Lu X, Li XF. Arsenic speciation. Anal Chem. 2004;76:26A–33A. [Google Scholar]
- 10.Gong ZL, Lu XF, Ma MS, Watt C, Le XC. Arsenic speciation analysis. Talanta. 2002;58:77–96. doi: 10.1016/s0039-9140(02)00258-8. [DOI] [PubMed] [Google Scholar]
- 11.Odanada Y, Tsuchiya N, Matano O, Goto S. Determination of inorganic arsenic and methylarsenic compounds by gas chromatography and multiple ion detection mass spectrometry after hydride generation-heptane cold trap. Anal Chem. 1983;55:929–32. doi: 10.1021/ac00257a025. [DOI] [PubMed] [Google Scholar]
- 12.Guerin T, Molenat N, Astruc A, Pinel R. Arsenic speciation in some environmental samples: a comparative study of HG-GC-QFAAS and HPLC-ICP-MS methods. Appl Organomet Chem. 2000;14:401–10. [Google Scholar]
- 13.Šlejkovec Z, van Elteren JT, Byrne AP. A dual arsenic speciation system combining liquid chromatographic and purge and trap-gas chromatographic separation with atomic fluorescence spectrometric detection. Anal Chim Acta. 1998;358:51–60. [Google Scholar]
- 14.Wickenheiser EB, Michalke K, Drescher C, Hirner AV, Hensel R. Development and application of liquid and gas-chromatographic speciation techniques with element specific (ICP-MS) detection to the study of anaerobic arsenic metabolism. Fresenius J Anal Chem. 1998;362:498–501. [Google Scholar]
- 15.Prohaska T, Pfeffer M, Tulipan M, Stingeder G, Mentler A, Wenzel WW. Speciation of arsenic of liquid and gaseous emissions from soil in a microcosmos experiment by liquid and gas chromatography with inductively coupled plasma mass spectrometer (ICP-MS) detection. Fresenius J Anal Chem. 1999;364:467–70. [Google Scholar]
- 16.Bouyssiere B, Baco F, Savary L, Garraud H, Gallup DL, Lobinski R. Investigation of speciation of arsenic in gas condensates by capillary gas chromatography with ICP-MS detection. J Anal At Spectrom. 2001;16:1329–32. [Google Scholar]
- 17.Pantsar-Kallio M, Korpela A. Analysis of gaseous arsenic species and stability studies of arsine and trimethylarsine by gas chromatography-mass spectrometry. Anal Chim Acta. 2000;410:65–70. [Google Scholar]
- 18.Kaise T, Ogura M, Nozaki T, Saitoh K, Sakurai T, Matsubara C, Watanabe C, Hanaoka K. Biomethylation of arsenic in an arsenic-rich freshwater environment. Appl Organomet Chem. 1997;11:297–304. [Google Scholar]
- 19.Howard AG, Arbab-Zavar MH. Determination of “Inorganic” arsenic (III) and arsenic (V), “Methylarsenic” and “Dimethylarsenic” species by selective hydride evolution atomic absorption spectroscopy. Analyst. 1981;106:213–20. [Google Scholar]
- 20.Burguera M, Burguera JL, Brunetto MR. Flow-injection atomic spectrometric determination of inorganic arsenic (III) and arsenic (V) species by use of an aluminium-column arsine generator and cold-trapping arsine collection. Anal Chim Acta. 1992;261:105–13. [Google Scholar]
- 21.Van Cleuvenbergen RJA, Van Mol WE, Adams FC. Arsenic speciation in water by hydride cold trapping-quartz furnace atomic absorption spectrometry: an evaluation. J Anal At Spectrom. 1988;3:169–76. [Google Scholar]
- 22.Hsiung TM, Wang JM. Cryogenic trapping with a packed cold finger trap for the determination and speciation of arsenic by flow injection/hydride generation/atomic absorption spectrometry. J Anal At Spectrom. 2004;19:923–28. [Google Scholar]
- 23.Le XC, Lu X, Ma M, Cullen WR, Aposhian V, Zheng B. Speciation of key arsenic metabolic intermediates in human urine. Anal Chem. 2000;72:5172–7. doi: 10.1021/ac000527u. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Zhou J, Lu X, Gong Z, Le XC. Arsenic speciation in urine from acute promyelocytic leukemia patients undergoing arsenic trioxide treatment. Chem Res Toxicol. 2004;17:95–103. doi: 10.1021/tx0341714. [DOI] [PubMed] [Google Scholar]
- 25.Lu X, Arnold LL, Cohen SM, Cullen WR, Le XC. Speciation of dimethylarsinous acid and trimethylarsine oxide in urine from rats fed with dimethylarsinic acid and dimercaptopropane sulfonate. Anal Chem. 2003;75:6463–8. doi: 10.1021/ac034868u. [DOI] [PubMed] [Google Scholar]
- 26.ACS Committee on Environmental Improvement. Guidelines for data acquisition and data quality evaluation in environmental chemistry. Anal Chem. 1980;52:2242–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.