Abstract
Lessons Learned
Local tolerance of intralesional treatment of melanoma skin metastases with BQ788 is excellent.
Hints of efficacy are observed, consisting of both direct effects (decreased expression of endothelin receptor B [EDNRB] and of survival factors, reduced proliferation) and indirect effects (enhanced immune cell infiltration and angiogenesis).
Patients in future trials should be screened for EDNRB expression level prior to treatment because only patients with high EDNRB-expressing melanomas (four of five) responded to BQ788.
Future studies should be performed for at least 2 weeks because reduced lesion growth was observed in the only patient that was treated for longer than 1 week.
Background.
This first-in-human proof-of-concept study aimed to check whether safety and preclinical results obtained by intratumoral administration of BQ788, an endothelin receptor B (EDNRB) antagonist, can be repeated in human melanoma patients.
Methods.
Three patients received a single intralesional BQ788 application of 3 mg. After 3–7 days, the lesions were measured and removed for analysis. The administered dose was increased to a cumulative dosage of 8 mg in patient 4 (4 × 2.0 mg, days 0–3; lesion removed on day 4) and to 10 mg in patient 5 (3 × 3.3 mg, days 0, 3, and 10; lesion removed after 14 days). Control lesions were simultaneously treated with phosphate-buffered saline (PBS). All samples were processed and analyzed without knowledge of the clinical findings.
Results.
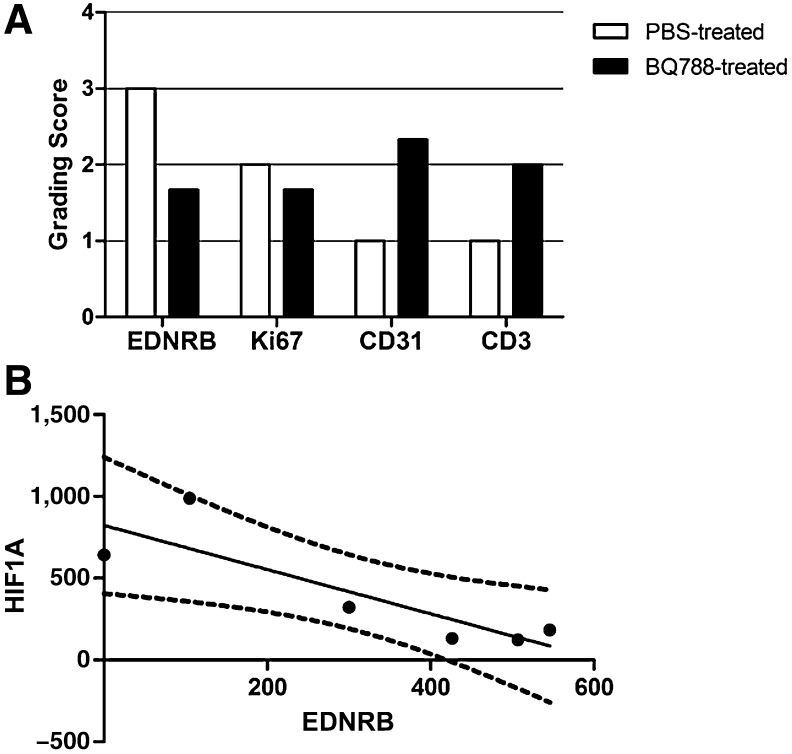
No statistical evaluation was possible because of the number of patients (n = 5) and the variability in the mode of administration. No adverse events were observed, regardless of administered dose. All observations were in accordance with results obtained in preclinical studies. Accordingly, no difference in degree of tumor necrosis was detected between BQ788- and PBS-treated samples. In addition, both EDNRB and Ki67 showed decreased expression in patients 2 and 5 and, to a lesser extent, in patient 1. Similarly, decreased expression of EDNRB mRNA in patients 2 and 5 and of BCL2A1 and/or PARP3 in patients 2, 3, and 5 was found. Importantly, semiquantitatively scored immunohistochemistry for CD31 and CD3 revealed more blood vessels and lymphocytes, respectively, in BQ788-treated tumors of patients 2 and 4. Also, in all patients, we observed inverse correlation in expression levels between EDNRB and HIF1A. Finally, in patient 5 (the only patient treated for longer than 1 week), we observed inhibition in lesion growth, as shown by size measurement.
Conclusion.
The intralesional applications of BQ788 were well tolerated and showed signs of directly and indirectly reducing the viability of melanoma cells.
Abstract
经验
• 病灶内注射 BQ788 治疗黑色素瘤皮肤转移的耐受性非常好。
• 观察到了有效性的迹象, 包括直接效应 [内皮素受体 B (EDNRB) 和存活因子的表达下调, 增殖减少] 和间接效应 (免疫细胞浸润及血管生成增加)。
• 将来的临床试验应在开始前对患者 EDNRB 表达水平进行筛查, 因为只有 EDNRB 高表达的黑色素瘤患者 (4/5 例) 对 BQ788 有治疗反应。
• 将来的研究应该至少持续 2 周, 因为我们只在治疗超过 1 周的患者中观察到病灶生长抑制。
摘要
背景. 本研究为首次应用于人体的概念验证研究, 旨在检验肿瘤内注射内皮素受体 B (EDNRB) 拮抗剂 BQ788 的安全性及其临床前结果在人类黑色素瘤患者中是否具有重现性。
方法. 3 例患者接受单次肿瘤内注射 BQ788 3 mg。3 ∼ 7 天后对病灶进行测量和切除以进行分析。第 4 例患者的给药剂量上调至累积剂量 8 mg (4×2.0 mg, 0 ∼ 3天, 在第 4 天切除病灶), 第 5 例患者上调至 10 mg(3×3.3 mg, 0、3、 10 天, 14 天后切除病灶)。同时使用磷酸盐缓冲液 (PBS) 治疗对照病灶。所有标本均在研究者不知晓临床结果的情况下进行处理和分析。
结果. 由于患者数少 (n = 5) 以及给药模式的变异性, 我们无法进行统计学评价。我们在所有给药剂量水平均未观察到不良事件。所有观察结果均与临床前研究获得的结果相一致。相应地, 在 BQ788 和 PBS 处理标本中未观察到肿瘤坏死的程度存在差异。此外, 患者 2 和 5 的 EDNRB 和 Ki67 表达下调, 而患者 1 的下调幅度较小。与此相似, 患者 2 和 5 的 EDNRB mRNA 表达下调, 患者 2 、 3 和 5 的 BCL2A1 和/或 PARP3 表达下调。CD31 和 CD3 免疫组化结果的半定量评分分别提示, 接受 BQ788 治疗的患者 2 和 4 分别出现血管和淋巴细胞增加, 这一点非常重要。同时, 我们在所有患者中均观察到 EDNRB 和 HIF1A 表达水平呈负相关。最后, 我们通过测量病灶发现患者 5 (唯一 1 例治疗超过 1 周的患者) 病灶生长受到抑制。
结论. 病灶内注射 BQ788 耐受良好, 且直接和间接迹象均提示治疗减少了黑色素瘤细胞的存活力。The Oncologist 2015;20:1121–1122
Author Summary
Discussion
In our preclinical studies, daily intratumoral administration of BQ788 for 9 days resulted in inhibition of tumor growth with associated enhanced angiogenesis, which, in accordance with in vitro data, was preceded by decreases in levels of EDNRB, BCL2A1, and PARP3 and enhanced HIF1A expression [1, 2]. In the current first-in-human study, we tested safety and reproducibility of preclinical results by administering BQ788 (doses shown to be safe in human cardiovascular studies [3, 4]) in lesions of patients with melanoma. Our study, which compared increasing cumulative doses (3–10 mg) and various short treatment modalities (3–14 days), established excellent local tolerance of the drug and did not reveal any side effects. Although safety was ensured, the duration of treatment (administered to the first four patients) was too short (3–7 days) to evaluate whether the growth of tumors was inhibited, as in preclinical studies [1, 2]. In patient 5, however (the only patient treated for longer than 1 week, as in the preclinical study), we observed inhibition in lesion growth, as shown by size measurement. Our study stopped at this point because of lack of funding; however, the latter finding indicates that future studies should be performed for at least 2 weeks. As in preclinical studies [1, 2], early direct effects of BQ788 administration on cancer cells were detected, such as decreased EDNRB, BCL2A1, and PARP3, and reduced proliferation, as shown by Ki67 staining (immunohistochemistry results for patient 2 are shown in Fig. 1A). Despite inhibition of tumor growth, hematoxylin and eosin staining did not show any difference in degree of tumor necrosis between BQ788- and vehicle-treated samples in preclinical studies [2]. Again, we did not observe any difference in degree of necrosis in this study as well. Patients in future trials should be screened for EDNRB expression level prior to treatment because only patients with high EDNRB expression (four of five) responded to BQ788. BQ788 can also kill cancer cells indirectly by improving the extravasation of immune cells from the blood vessel into the tumor [5]. In preclinical in vivo analyses, this indirect effect was manifested by an increase in HIF1A expression and angiogenesis [1, 5]. The same observation was repeated in this study, as shown by the inverse correlation between HIF1A and EDNRB expression (Fig. 1B). Semiquantitatively scored immunohistochemical stainings for CD31 and CD3 revealed a higher abundance of blood vessels and lymphocytes, respectively, in BQ788-treated tumors, indicative of an indirect effect of BQ788. If these observed signs of enhanced immune cell infiltration and angiogenesis hold up in larger clinical trials, future investigation should assess the potentially beneficial effect of BQ788 administration on the efficacy of anti-programmed death-1 receptor immunotherapy. Finally, emerging evidence shows that EDN1-dependent EDNRB signal transduction is crucial for paracrine resistance to BRAF inhibitors in heterogeneous tumors [6, 7]; BQ788 can be tested in combination with BRAF inhibitors.
Figure 1.
Effects of BQ788 on protein and gene expression in human melanoma. (A): Summary of semiquantitatively scored immunohistochemical stainings for EDNRB, Ki67, CD31, and CD3 in PBS- and BQ788-treated tumors of patient 2. (B): Pearson correlation analysis of HIF1A and EDNRB mRNA expression on all frozen samples (n = 6 in total; Pearson r = −.86; p = .0284; dashed line, 95% confidence interval of the best-fit line).
Abbreviations: EDNRB, endothelin receptor B; PBS, phosphate-buffered saline.
Supplementary Material
Footnotes
Access the full results at: Wouters-15-139.theoncologist.com
ClinicalTrials.gov Identifier: NCT02442466
Sponsor(s): MelCure SA
Principal Investigators: Ronit Lahav-le Coutre, Joost J. van den Oord
IRB Approved: Yes
Author disclosures and references available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.