Abstract
Background
The National Cancer Registry (NCR) was established as a pathology-based cancer reporting system. From 2005 to 2007, private health laboratories withheld cancer reports owing to concerns regarding voluntary sharing of patient data.
Objectives
To estimate the impact of under-reported cancer data from private health laboratories.
Methods
A linear regression analysis was conducted to project expected cancer cases for 2005 – 2007. Differences between actual and projected figures were calculated to estimate percentage under-reporting.
Results
The projected NCR case total varied from 53 407 (3.8% net increase from actual cases reported) in 2005 to 54 823 (3.7% net increase) in 2007. The projected number of reported cases from private laboratories in 2005 was 26 359 (19.7% net increase from actual cases reported), 27 012 (18.8% net increase) in 2006 and 27 666 (28.4% net increase) in 2007.
Conclusion
While private healthcare reporting decreased by 28% from 2005 to 2007, this represented a minimal impact on overall cancer reporting (net decrease of <4%).
The National Cancer Registry (NCR) within the National Health Laboratory Service (NHLS) is the principal cancer surveillance system in South Africa (SA) and maintains the largest repository of cancer data in the country. The NCR is mandated through recent (2011) legislation to monitor SA’s national cancer burden.[1] Established in 1986 as a voluntary, pathology-based cancer reporting system, it now receives over 100 000 cancer reports annually. Approximately 80 000 are new cases, on the basis of which cancer incidence is calculated. Data collected from the system are used for research, for educational purposes and to inform decision-making for cancer prevention and control policies in SA.
Surveillance and research activities at the NCR have made a significant contribution to the scope of cancer knowledge both locally and internationally. In addition to describing the overall cancer burden in SA, the registry data have been used to highlight cancers of special interest such as skin, prostate and oral cancers.[2–4] Of importance in the SA context, the data from the Johannesburg Cancer Case Control Study (JCCCS), conducted by the research arm of the NCR, the Cancer Epidemiology Research Group (CERG), have been used to extensively describe the epidemiology of HIV-related cancers in SA and particularly to explore the relationship between Kaposi’s sarcoma and HIV.[5–11] The JCCCS has also contributed to risk factor analysis in the International Collaboration of Epidemiological Studies of Cervical Cancer, including the link between oral and injectable contraceptive use and female cancers.[12–18]
The NCR manages cancer surveillance in the context of a dual health system in SA: a large public health infrastructure serving approximately 84% of the population, and a smaller private health system catering to 16%.[19] The NCR achieves its objectives by estimating cancer incidence rates by age, race and gender, using pathology reports received from all public and private healthcare laboratories nationally.[20]
Data reporting among private systems was consistent throughout the early 2000s. However, concerns regarding voluntary sharing of patient data led some private healthcare laboratories to withhold cancer pathology reports, beginning in 2005. We undertook an analysis to measure the impact of withheld private data on cancer surveillance in SA.
Methods
NCR methodology
The NCR methodology follows that recommended by the International Agency for Research on Cancer.[21] Pathology reports are received in electronic or hard-copy format, and from these appropriate data items, namely demographic and tumour information, are abstracted. A hot-deck imputation method[22] is used to allocate population group to cases without this information. Following international practice, cancers are classified by anatomical site/topography using the International Classification of Diseases – Oncology, Version 3 (ICD-O-3).[23]
Mid-year population estimates from Statistics South Africa are used as the denominator, stratified by population, gender and 5-year age groups. Analyses include crude incidence rates, age-standardised incidence rates (ASRs) using the Doll et al.[24] world population as the standard, 95% confidence intervals for the ASRs, and cumulative lifetime incidence risk (the likelihood of developing a cancer in one’s lifetime if one lives to age 74).[25] The ASR and the lifetime incidence risk are adjusted for the proportion of cases in the unknown age category. The rate calculations represent incident cancers, excluding basal and squamous cell carcinomas of the skin.
Analysis of under-reporting
Using actual numbers of cases reported by private health laboratories for 1995 – 2004, a linear regression analysis was performed. Based on this analysis, we were able to project the expected cases for 2005, 2006 and 2007 from private laboratories. The calculated number of projected cases for each year was used to estimate the number of missed cases reported per annum from private laboratories. Differences between actual and projected figures were calculated to establish the percentage of under-reporting.
Results
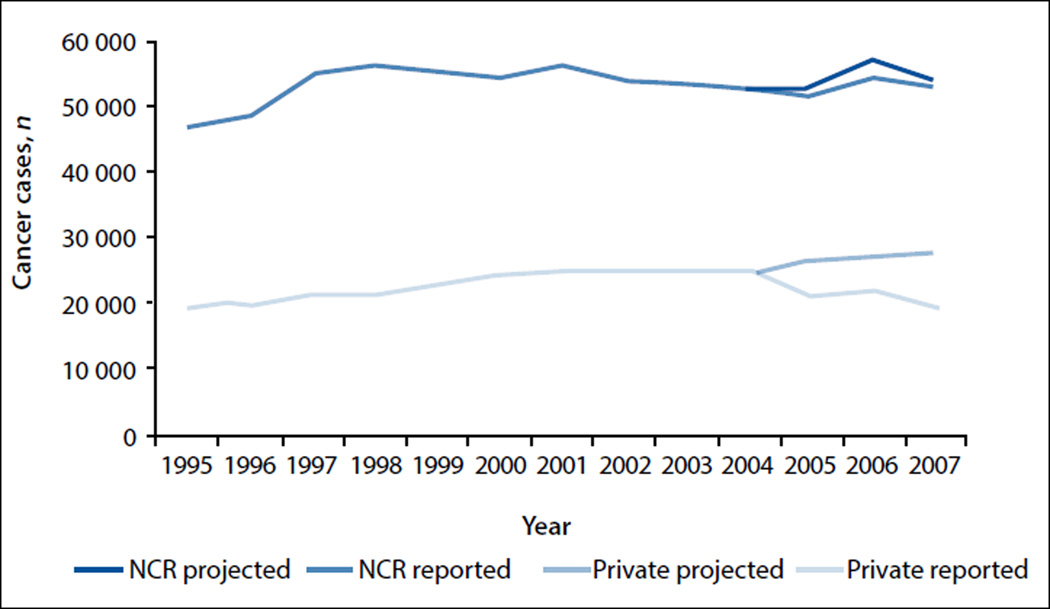
In 1995, a total of 46 769 cases of cancer were reported from all laboratories (both private and public system laboratories), which increased to 52 887 cases in 2004, then decreased to 52 816 in 2007 (Fig. 1). The projected increase in cases in 2005 was 53 407 (3.8% net increase from actual cases reported), followed by 56 679 (3.5% net increase) in 2006 and 54 823 (3.7% net increase) in 2007.
Fig. 1.
Actual and projected case reporting from private laboratories and to the NCR, 1995 – 2007.
In 1995, a total of 19 137 cases of cancer were reported from private laboratories, which increased to 24 473 cases in 2004, then decreased to 19 803 in 2007. The projected number of reported cases of cancer in 2005 was 26 359 (19.7% net increase from actual cases reported), followed by 27 012 (18.8% net increase) in 2006 and 27 666 (28.4% net increase) in 2007.
Discussion
This is the first study to assess the impact of missing cancer cases from private healthcare laboratories on NCR surveillance in SA. While private healthcare reporting decreased by 28% from 2005 to 2007, this represented a minimal impact on overall cancer reporting (net decrease of <4%). Despite missing data, the NCR cancer data therefore provide an accurate estimation of overall pathology-diagnosed cancer incidence in SA.
This finding can be explained by healthcare use patterns, particularly the two-tiered healthcare system mentioned previously. Given that a relatively large proportion of the population accesses the public healthcare system (84%) as opposed to private healthcare facilities,[19] the bulk of cancer reports to the NCR originate in the public health system from the more than 300 laboratories operated by the NHLS. While the overall decrease in reporting may represent a small proportion, missing data may be over-represented in certain population groups. Membership in medical schemes, and therefore ability to access private healthcare, is concentrated in the wealthiest 20% of the SA population[26] and correlates with population group. More than two-thirds of white South Africans belong to a medical aid, as opposed to 8% of blacks.[27] The NCR may therefore be underreporting cancers in these groups for the specified period.
Our results also reflect some underreporting or under-diagnosis of cancer among public healthcare facilities. Sixteen per cent of South Africans (all race groups) belong to a medical aid,[19] yet the private health service represents just under half of the cancer cases reported. Despite the lifestyle risk factors for cancer associated with higher socioeconomic status, one would expect more cancer cases from the public health service than the approximately 40 000 received by the NCR. The NCR receives and processes all cancer pathology reports from the public healthcare facilities. The rate of under-diagnosis and under-ascertainment of cancers in the SA health service must be quantified so that a more accurate illustration of the cancer burden can be provided.
Several strengths and limitations are evident in this study. The NCR is the largest and most representative cancer surveillance system in SA. The registry methods are robust, with staff available to process the country’s cancer burden with a quality-assured output. Since the registry is pathology based, specific and detailed histological diagnoses are available. However, this also implies that cases without a pathology diagnosis will be missed, resulting in underestimates of cancer burden. Although publication of cancer incidences for SA is delayed, the reporting time will soon be comparable to more comprehensive international registries such as the US Centers for Disease Control’s National Program of Cancer Registries, which has a reporting time of approximately 3 years from the time a patient is diagnosed to the time national data are reported.[28]
The delays in cancer reporting are attributable to a number of factors, including lack of a champion for cancer registration. The NCR was without a director from 2002 to 2009, which led to leadership inconsistencies and high staff turnover. During this time, competing health priorities such as HIV prevention and control emerged as the public health priority in SA. As a result, the NCR did not receive the same level of resources for cancer surveillance as in the past, which impacted negatively on cancer reporting and data analysis.
However, with the appointment of the new management in 2009 and promulgation of new legislation in 2011, there have been significant gains in efficiency. The NCR has established electronic receipt of pathology data from public sector laboratories, thereby ensuring a more timely acquisition of morbidity information. Relationships with private sector laboratories have been renewed, and a standard system has been established to receive private sector pathology data electronically.
Promulgation of Regulation 380 of the National Health Act[1] by the Department of Health in 2011 marked a milestone in cancer care and control by formally establishing the NCR as the main cancer surveillance agency and requiring mandatory reporting of all confirmed cancers in SA to the NCR. The legislation ensured that cancer reporting to the NCR was no longer voluntary and precluded the drop-off of reporting that has occurred in the past.
Regulation 380 also allows the NCR to implement population-based cancer registration in selected surveillance sites. In response to the legislation, the NCR has developed a 10-year business plan for the implementation of the population-based registries for the country, as well as tackling the pathology-based registry backlog. Fund-raising activities have commenced, and a pilot population-based registry is operational in Ekurhuleni District, Gauteng Province. Population and pathology-based cancer registration complemented by novel research from the NCR’s CERG will provide a comprehensive description of the cancer burden across SA.
In an era of growing prioritisation of non-communicable diseases, and with global cancer burdens estimated to increase significantly,[29] the NCR has an invaluable role to play in the health and health planning landscape of SA. In view of the progressive health developments in the country, such as introduction of National Health Insurance,[5] there is an imperative to accurately quantify the cancer burden, and thus the cost of cancer services to be provided to the SA population.
Conclusion
The withholding of private laboratory cancer data from 2005 to 2007 resulted in a 4% decrease in overall cancer reporting to SA’s NCR, despite a relatively larger amount (28%) of under-reporting of private healthcare cancers. This probably reflects the reality that four out of every five SA citizens receive care in the public healthcare system. This analysis does not address undiagnosed cancer cases in the public health system, an issue that requires further investigation. The NCR is an invaluable source of cancer data for the country. Recent parliamentary legislation, investment in the NCR and improved access to quality healthcare will allow the NCR to remain SA’s leading resource for national cancer data.
Footnotes
Authorship. All authors have read and approved the manuscript, and there are no financial disclosures, conflicts of interests and/or acknowledgements necessary. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.National Department of Health. National Health Act (Act No. 61 of 2003): Regulations Relating to Cancer Registration. No. R 380. [(accessed 12 January 2015)];Republic of South Africa. 2011 http://www.nioh.ac.za/assets/files/Gazetted%20Version%2026_4_2011%20(2).pdf. [Google Scholar]
- 2.Norval M, Kellett P, Wright CY. The incidence and body site of skin cancer in the population groups of South Africa. Photodermatol Photoimmunol Photomed. 2014;30(5):262–265. doi: 10.1111/phpp.12106. [ http://dx.doi.org/10.1111/phpp.12106] [DOI] [PubMed] [Google Scholar]
- 3.Abram MH, van Heerden WFP, Rheeder P, Birdler-Brown BV, van Zyl W. Epidemiology of oral squamous cell carcinoma. SADJ. 2012;67(10):550–553. [PubMed] [Google Scholar]
- 4.Babb C, Urban M, Kielkowski D, Kellett P. Prostate cancer in South Africa: Pathology based National Cancer Registry data (1986–2006) and mortality rates (1997–2009) Prostate Cancer. 2014 doi: 10.1155/2014/419801. 2014, Article ID 419801. [ http://dx.doi.org/10.1155/2014/419801] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sitas F, Pacella-Norman R, Carrara H, et al. The spectrum of HIV-1 related cancers in South Africa. Int J Cancer. 2000;88(3):489–492. doi: 10.1002/1097-0215(20001101)88:3<489::aid-ijc25>3.0.co;2-q. [ http://dx.doi.org/10.1002/1097-0215(20001101)88:3<489::AID-IJC25>3.0.CO;2-Q] [DOI] [PubMed] [Google Scholar]
- 6.Stein L, Urban MI, O’Connell D, et al. The spectrum of human immunodeficiency virus-associated cancers in a South African black population: Results from a case-control study, 1995–2004. Int J Cancer. 2008;122(10):2260–2265. doi: 10.1002/ijc.23391. [ http://dx.doi.org/10.1002/ijc.23391] [DOI] [PubMed] [Google Scholar]
- 7.Sitas F, Newton R. Kaposi’s sarcoma in South Africa. J Natl Cancer Inst Monogr. 2000;2000(28):1–4. doi: 10.1093/oxfordjournals.jncimonographs.a024250. [ http://dx.doi.org/10.1093/oxfordjournals.jncimonographs.a024250] [DOI] [PubMed] [Google Scholar]
- 8.Sitas F, Bezwoda WR, Levin V, et al. Association between human immunodeficiency virus type 1 infection and cancer in the black population of Johannesburg and Soweto, South Africa. Br J Cancer. 1997;75(11):1704–1707. doi: 10.1038/bjc.1997.290. [ http://dx.doi.org/10.1038/bjc.1997.290] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Collaboration on HIV and Cancer. Highly active retroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst. 2000;92(22):1823–1830. doi: 10.1093/jnci/92.22.1823. [ http://dx.doi.org/10.1093/jnci/92.22.1823] [DOI] [PubMed] [Google Scholar]
- 10.Weiss RA, Sitas F. Kaposi’s sarcoma in AIDS. J Registry Manage. 2001;28(2):93–96. [Google Scholar]
- 11.Beral V, Newton R, Sitas F. Human herpesvirus 8 and cancer. J Natl Cancer Inst. 1999;91(17):1440–1441. doi: 10.1093/jnci/91.17.1440. [ http://dx.doi.org/10.1093/jnci/91.17.1440] [DOI] [PubMed] [Google Scholar]
- 12.Plummer M, Peto J, Franceschi S for International Collaboration of Epidemiological Studies of Cervical Cancer (incl. Urban MI) Time since first sexual intercourse and the risk of cervical cancer. Int J Cancer. 2012;130(11):2638–2644. doi: 10.1002/ijc.26250. [ http://dx.doi.org/10.1002/ijc.26250] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plummer M, Peto J, Franceschi S for International Collaboration of Epidemiological Studies of Cervical Cancer (incl. Urban MI) Cervical carcinoma and sexual behaviour: Collaborative reanalysis of individual data on 15,461 women with cervical carcinoma and 29,164 women without cervical carcinoma from 21 epidemiological studies. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1060–1069. doi: 10.1158/1055-9965.EPI-08-1186. [ http://dx.doi.org/10.1158/1055-9965.EPI-08-1186] [DOI] [PubMed] [Google Scholar]
- 14.Plummer M, Peto J, Franceschi S for International Collaboration of Epidemiological Studies of Cervical Cancer (incl. Urban MI) Cervical cancer and hormonal contraceptives: Collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370(9599):1609–1621. doi: 10.1016/S0140-6736(07)61684-5. [ http://dx.doi.org/10.1016/S0140-6736(07)61684-5] [DOI] [PubMed] [Google Scholar]
- 15.Plummer M, Peto J, Franceschi S for International Collaboration of Epidemiological Studies of Cervical Cancer (incl. Urban MI) Cervical carcinoma and reproductive factors: Collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33,542 women without cervical carcinoma from 25 epidemiological studies. Int J Cancer. 2006;119(5):1108–1124. doi: 10.1002/ijc.21953. [ http://dx.doi.org/10.1002/ijc.21953] [DOI] [PubMed] [Google Scholar]
- 16.Plummer M, Peto J, Franceschi S for International Collaboration of Epidemiological Studies of Cervical Cancer (incl. Urban MI) Carcinoma of the cervix and tobacco smoking: Collaborative reanalysis of individual data on 13 541 women with carcinoma of the cervix and 23 017 woman without carcinoma of the cervix from 23 epidemiological studies. Int J Cancer. 2006;118(6):1481–1495. doi: 10.1002/ijc.21493. [ http://dx.doi.org/10.1002/ijc.21493] [DOI] [PubMed] [Google Scholar]
- 17.Plummer M, Peto J, Franceschi S for International Collaboration of Epidemiological Studies of Cervical Cancer (incl. Urban MI) Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: Collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies. Int J Cancer. 2006;120(4):885–891. doi: 10.1002/ijc.22357. [ http://dx.doi.org/10.1002/ijc.22357] [DOI] [PubMed] [Google Scholar]
- 18.Urban M, Banks E, Egger S, Canfell K, O’Connell D, Beral V, Sitas F. Injectable and oral contraceptive use and cancers of the breast, cervix, ovary, and endometrium in black South African women: Cancer case-control. PLoS Med. 2012;9(3):e1001182. doi: 10.1371/journal.pmed.1001182. [ http://dx.doi.org/10.1371/journal.pmed.1001182] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green Paper: National Health Insurance in South Africa. A Policy Paper. [(accessed 25 April 2014)];National Department of Health, Republic of South Africa. http://www.hst.org.za/publications/green-paper-national-health-insurance-south-africa. [Google Scholar]
- 20.Cancer in South Africa Full Reports: 2000–2007. [(accessed 9 July 2014)]; www.ncr.ac.za. [Google Scholar]
- 21.National Health Laboratory Service. National Cancer Registry Methodology. [(accessed 1 July 2014)];2006 www.nioh.ac.za/assets/files/NCR_methodology.pdf. [Google Scholar]
- 22.Little RJA, Rubin DB. The analysis of social science data with missing values. In: Fox J, Long JS, editors. Modern Methods of Data Analysis. London: Sage Publications; 1990. [Google Scholar]
- 23.Fritz A, Percy C, Jack A, Kanagaratnam S, Sobin L, Parkin DM, et al., editors. International Classification of Diseases for Oncology. 3rd ed. Geneva: World Health Organization; 2000. [Google Scholar]
- 24.Doll R, Payne PM, Waterhouse JAH. International Union Against Cancer. Berlin: Springer-Verlag; 1966. Cancer Incidence in Five Countries. [ http://dx.doi.org/10.1007/978-3-642-85849-9] [Google Scholar]
- 25.Jensen OM, Parkin DM, Maclennan R, Muir CS, Skeet RG. IARC Scientific Publications No. 95. Lyon: International Agency for Research on Cancer; 1991. Cancer Registration: Principles and Methods. [ http://dx.doi.org/10.1038/bjc.1991.142] [Google Scholar]
- 26.McIntyre D. Private Sector Involvement in Funding and Providing Health Services in South Africa: Implications for Equity and Access to Health Care. EQUINET Discussion Paper Series 84. Health Economics Unit, University of Cape Town; ISER, Rhodes University, Grahamstown, Eastern Cape; EQUINET, Harare. 2010 [Google Scholar]
- 27.Statistics South Africa. General Household Survey 2011. [(accessed 25 June 2014)];Statistical Release P0318. www.statsa.gov.za.
- 28.US Cancer Statistics Working Group. Incidence and Mortality Web-based Report. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2013. [(accessed 9 July 2014)]. United States Cancer Statistics: 1999–2010. www.cdc.gov/uscs. [Google Scholar]
- 29.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): A population-based study. Lancet Oncology. 2012;13(8):790–801. doi: 10.1016/S1470-2045(12)70211-5. [ http://dx.doi.org/10.1016/S1470-2045(12)70211-5] [DOI] [PubMed] [Google Scholar]