Abstract
Brevetoxins are neurotoxins produced by the marine dinoflagellate Karenia brevis. Histopathologic examination of marine mammals dying following repeated exposure of brevetoxins during red tide events suggests that the respiratory tract, nervous, hematopoietic, and immune systems are potential targets for toxicity in repeatedly exposed individuals. The purpose of this experiment was to evaluate the effects of repeated inhalation of K. brevis extract on these potential target systems in rats. Male Sprague-Dawley rats were exposed four hours/day, five days/week for up to four weeks to target concentrations of 200 and 1000 μg/L K. brevis extract (approximately 50 and 200 μg/L brevetoxin-like compounds; positive neurotoxicity in a fish bioassay). Control rats were sham exposed to air. Immunohistochemical staining of pulmonary macrophages indicated deposition of brevetoxin-like compound within the lung. However, exposure resulted in no clinical signs of toxicity or behavioral changes. There were no adverse effects on hematology or serum chemistry. No histopathological changes were observed in the nose, lung, liver, kidneys, lymph nodes, spleen, or brain of exposed rats. Immune suppression was suggested by reduced responses of spleen cells in the IgM-specific antibody-forming plaque cell response assay and reduced responses of lymphocytes to mitogen stimulation in vitro. Differences between responses observed in rats in this study and those observed in manatees may be a function of dose or species differences in sensitivity.
Introduction
Brevetoxins (PbTxs) are potent neurotoxins produced by the marine dinoflagellate Karenia brevis. Karenia brevis blooms are responsible for the red tides occurring almost annually in the Gulf of Mexico and Atlantic coast of Florida (Baden, 1989). Inhalation of aerosolized PbTxs in sea spray results in almost immediate irritation of the eyes and respiratory tract that generally abates when people leave the beach area (Baden, 1989; Kirkpatrick et al, in press).
Brevetoxins also produce respiratory tract responses at extremely low concentrations. Recently, Backer and colleagues (2003) correlated the extent of respiratory tract symptoms experienced by individuals recreationally exposed to aerosolized PbTxs during a K. brevis red tide and the brevetoxin concentration in the air. Significant increases in eye and throat irritation and cough and chest tightness were reported by individuals exposed to <10 to 36 ng total PbTx/m3, while significant increases in nasal congestion and wheezing were reported by individuals exposed to 20–93 μg PbTx/m3. Little is known about the long-term health effects associated with inhalation of aerosolized PbTxs during red tide events. Examination of manatees dying as a result of a K. brevis event suggest that the respiratory tract, nervous, immune, and hematopoietic systems are potential targets for toxicity upon repeated inhalation and/or ingestion, but dose-response relationships have not been established (Bossart et al, 1998). The purpose of this study was to initiate examination of the health effects associated with inhalation of aerosolized K. brevis extract for up to four weeks.
Materials and Methods
The two batches of extract used for this study were prepared at the Center for Marine Sciences, University of North Carolina at Wilmington, NC, by extracting K brevis cultures with chloroform (1 L chloroform per 10 L culture). The chloroform layer was removed, dried, and analyzed for total brevetoxin by ELISA (Naar et al, 2002). The extracts were provided in aliquots of 10 mg of brevetoxin-positive material. High-pressure liquid chromatographic analysis (with UV detection) indicated K. brevis contained three main components (relative percent): PbTx2 (82), PbTx3 (12.6), and the potent PbTx antagonist, AJB6.0p (6.1). The first preparation was not chemically characterized, but the relative concentration of major components is expected to be similar to that of the second extract preparation used. Characteristics of the antagonist have recently been described (Bourdelais et al, 2003).
Male Sprague-Dawley rats, five to six weeks old, were purchased from Charles River Laboratories (Wilmington, MA). The study was conducted under an IACUC-approved protocol, and animals were treated in accordance with the Guide for Care and Use of Laboratory Animals (National Research Council, 1996). The rats were randomized by weight into three groups: 1) control (sham exposed to filtered air), 2) the low dose group (50 μg brevetoxin equivalents/m-3), and high dose group (200 μg brevetoxin equivalents/m3). For the core sub group, six rats/level were sacrificed after one and four weeks of exposure and four weeks after termination of exposure. For the immunology group, five rats/level were sacrificed after one and four weeks of exposure and four weeks after termination of exposure. The neurotoxicity subgroup consisted of three rats/level sacrificed after one and four weeks of exposure.
The rats were exposed for four hours/day, five days/week, for up to four weeks. Aerosols were generated by nebulization from a solution containing 0.67 mg brevetoxin equivalents/mL of vehicle (33% ethanol in water containing 0.05% Alkamuls® EL620A). The rats were exposed in 96-port nose-only chambers. Total aerosol mass concentration was determined gravimetrically. Brevetoxin concentration was estimated by knowing the fraction of the total solute represented by brevetoxin and was confirmed by ELISA on selected filter samples (Naar et al, 2002). The aerosol size distribution, volume median aerodynamic diameter (geometric standard deviations) for the low- and high-exposure concentrations were 0.66 μm (2.2) and 1.4 μm (2.5), respectively.
Body weights and detailed observations were recorded the day before exposures began and weekly thereafter. Core rats were sacrificed by intraperitoneal injection of Eutha-sol®. Blood was collected by cardiac puncture for evaluation of hematology (erythrocyte count, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, platelet count, reticulocyte count, and total and differential leukocyte count), serum chemistry (blood urea nitrogen, creatinine, aspartate transaminase, alanine transaminase, alkaline phosphatase, gamma glutamyl-transferase, inorganic phosphorus, cholesterol, triclycerides, total bilirubin, glucose, total protein, albumin and globulin, and electrolytes). The respiratory tract (except right lung), brain, liver, kidney, peripheral nerve, spinal chord, spleen, thymus, and thyroid were weighed and fixed in 10% neutral buffered formalin. Tissues were trimmed, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin for evaluation. Before separation from the whole lung, the right lungs were lavaged via the trachea with saline. After centrifugation, the liquid portion of each lavage was analyzed for lactate dehydrogenase (an indicator of cell damage and death) and total protein (an indicator of pulmonary inflammation). Recovered leukocytes were resuspended in saline and manually counted by light microscopy using a hemocytometer. Cytospin preparations were prepared, stained with Kwik™ Diff, and a differential cell count was performed. Cytospin preparations made on ProbOn™ Plus sides were immunohistochemically stained for brevetoxin (Bossart et al, 1998).
Rats designated for evaluation of effects on the immune system were immunized by tail vein injection of a 15% suspension of sheep red blood cells (SRBC) in phosphate buffered saline. At five days post immunization, the rats were euthanized as described above and spleens recovered. Cells were isolated from weighed portions of spleen, washed, and then resuspended in complete RPMI culture medium to a final concentration of 1 × 106 cells/mL. The IgM antibody forming cell (AFC) response to the T-cell dependent antigen, SRBC, was assessed with a modified plaque-forming assay (Cunningham and Szensberg, 1968). The number of AFC was determined by light microscopy and reported as AFC/106 lymphoid cells. The proliferative response of the spleen cells to the mitogen, Concanavalin A (ConA), was assessed. Spleen cells (5 × 105/100 μL complete RPMI) were incubated with 0.1, 0.3, and 1.0 μg ConA/50 μL at 37°C in the presence of 5% CO2 for 54 hours. At that time cells were pulsed with 0.5 μCi 3H-tymidine and incubated for an additional 18 hours. Cells were collected on filter paper using a cell harvester, and 3H activity was determined by liquid scintillation spectrometry.
Rats in the neurotoxicity group were sacrificed and fixed in situ by whole body perfusion with 4% paraformaldehyde. Samples were shipped in 4% paraformaldehyde to Neuro-Sciences Associates (Knoxville, TN) for analysis of neuronal toxicity, as indicated by silver stain uptake.
Results and Discussion
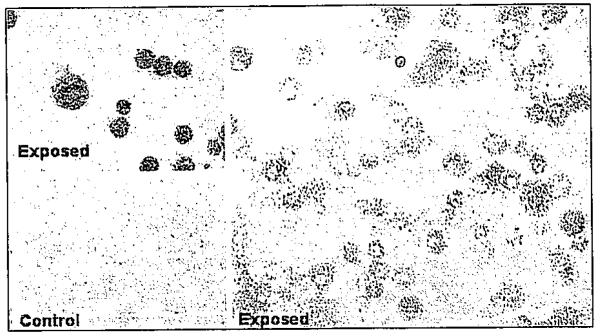
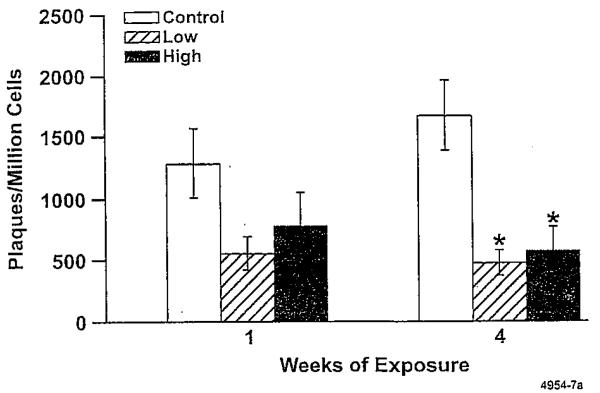
Alveolar macrophages obtained from lungs of K. brevis-ex-posed rats, but not from control rats, stained positively for brevetoxin, indicating compound deposition in the brevetoxin-exposed rats (Fig. 1). Exposure for up to four weeks resulted in no clinical signs of toxicity or adverse effects on body weight. There were no gross lesions observed at necropsy at any sacrifice time. Weights of brain, lung, liver, kidney, spleen, and thymus were unaffected. There were no adverse effects on hematology (including reticulocyte counts) or serum chemistry parameters (including blood urea nitrogen, aspartate transaminase, gamma glutamyltransferase, and total bilirubin). Lavage fluid endpoints were negative for cytotoxicity (lactate dehydrogenase) and inflammation (total protein, total and differential white blood cell count). There were no histopathological changes in any tissue examined. There was no evidence of neuronal toxicity by silver stain analysis in the brain. Karenia brevis extract inhalation reduced the number of plaque-forming lymphocytes in the spleen (Fig. 2). The effect was statistically significant after four weeks of exposure, but normal responses were observed after the four-week hold period. However, exposure did not affect lymphocyte profiles in the bronchial lymph nodes or spleen at any time.
Figure 1.
Immunohistochemical staining of alveolar macrophages from exposed animals (top left and right panel). Cells from control animals (bottom left panel) did not stain positive for brevetoxin. Color figure p. 575
Figure 2.
Effect of K. brevis extract exposure on numbers of plaque-forming spleen lymphocytes. Error bars represent SEM. * indicates values are significantly different from control (analysis of variance, adjusted for multiple comparisons, P < 0.05).
The purpose of this study was to evaluate the effects of repeated K. brevis extract inhalation in rats. Endpoints focused on assessment of targets for toxicity suggested by findings in manatees exposed for extended periods by inhalation and/or ingestion to high concentrations of K. brevis (Bossart et al, 1998). Repeated inhalation exposure to the mixture of brevetoxins at concentrations orders of magnitude greater than measured along Gulf Coast beaches (Pierce et al, 2003; Cheng et al, 2002) did not cause toxic effects in the respiratory, nervous, or hematopoietic systems. An important finding of this study was the significant reduction in spleen AFC response among rats exposed to even the lowest K. brevis extract concentration for one week in the absence of overt toxicity in the spleen. Disparity between other effects in marine mammals and rats in this study may be due to differences in dose or interspecies differences in sensitivity to the toxins. The contribution of the antagonist AJB6.0p (6.1) to the overall response is uncertain but will be the focus of future investigations.
Acknowledgements
This research funded under NIH P01-ES10594ZES. The authors are grateful to the technical staff of the Lovelace Respiratory Research Institute for support of this study.
References
- Backer LC, Fleming LE, Cheng Y-S, Benson J, Pierce RH, Zaias J, Bean J, Bossart GD, Johnson D, Quimbo R, Baden DG. Harmful Algae. 2003;2:19–28. [Google Scholar]
- Baden DG. FASEB J. 1989;3:1807–1817. doi: 10.1096/fasebj.3.7.2565840. [DOI] [PubMed] [Google Scholar]
- Bossart GD, Baden DG, Ewing RY, Roberts B, Wright SD. Toxicol. Pathol. 1998;26:276–282. doi: 10.1177/019262339802600214. [DOI] [PubMed] [Google Scholar]
- Bourdelais AJ, Campbell S, Kubanek J, Wright J, Baden DG. Book of Abstracts, 10th International Conference on Harmful Algae; 2002. p. 34. [Google Scholar]
- Cheng YS, Zhou Y, Gao J, Villareal TA, Pierce RH, Wetzel D, Naar J, Baden DG. Book of Abstracts, 10th International Conference on Harmful Algae; 2002. p. 51. [Google Scholar]
- Cunningham AJ, Szensberg A. Immunol. 1968;14:599–600. [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Fleming LE, Squicciarini D, Backer LC, Clark R. Harmful Algae. doi: 10.1016/j.hal.2003.08.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naar J, Bourdelais A, Tomas C, Kubanek J, Whitney PL, Flewelling L, Steidinger K, Lancaster J, Baden DG. Environ. Health Perspect. 2002;110:179–185. doi: 10.1289/ehp.02110179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RH, Henry MS, Blum PC, Lyons J, Cheng YS, Yazzie D, Zhou Y. Bull. Environ. Contam. Toxicol. 2003;70:161–165. doi: 10.1007/s00128-002-0170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]