Abstract
Background
The association between abacavir (ABC) and cardiovascular disease (CVD) risk in HIV-infected individuals is unclear. Putative mechanisms for an effect of ABC on CVD risk including endothelial dysfunction have been proposed; however, a biological mechanism has not been established.
Methods
This was a cross-sectional study of HIV-infected subjects with HIV RNA levels <400 copies/ml, who were randomly assigned to ABC or tenofovir (TDF) as initial therapy during a prior clinical trial. A small cohort of subjects on zidovudine (AZT; not randomly assigned) were studied to explore long-term exposure to this agent. All underwent brachial artery ultrasound for flow-mediated dilation (FMD), and D-dimer, high-sensitivity C-reactive protein (hsCRP), interleukin-6 (IL-6) and fasting lipids were measured. Between-arm differences were evaluated by multivariable linear or logistic regression modelling.
Results
There were 148 subjects (46 on ABC, 72 on TDF and 30 on AZT). Demographic characteristics were balanced across the groups except, as expected, AZT-treated participants were older, had higher CD4+ T-cell counts, and longer antiretroviral therapy duration. After adjusting for age, brachial artery diameter, and treatment duration, FMD was similar in those on ABC (3.9%) and TDF (5.4%; P=0.181). FMD was higher in those on AZT (6.1%; P<0.005). Levels of IL-6, hsCRP and detectable D-dimer were similar between groups.
Conclusions
Among individuals assigned to ABC or TDF in randomized clinical trials there were no significant differences in FMD or markers of inflammation and coagulation. Whether ABC contributes to risk of CVD remains unclear, but our results suggest that endothelial dysfunction, heightened inflammation, and altered coagulation are unlikely to be mechanisms by which the drug could increase CVD risk above that seen with TDF.
Introduction
The role of abacavir in the development of cardiovascular disease (CVD) in HIV-infected patients is unclear. Observational cohort studies, in particular the D:A:D and French Hospital Database on HIV studies, have reported an association between exposure to abacavir and CVD risk [1–3]; however, retrospective analyses conducted by researchers in the Veterans Administration and the AIDS Clinical Trials Group (ACTG), as well as two meta-analyses of clinical trials involving abacavir found no such link [4–7].
Observational data may be subject to channelling bias, whereby the real or perceived risk of an adverse effect influences medication selection. In the Veterans Administration study, patients with renal insufficiency, a risk factor of increased CVD, were more likely to be prescribed abacavir than tenofovir and, after adjustment for renal disease, no association between abacavir and CVD was detected [4]. Similarly, in the French Hospital Database on HIV study, the association between abacavir and myocardial infarction was lost after accounting for differential rates of cocaine and intravenous drug use [3].
Compounding the uncertainty regarding the association between abacavir and CVD is the absence of a mechanism to explain how the drug could promote the development of CVD. Putative mechanisms, such as endothelial dysfunction, hypercoagulability, and direct pro-inflammatory effects have been suggested [2,7–12]; however, studies examining an effect of abacavir on biomarkers for CVD and inflammation have provided inconsistent results [13–16], and in vitro investigations of the effects of abacavir on cellular processes, such as platelet aggregation or stimulation of Toll-like receptor-8 signalling are of unclear significance [7,12].
To further examine the role of abacavir in CVD risk, we conducted a cross-sectional analysis of a sample of HIV-infected patients who had previously been randomized to abacavir or tenofovir as initial HIV therapy within the context of a clinical trial. Measurements of interest included endothelial function (flow-mediated vasodilation), markers of inflammation (interleukin-6 [IL-6], high-sensitivity C-reactive protein [hsCRP]), coagulation (D-dimer) and lipids (fasting total, low-density lipoprotein [LDL] and high-density lipoprotein [HDL] cholesterol, and triglyceride levels). Recruitment of patients randomly assigned to their nucleoside/nucleotide was intended to reduce channelling bias that could affect the outcomes of interest. A third group of patients treated long-term with zidovudine was also enrolled to examine markers of endovascular function, inflammation and coagulation in those treated with this nucleoside analogue and to explore differences between these and the abacavir- and tenofovir-treated patients.
Methods
Participants
Patients age ≥18 years with documented HIV infection, receiving continuous HIV therapy for ≥6 months prior to study entry and evaluation, having plasma HIV-1 RNA levels below the limits of detection of the assay in use at the clinical site at least twice during the 48 weeks prior to and at study entry, and who were not pregnant were eligible to enrol provided they met the following antiretroviral treatment criteria: previously assigned randomly to abacavir or tenofovir as part of initial therapy for HIV infection during any clinical trial, remained on that nucleoside/nucleotide at the time of study entry, were not receiving both drugs at the same time, and were not taking zidovudine. Patients who had components of their initial antiretroviral regimen other than their abacavir or tenofovir changed, such as for intolerance or convenience, were not excluded.
In addition to the abacavir- and tenofovir-treated patients, a small cohort of patients receiving zidovudine as a component of initial therapy for HIV was recruited. By design, the zidovudine-treated participants were not required to have been assigned therapy in a clinical trial and were included in order to examine endothelial function, inflammation and coagulation markers in a cohort with long-term zidovudine exposure.
The study was conducted at eight academic clinical centres across the US. The institutional review boards at each participating institution approved the study. All participants provided written informed consent.
Evaluations
Endothelial function
Endothelial function was evaluated by flow-mediated dilation (FMD) of the brachial artery, a well-established measure of endothelium-dependent vasodilation, using procedures that have been described elsewhere [17–21]. Brachial artery reactivity studies were performed on the same day as phlebotomy. Subjects were required to be fasting and not use any tobacco-containing products for 8 h before the study. A blood pressure cuff was placed on the widest part of the proximal right forearm approximately 1 cm distal to the antecubital fossa. Using a high resolution (≥7 MHz) linear array vascular ultrasound transducer, the brachial artery was located above the elbow and scanned in longitudinal sections with the focus zone set to the depth of the far wall. Time-gain compensation and overall gain settings were used to optimize images of the lumen/arterial wall interface. The extravascular landmarks were identified and labelled to assure that the imaged segment of the brachial artery was reproduced within and between studies. After recording baseline B-mode images of the brachial artery and spectral Doppler images of flow, the forearm cuff was inflated to 250 mmHg for 4.5 min to induce reactive hyperaemia. Immediately after deflation, spectral Doppler images were obtained to verify hyperaemia. FMD of the brachial artery was measured at 60 and 90 s after cuff deflation. FMD was calculated as the ratio of the maximum brachial artery diameter after reactive hyperaemia to the baseline diameter and was expressed as a percentage change. Each study was recorded digitally and sent to the University of Wisconsin core ultrasound laboratory (Madison, WI, USA). Artery diameters were measured in triplicate with a digital border tracing tool (Access Point Web, Freeland Systems, Westminster, CO, USA). Measurements were performed by a single reader blinded to subject information and treatment.
All sonographers completed training supervised by the University of Wisconsin core ultrasound laboratory. After on-site training, each sonographer was required to submit a minimum of three paired mock studies to demonstrate adherence to the study protocol and consistent display of high-quality images and reproducibility of landmarks. Before study initiation, ultrasound equipment at each laboratory was evaluated and calibrated using a small parts ultrasound phantom. Blinded re-reading of 25 contemporaneous scans revealed a strong Pearson correlation of 0.94 (P<0.001) and a small mean difference of 0.1% (sd 0.9%; P=0.589). The two outliers on limits of agreement analyses were due to measurements that differed by <1 digital pixel.
Laboratory evaluations
After FMD was performed, blood was obtained for measurement of hsCRP, IL-6, D-dimer and lipid panel (total, LDL and HDL cholesterol, and triglycerides), and HLA-B*5701 testing. All blood specimens were drawn at the local site and shipped to the University of North Carolina (Chapel Hill, NC, USA) for analysis. hsCRP was performed by turbidometric analysis using the VITROS Chemistry System (Ortho Clinical Diagnostics, Rochester, NY, USA) and IL-6 levels were determined with Quantikine Human IL-6 Immunoassay (R&D Systems, Inc., Minneapolis, MN, USA). D-dimer levels were measured using latex agglutination. HLA-B*5701 testing (LabType SSO high definition HLA-B kit, One Lambda, Inc., Canoga Park, CA, USA) was conducted on all participants.
Statistical analyses
The primary objective was to determine whether there are significant differences in FMD between participants receiving abacavir compared to tenofovir. The secondary objective was to determine if there were significant differences in markers of inflammation and coagulation between participants receiving abacavir compared to tenofovir. Comparisons with zidovudine were considered exploratory and were performed to provide context to the results from the other two groups. To detect an FMD difference of 2% (sd 4%) between abacavir and tenofovir, 70 subjects per group were required (α=0.05, 1-β=0.80). For descriptive purposes, a target of 30 zidovudine-receiving patients was planned. Recruitment targets were achieved for the tenofovir and zidovudine groups. Enrolment of the abacavir group was halted at 65% of target sample size due to lack of additional available patients at the study sites. Between-group differences were evaluated by ANOVA and multivariable linear regression modelling; the binomial test was used for proportions. A series of multivariable linear regression models were created to determine if drug treatment group was associated with differences in FMD, IL-6, hsCRP, D-dimer, cholesterol fractions and triglyceride levels after adjustment for age and antiretroviral therapy duration. Adjustment for baseline brachial artery diameter (as recommended by the American Society of Echocardiography) was also performed for analysis of FMD [22]. Exploratory analyses investigated the effects of parameters that differed by treatment group, including CD4+ T-cell count, use of protease inhibitors, and use of statins. All variables are described as means (sem).
Results
Participant characteristics
A total of 148 participants were recruited: 46 receiving abacavir, 72 receiving tenofovir and 30 receiving zidovudine. The characteristics of the participants are included in Table 1. The majority of the participants receiving abacavir and tenofovir were previously enrolled in ACTG study A5202 (78% and 96%, respectively), with the remainder formerly in other ACTG, investigator-initiated, or industry-sponsored studies. Overall, the abacavir and tenofovir groups were balanced in terms of demographic characteristics; however, participants on abacavir had a slightly but statistically significant longer duration of antiretroviral therapy than those on tenofovir (4.0 versus 3.6 years; P=0.015) and were more likely to be receiving protease inhibitors (67% versus 48%; P=0.041). As anticipated, the zidovudine-receiving participants had a longer duration of HIV therapy (P<0.001 for both), were older (P<0.08 versus abacavir and P<0.009 versus tenofovir), and had greater statin use (P<0.029 for both) than those in the abacavir and tenofovir groups.
Table 1. Participant characteristics.
| Characteristic | ABC (n=461) | TDF (n=72) | AZT (n=30) | P-value, ABC versus THF | P-value, ABC versus AZT | P-value, TDF versus AZT |
|---|---|---|---|---|---|---|
| Male, % | 89 | 89 | 73 | 0.967 | 0.078 | 0.046 |
| Mean age, years (3D) | 46 (9.7) | 44 (9.6) | 50 (10.9) | 0.310 | 0.030 | 0.009 |
| African American, % | 39 | 39 | 40 | 0.989 | 0.964 | 0.934 |
| White, % | 57 | 58 | 53 | 0.871 | 0.811 | 0.668 |
| Mean CD4+T-cell count, cells/mm3 (3D) | 656 (232.4) | 585 (238.8) | 725 (370.3) | 0.167 | 0.274 | 0.018 |
| Mean NRTI therapy duration, days (3D) | 1,462 (277.7) | 1,303 (424.1) | 3,761 (1,499.3) | 0.015 | <0.001 | <0.001 |
| Current smoker, % | 22 | 33 | 30 | 0.210 | 0.490 | 0.834 |
| Current statin treatment, % | 8.7 | 11.1 | 30.0 | 0.740 | 0.029 | 0.022 |
| Companion ART | ||||||
| NNRT1, % | 33 | 43 | 67 | 0.294 | 0.054 | 0.219 |
| PI, % | 67 | 48 | 27 | 0.041 | <0.001 | 0.059 |
| Other, % | 0 | 9 | 17 | 0.031 | 0.005 | 0.355 |
ABC, abacavir; ART, antiretroviral therapy; AZT, zidovudine; NNRTI, non-nucleoskJe reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TDF, tenofovir.
Endothelial function
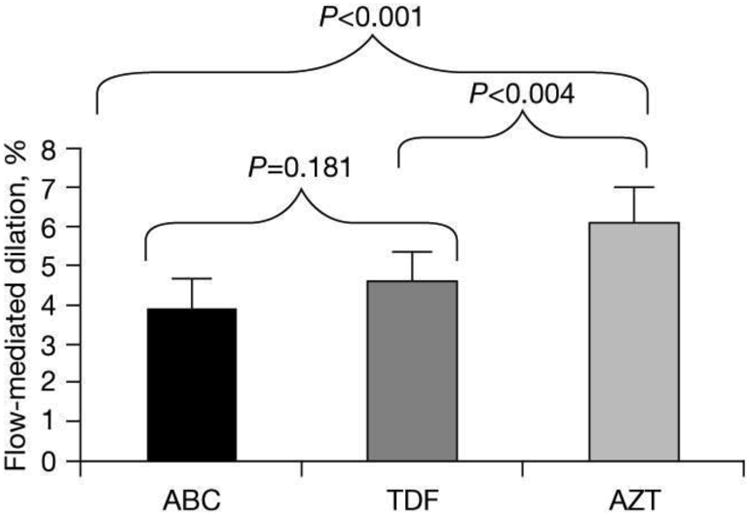
FMD in the abacavir and tenofovir groups was not significantly different from each other (4.2% [2.83%] versus 4.8% [2.82%]; P=0.2). After adjusting for age, brachial artery diameter and antiretroviral therapy duration, FMD in the abacavir and tenofovir groups was not significantly different (3.9% [0.4%] versus 4.5% [0.3%]; P=0.181; Table 2 and Figure 1). Age, CD4+ T-cell count, current treatment with protease inhibitors, and current statin treatment were not independent predictors of FMD and addition of these variables did not improve models of FMD (data not shown).
Table 2. Markers of endothelial function, inflammation and coagulation, and fasting lipids in patients receiving ABC, TDF and AZT.
| Marker | ABC (n=46) | TDF (n=72) | AZT (n=30) | P-value, ABC versus TDF | P-value, ABC versus AZT | P-value, TDF versus AZT |
|---|---|---|---|---|---|---|
| Mean FMD, % (SD)a | 3.9 (2.74) | 4.5 (2.74) | 6.1 (2.74) | 0.181 | <0.001 | 0.004 |
| IL-6>0.70 pg/ml, % | 28.9 | 15.5 | 27.6 | 0.121 | 0.967 | 0.299 |
| Mean hsCRP, mg/1 (SD) | 3.0 (3.5) | 2.2 (2.8) | 3.8 (4.5) | 0.197 | 0.404 | 0.086 |
| D-dimer >150 units, % | 9.3 | 13.2 | 26.7 | 0.689 | 0.064 | 0.122 |
| Mean LDL cholesterol, mg/dl (SD) | 120 (35.6) | 117 (30.9) | 108 (34.7) | 0.668 | 0.149 | 0.235 |
| Mean HDL cholesterol, mg/dl (SD) | 55 (17.3) | 53 (37.8) | 52 (14.6) | 0.762 | 0.644 | 0.815 |
| Mean triglycerides, mg/dl (3D) | 174 (191.5) | 115 (51.6) | 201 (202.0) | 0.037 | 0.430 | 0.008 |
Adjusted for baseline brachial artery diameter, age. and antiretroviral therapy duration. ABC, abacavir; AZT. zidovudine; FMD. flow-mediated dilation; HDL. high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; LDL, low-density lipoprotein; TDF, tenofovir.
Figure 1.
Inflammation, coagulation and lipids
There were no significant differences between abacavir and tenofovir in hsCRP levels or levels of LDL or HDL cholesterol. Few participants in either group had levels of IL-6 or D-dimer that were above the level of assay detection (0.70 pg/ml for IL-6 and 150 units for D-dimer). IL-6 levels above the limits of assay detection were recorded in 11.3% (5/44) of the abacavir group and 8.5% (6/70) in the tenofovir group. For D-dimer, 7.0% (9/69) in the abacavir group had detectable levels as did 13.0% in the tenofovir group. The proportion with detectable IL-6 or D-dimer levels did not significantly differ in the abacavir and tenofovir groups. Triglycerides were significantly higher among those taking abacavir compared to those taking tenofovir (P=0.037; Table 2).
Among those in the zidovudine group, 16.7% (5/30) had an IL-6 value above the limits of assay detection and 27% (8/30) had quantifiable D-dimer levels. Triglycerides, but no other laboratory parameter, were significantly different among those treated with zidovudine compared to those treated with either abacavir or tenofovir (Table 2). There was a trend toward a lower proportion with detectable D-dimer in the abacavir group compared with the zidovudine group.
Discussion
Using a study design intended to minimize channelling bias that could lead to increased prescriptions for abacavir among individuals at greater risk of CVD, we found no significant difference in FMD among HIV-infected patients with controlled HIV infection receiving abacavir versus tenofovir, after adjustment for major confounders including brachial artery diameter, age and antiretroviral therapy duration, as well as CD4+ T-cell count, use of protease inhibitors, and use of statins. Notably, the abacavir and tenofovir groups were well-balanced with the exception of a 5 month longer mean duration in HIV therapy and a higher proportion receiving protease inhibitor therapy in the abacavir group. Furthermore, measurements of hsCRP, IL-6 and D-dimer – markers of inflammation and coagulation – were not significantly different between the abacavir and tenofovir groups. Collectively, these results suggest that abacavir is no more associated with an inflammation-mediated impairment in endothelial function than is tenofovir.
Our FMD findings stand in contrast to a previous report, which described an independent association of abacavir use with impaired endothelial function in a cross-sectional study of antiretroviral-treated patients with suppressed HIV replication [9]. However, that study was from a single centre, was relatively small (n=61; 30 receiving abacavir), and participants were not randomly assigned to treatment with abacavir, leading to potentially important imbalances in the characteristics of those receiving and not receiving abacavir.
Additionally, markers of inflammation in our study were not significantly different among those treated with abacavir and tenofovir. By contrast, in A5224s, a recent metabolic substudy of the A5202 trial, participants receiving abacavir/lamivudine along with atazanavir/ritonavir or efavirenz experienced a significant increase in hsCRP levels over 96 weeks, while among those receiving tenofovir/emtricitabine hsCRP levels changed little from baseline [10]. Interestingly, efavirenz was associated in this study with a sharp increase in hsCRP at week 24 but at 96 weeks was not significantly different than baseline. Although the majority of participants treated with abacavir and tenofovir in our study were originally enrolled in A5202, differences in the study designs, treatment duration, companion medications, and comorbid conditions may account for these discordant findings. It is notable that IL-6 levels in A5224s declined over time in both those treated with abacavir/lamivudine- and tenofovir/emtricitiabine-containing regimens, although there was a lag in the timing of this decrease for abacavir/lamivudine relative to tenofovir/emtricitiabine. Furthermore, in the HEAT Trial, in which treatment-naive HIV-infected participants were randomized to abacavir/lamivudine or tenofovir/emtricitiabine plus lopinavir/ritonavir, hsCRP and IL-6, as well as sVCAM-1 (a marker of endothelial dysfunction) declined during study follow-up similarly in both study arms [23]. However, those patients were viraemic at the onset of antiretroviral treatment.
As has been observed in previous studies, triglyceride levels were higher among participants receiving abacavir versus tenofovir [24,25]; however, it should be noted that a greater proportion of participants in the abacavir group were also receiving a protease inhibitor. These differences in triglycerides are modest and are unlikely to be responsible for an increased CVD risk with abacavir. Triglycerides were not significantly correlated with FMD.
In this study we also examined endothelial function, inflammation and coagulation in a small cohort of patients treated with zidovudine. As expected, these patients differed markedly from those in the abacavir and tenofovir groups in that they were older, had been on HIV therapy much longer, had higher CD4+ T-cell counts, and greater statin use than the other two groups. Furthermore, they were not assigned HIV therapy in a clinical trial. Therefore, given the differences between the zidovudine and the abacavir and tenofovir groups, any comparisons should be interpreted with a high level of caution. However, the zidovudine-treated patients provide some insights regarding the effect of long-term zidovudine-containing antiretroviral therapy on the parameters of interest. Endothelial function was observed to be better in the cohort of individuals receiving zidovudine compared with younger patients receiving abacavir or tenofovir for shorter durations; however, despite their lower FMD results, the zidovudine group tended to have higher hsCRP and D-dimer levels than the abacavir- and tenofovir-treated participants.
There are limitations to our study. Foremost, this was an observational cross-sectional study, and changes in the study outcomes over time could not be assessed. Furthermore, although major potentially confounding variables including age, antiretroviral therapy duration, statin therapy and CD4+ T-cell count were included in the modelling of our results, unmeasured factors that could have influenced the outcomes may have been missed. However, initial therapy with either abacavir or tenofovir was assigned in a randomized clinical trial, which would minimize imbalances between these groups. While this is the largest study to date to evaluate endothelial function among abacavir-treated patients, enrolment to the abacavir group did not reach the sample size goal and, therefore, modest FMD differences between abacavir and tenofovir may have been unrecognized. The International Brachial Artery Task Force [17] has stated that ‘typically, significant improvement in FMD can be seen with … 40 to 60 patients in a parallel-group design study. In studies of this size, the minimal statistically significant improvement that can be detected with intervention is an absolute change in FMD of 1.5% to 2%.’ The American Society of Echocardiography [22] stated that ‘in experienced laboratories with excellent reproducibility, a 2% to 3% improvement in FMD can be detected in … parallel-group trials with about 25 to 45 subjects per treatment arm.’ Our power analysis and recruitment targets are consistent with these recommendations. The study was conducted at multiple research centres, potentially adding variability to FMD assessments. However, a number of multicentre FMD studies have been successfully conducted, including among HIV-infected patients [26–29]. To minimize variability in FMD, ultrasonographers across centres were trained and certified by a central reading centre with rigorous quality assurance procedures in place. As mentioned above, the zidovudine group was small and was included in the study in order to examine markers of endothelial function, inflammation and coagulation in patients receiving long-term therapy with this nucleoside analogue. Comparisons between this and the other groups was exploratory and should be interpreted with caution given differences between groups.
In summary, in this cross-sectional study, we found that individuals who were initially assigned randomly to abacavir or tenfovir in clinical trials did not differ significantly on measures of FMD, or markers of inflammation and coagulation. Whether abacavir contributes to risk of CVD events remains unclear, however, and our results suggest that impairment of endothelial function, heightened inflammation, and altered coagulation are, at most, of small magnitude and not likely to be mechanisms by which the drug could significantly increase CVD risk above that seen with tenofovir.
Acknowledgments
The authors wish to thank the participants for their generosity and the Centers for AIDS Research (CFARs) at the University of North Carolina (AI 50410), Case Western Reserve University (Cleveland, OH, USA; AI036219), Duke University Medical Center (Durham, NC, USA; AI064518) and the University of Pennsylvania (Philadelphia, PA, USA; AI045008). This research was supported by an investigator-initiated grant from GlaxoSmithKline. We also acknowledge study site staff including: Debbie McMullen (Duke University Medical Center), Patricia Walton and Felicia Williams (Case Western Reserve University), Karen Coleman (Northwestern University, Evanston, IL, USA), Tom Kimball, Jenifer Baer and Connie McCoy (University of Cincinnati, Cincinnati, OH, USA), Graham Ray and Stephen Belcher (NIH grant RR025780; University of Colorado Denver, and the Colorado Clinical Translational Science Institute, Denver, CO, USA), Joseph Quinn (University of Pennsylvania; ACTG AI069467), and Faith Ashton, Susan Pedersen and Melissa Caughey (University of North Carolina).
DAW has received grant support for research from GSK, Gilead and Merck, and has been an advisory board member for Gilead and Janssen. CJF has received grant support for research from Abbott, Janssen and Pfizer, and has been a speaker for Janssen and Gilead. BT has been a consultant and/or received research funding to Northwestern University from Tibotec, ViiV, Pfizer, GlaxoSmithKline and Monogram. CH has received grant support from and is an advisory board member for Argos, BMS, Gilead, Janssen, ViiV and Merck. GAM has been a consultant or speaker for BMS, Gilead and Tibotec/Janssen and a DSMB Chair for Pfizer, and has received research grant support from BMS, Astra Zeneca and GSK. PS has been a consultant to or Scientific Advisory Board member for BMS, Gilead, GSK, Merck and Janssen, and has received grant support for research from BMS, Gilead, Merck and GSK. PT has been a DSMB member for Cytheris and GSK, and is a consultant for Merck. BH has been an employee of ViiV Healthcare/GSK who provided financial support for this study. JHS has been a DSMB member for Abbott, Lilly and Takeda for clinical trials not involving HIV, and has received royalties from Wisconsin Alumni Research Foundation for a patent regarding carotid ultrasound and vascular age.
Footnotes
Disclosure statement: All other authors declare no competing interests.
References
- 1.Sabin CA, Worm SW, Weber R, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multicohort collaboration. Lancet. 2008;371:1417–1426. doi: 10.1016/S0140-6736(08)60423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strategies for Management of Anti-Retroviral Therapy/INSIGHT Study Group, DAD Study Group. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients. AIDS. 2008;22:F17–F24. doi: 10.1097/QAD.0b013e32830fe35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang S, Mary-Krause M, Cotte L, et al. Impact of individual antiretroviral drugs on the risk of myocardial infarction in human immunodeficiency virus-infected patients: a case-control study nested within the French Hospital Database on HIV ANRS cohort CO4. Arch Intern Med. 2010;170:1228–1238. doi: 10.1001/archinternmed.2010.197. [DOI] [PubMed] [Google Scholar]
- 4.Bedimo RJ, Westfall AO, Drechsler H, Vidiella G, Tebas P. Abacavir use and risk of acute myocardial infarction and cerebrovascular events in the highly active antiretroviral therapy era. Clin Infect Dis. 2011;53:84–91. doi: 10.1093/cid/cir269. [DOI] [PubMed] [Google Scholar]
- 5.Ribaudo HJ, Benson CA, Zheng Y, et al. No risk of myocardial infarction associated with initial antiretroviral treatment containing abacavir: short and long-term results from ACTG A5001/ALLRT. Clin Infect Dis. 2011;52:929–940. doi: 10.1093/cid/ciq244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding X, Andraca-Carrera E, Cooper C, et al. No association of myocardial infarction with ABC use: an FDA meta-analysis. 18th Conference on Retroviruses & Opportunistic Infections; 27 February–2 March 2011; Boston, MA, USA. Abstract 808. [Google Scholar]
- 7.Cruciani M, Zanichelli V, Serpelloni G, et al. Abacavir use and cardiovascular disease events: a meta-analysis of published and unpublished data. AIDS. 2011;25:1993–2004. doi: 10.1097/QAD.0b013e328349c6ee. [DOI] [PubMed] [Google Scholar]
- 8.Satchell CS, O'Halloran JA, Cotter AG, et al. Increased platelet reactivity in HIV-1-infected patients receiving abacavir-containing antiretroviral therapy. J Infect Dis. 2011;204:1202–1210. doi: 10.1093/infdis/jir509. [DOI] [PubMed] [Google Scholar]
- 9.Hsue PY, Hunt PW, Wu Y, et al. Association of abacavir and impaired endothelial function in treated and suppressed HIV-infected patients. AIDS. 2009;23:2021–2027. doi: 10.1097/QAD.0b013e32832e7140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McComsey GA, Kitch D, Daar ES, et al. Inflammation markers after randomization to abacavir/lamivudine or tenofovir/emtricitabine with efavirenz or atazanavir/ritonavir: ACTG A5224s, A5202 substudy. AIDS. 2012;26:1371–1385. doi: 10.1097/QAD.0b013e328354f4fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shikuma CM, Ribaudo HJ, Zheng Y, et al. Change in high-sensitivity C-reactive protein levels following initiation of efavirenz-based antiretroviral regimens in HIV-infected individuals. AIDS Res Hum Retroviruses. 2011;27:461–468. doi: 10.1089/aid.2010.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacLeod I, Rowley C, Essex M. Molecular basis for the immunostimulatory activity of abacavir via TLR-8. 19th Conference on Retroviruses and Opportunistic Infections; 5–8 March 2012; Seattle, WA, USA. Abstract 816. [Google Scholar]
- 13.Padilla S, Masia M, Garcia N, Jarrin I, Tormo C, Gutierrez F. Early changes in inflammatory and pro-thrombotic biomarkers in patients initiating antiretroviral therapy with abacavir or tenofovir. BMC Infect Dis. 2011;11:40. doi: 10.1186/1471-2334-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palella FJ, Gange SJ, Benning L, et al. Inflammatory biomarkers and abacavir use in the Women's Interagency HIV Study and the Multicenter AIDS Cohort Study. AIDS. 2010;24:1657–1665. doi: 10.1097/QAD.0b013e3283389dfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin A, Amin J, Cooper DA, et al. Abacavir does not affect circulating levels of inflammatory or coagulopathic biomarkers in suppressed HIV: a randomized clinical trial. AIDS. 2010;24:2657–2663. doi: 10.1097/QAD.0b013e32833f147f. [DOI] [PubMed] [Google Scholar]
- 16.Martínez E, Larrousse M, Podzamczer D, et al. Abacavir-based therapy does not affect biological mechanisms associated with cardiovascular dysfunction. AIDS. 2010;24:F1–F9. doi: 10.1097/QAD.0b013e32833562c5. [DOI] [PubMed] [Google Scholar]
- 17.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 18.Joannides R, Haefeli WE, Linder L, et al. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 19.Takase B, Uehata A, Akima T, et al. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol. 1998;82:1535–1539. doi: 10.1016/s0002-9149(98)00702-4. [DOI] [PubMed] [Google Scholar]
- 20.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568:357–369. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uehata A, Lieberman EH, Gerhard MD, et al. Noninvasive assessment of endothelium-dependent flow-mediated dilation of the brachial artery. Vasc Med. 1997;2:87–92. doi: 10.1177/1358863X9700200203. [DOI] [PubMed] [Google Scholar]
- 22.Gottdiener JS, Bednarz J, Devereux RM, et al. American Society of Echocardiography recommendations for use of echocardiography in clinical trials: a report from the American Society of Echocardiography's Guidelines and Standards Committee and The Task Force on Echocardiography in Clinical Trials Writing Committee. J Am Soc Echocardiogr. 2004;17:1086–1119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 23.McComsey G, Smith K, Patel P, et al. Similar reductions in markers of inflammation and endothelial activation after initiation of abacavir/lamivudine or tenofovir/emtricitabine: the HEAT Study. 16th Conference on Retroviruses and Opportunistic Infections; 8–11 February 2009; Montreal, QC, Canada. [Google Scholar]
- 24.Moyle GJ, Sabin CA, Cartledge J, et al. A randomized comparative trial of tenofovir DF or abacavir as replacement for a thymidine analogue in persons with lipoatrophy. AIDS. 2006;20:2043–2050. doi: 10.1097/01.aids.0000247574.33998.03. [DOI] [PubMed] [Google Scholar]
- 25.Post FA, Moyle GJ, Stellbrink HJ, et al. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral-naive, HIV-1-infected adults: 48-week results from the ASSERT study. J Acquir Immune DeficSyndr. 2010;55:49–57. doi: 10.1097/QAI.0b013e3181dd911e. [DOI] [PubMed] [Google Scholar]
- 26.Murphy RL, Berzins B, Zala C, et al. Change to atazanavir/ritonavir treatment improves lipids but not endothelial function in patients on stable antiretroviral therapy. AIDS. 2010;24:885–890. doi: 10.1097/QAD.0b013e3283352ed5. [DOI] [PubMed] [Google Scholar]
- 27.Torriani FJ, Komarow L, Parker RA, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52:569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeboah J, Crouse JR, Hsu FC, et al. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 29.Yeboah J, Folsom AR, Burke GL, et al. Events in a population-based study: the multi-ethnic study of atherosclerosis predictive value of brachial flow-mediated dilation for incident cardiovascular. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]


