The significance of hypertension developing during vascular endothelial growth factor receptor tyrosine kinase inhibitor treatment and a group of cytokines and angiogenic factors in advanced non-clear cell renal cell carcinoma patients treated with sunitinib in a phase II study were evaluated. No association between hypertension and survival or response was found. Elevated baseline soluble tumor necrosis factor receptor I, interleukin-8, growth-regulated oncogene, transforming growth factor-a, and VEGFR-2 levels were associated with an increased risk of death.
Keywords: Cytokines and angiogenic factors, Biomarkers, Hypertension, Non-clear cell renal cell carcinoma, Nitric oxide, Sunitinib
Abstract
Background.
We evaluated the significance of hypertension developing during vascular endothelial growth factor (VEGF) receptor tyrosine kinase inhibitor (VEGFR-TKI) treatment and a group of cytokines and angiogenic factors (CAFs) in advanced non-clear cell renal cell carcinoma (nccRCC) patients treated with sunitinib in a phase II study.
Materials and Methods.
Using multiplex assays, we analyzed the levels of 38 CAFs in plasma at baseline and after 4 weeks of sunitinib therapy. Sunitinib benefit was defined as a partial response or stable disease using the Response Evaluation Criteria in Solid Tumors lasting ≥4 months. Cox proportional hazards regression models were used to assess the associations among hypertension, CAFs, and progression-free (PFS) and overall survival (OS).
Results.
Fifty-seven patients were evaluable; 53 had baseline CAF levels available. The median PFS and OS were 2.9 months (95% confidence interval [CI], 1.4–5.5) and 16.8 months (95% CI, 10.7–27.4), respectively. Sunitinib benefit was observed in 21 patients (37%). However, 33 patients (60%) developed hypertension during treatment, although no association was found with survival or response. Elevated baseline soluble tumor necrosis factor (TNF) receptor I, interleukin-8, growth-regulated oncogene, transforming growth factor-α, and VEGFR-2 levels were associated with an increased risk of death on multivariate analysis.
Conclusion.
We found no association between the development of hypertension and survival or sunitinib benefit in advanced nccRCC. TNF and angiogenic/immunomodulatory mediators were identified for evaluation as markers of prognosis and VEGFR-TKI benefit in future studies.
Implications for Practice:
The present study describes the first analysis of hypertension and a relatively large set of circulating cytokines and angiogenic factors in patients with advanced non-clear cell renal cell carcinoma (nccRCC) treated with sunitinib. No association was found between hypertension and patient outcomes. However, a group of candidate circulating biomarkers was identified, in particular, those associated with tumor necrosis factor and CXCR1/2 signaling, with probable biological and clinical significance in nccRCC, warranting confirmation in future studies.
Introduction
Roughly 25% of renal cell carcinoma (RCC) cases are classified as non-clear cell types (nccRCC), which, in descending order of prevalence, include papillary, chromophobe, and rare forms such as collecting duct, medullary, TFE3 translocation-associated, and unclassified histological subtypes [1]. Each subtype is characterized by distinctive biology and unique morphology (although some tumors could have mixed histological characteristics) [2]. In clinical practice, the behavior and responsiveness to treatment of the different forms of nccRCC are also diverse.
Because of their heterogeneity and lower prevalence than that of the conventional clear cell type, non-clear cell tumors have been traditionally underrepresented in or excluded from clinical trials. Thus, the optimal treatment for nccRCC patients remains poorly defined, and, in the absence of better alternatives, standard therapies for ccRCC (vascular endothelial growth factor [VEGF] and mammalian target of rapamycin [mTOR] inhibitors) have been used. We recently reported the results of a single-arm phase II clinical trial aimed at prospectively determining the responsiveness of the different nccRCC types to the multitargeted inhibitor of angiogenesis and VEGF receptors (VEGFRs), sunitinib [3]. Our findings were consistent with those of other studies, showing generally lower activity of VEGF- and mTOR-targeted agents against nccRCC than against ccRCC [4]. More importantly, the results of these clinical trials have made it evident that, to achieve better outcomes for nccRCC patients, improvements in understanding the underlying biology need to be paired with correlative efforts comparing the antitumor responses with the molecular profiles in individual histotypes.
Sunitinib exerts antiangiogenic and antitumor effects via tyrosine kinase (TK) inhibition in signaling cascades (including VEGF, platelet-derived growth factor [PDGF], and stem cell factor [SCF]) that regulate tumor angiogenesis, survival, and growth. Although the ccRCC-defining loss of function of the von Hippel-Lindau gene (VHL) was thought uncommon in nccRCC, a recent study reported an incidence of ∼16% in sporadic cases [5]. Moreover, amplification or activating mutations in MET (encoding the hepatocyte growth factor [HGF] receptor), found in a subset of patients with sporadic papillary RCC (the most common nccRCC type), often result in tumor hypoxia, leading to overexpression of angiogenic mediators. As a consequence of these or other, still unclear, alterations, VEGF ligands and receptors are known to be expressed at least by papillary and chromophobe nccRCC (which also distinctively expresses the SCF receptor) [6–8]. However, as hinted at by the clinical results, the relative importance of angiogenesis as a driver of nccRCC progression is likely much less significant than for conventional RCC.
Biomarkers are critically needed to improve prognostication and guide drug selection in view of the complex heterogeneity of nccRCC. Unfortunately, although the biological targets of sunitinib and other inhibitors of angiogenesis have been validated [9], the molecular determinants and biomarkers of clinical benefit remain unclear. The primary objective of the present study was to investigate the associations between cytokines and angiogenic factors (CAFs) in plasma and the outcomes in patients participating in the aforementioned clinical trial of sunitinib in nccRCC. Our previous work using CAF profiling for blood biomarker screening resulted in the identification of markers of prognosis (interleukin [IL]-6, IL-8, osteopontin, HGF, and tissue inhibitor of metalloproteinase 1) and even the prediction of anti-VEGF treatment benefit (IL-6, 6-CAF signature) in ccRCC [10, 11]. In the present study, we sought to expand our experience to nccRCC. Because hypertension is one of the most common side effects of sunitinib and other VEGFR-TK inhibitors [12], and because development of hypertension during treatment has been linked to improved clinical results in patients with metastatic ccRCC [13], we additionally evaluated the influence of treatment-related hypertension on nccRCC outcomes. Because alterations in nitric oxide (NO) bioavailability have been implicated in the pathogenesis of sunitinib-induced hypertension [14], we also measured the levels of this marker in blood.
Materials and Methods
Patients
The clinical study has been previously described [3]. The primary endpoints were the objective response rate and progression-free survival (PFS). The secondary endpoints were safety and overall survival (OS).
Hypertension and Ejection Fraction
Blood pressure (BP) was measured with the patient in a seated position in the clinic after resting for 5 minutes on days 1 and 29 of each 6-week cycle, throughout the clinical trial. Hypertension was defined as the intensification of antihypertensive treatment or (a) systolic blood pressure >140 mmHg if the baseline was normal (<130 mmHg) or an increase of ≥20 mmHg if systolic hypertension was already present; and (b) diastolic blood pressure (DBP) >90 mmHg if the baseline BP was normal (<80 mmHg), or an increase of ≥10 mmHg if diastolic hypertension was already present. Prospective evaluation of systolic and diastolic function was performed by echocardiography before and after completion of sunitinib therapy and independently verified by us.
Plasma Sample Collection and CAF Analysis
Patients provided written institutional review board-approved informed consent for the collection of blood samples for biomarker analysis. Specimens were obtained at baseline (before treatment) and on day 29 of treatment. Venous blood was collected into an 8-mL sodium citrate cell preparation tube (BD, Franklin Lakes, NJ, http://www.bd.com) and processed as previously described [10].
The concentrations of 59 CAFs were measured in single or duplicate determinations (77 and 22 specimens, respectively) at the antibody-based proteomics core at Baylor College of Medicine (Houston, TX) using Luminex bead-based assays (MPXHCYTO60KPMX39, MPXHCYTO60K, HSCR-32K-PMX14, HCVD1-67AK, and HADK2-61K-B; EMD Millipore, Billerica, MA, http://www.emdmillipore.com) according to the manufacturer’s instructions. The plates were analyzed using the Bio-Plex 200 system (Bio-Rad, Hercules, CA, http://www.bio-rad.com). For each CAF, we examined the number of samples that were out of range (OOR) based on the standard curve. Factors with >25% of samples OOR were omitted from the analysis; thus, of the 59 CAFs, 21 (35.6%) were omitted. For the remaining 38 CAFs, we used the highest value on the curve for above-range OOR values and half the lowest value on the curve as an estimate for below-range OOR. NO levels were measured using a colorimetric assay kit (item no. 780001; Cayman Chemicals, Ann Arbor, MI, http://www.caymanchem.com).
Statistical Analysis
Sunitinib benefit was defined as a partial response or stable disease lasting ≥4 months. CAF levels were categorized as high or low relative to the median and characterized using both continuous and binary scales. Log transformation was applied to variables showing high asymmetry. Paired t tests were used to assess the change of left ventricular ejection fraction (LVEF) from baseline to end-of-study and the change in BP from baseline to day 29. Fisher exact tests were conducted to assess the association between baseline CAF levels and the change in ejection fraction (EF) from baseline to study end (≥0 vs. <0), and the association between histology (papillary vs. nonpapillary) and sunitinib benefit (yes vs. no). The Wilcoxon rank-sum test was used to compare the change in CAF between patients with and without hypertension during treatment and between EF changes (≥0 vs. <0). The same test was used to compare the baseline CAFs between histology groups (papillary vs. nonpapillary). PFS and OS were estimated using the Kaplan-Meier method and compared across subgroups of patients using the log-rank test. Cox proportional hazards models were used to assess the association between CAFs or hypertension and PFS and OS. Logistic models were used to evaluate the association between CAF levels or hypertension and sunitinib benefit. Significant predictors in the univariate models were included in the multivariate models through backward elimination, until all remaining predictors had p values <.05 in the final fitted model. Statistical analyses were performed using SAS, version 9.3 (SAS Institute Inc., Cary, NC, http://www.sas.com), and the data were plotted using R software (R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org).
Results
Patient Outcomes and Disease Characteristics
A total of 57 patients receiving sunitinib in the phase II clinical trial were evaluable for the present study. The disease characteristics have been previously summarized [3]. Of the 57 patients, 27 (47%) had papillary RCC (2 with type I, 11 with type II, and 14 not specified), 8 (14%) had an unclassified variant, 7 (12%) had sarcomatoid, 6 (11%) had collecting duct/renal medullary, 5 (9%) had chromophobe, and 4 (7%) had other forms of nccRCC. With a median follow-up time of 16.8 months (range, 0.9–82.6), 52 (91%) of the 57 patients had progressive disease or had died, and 5 (9%) were alive without progression at the last follow-up visit. The median PFS was 2.9 months (95% confidence interval [CI], 1.4–5.5), and the 1-year PFS rate was 18% (95% CI, 10%–31%). The median OS was 16.8 months (95% CI, 10.7–27.4), the 1-year OS rate was 61% (95% CI, 50%–75%). Two patients were not evaluable for response. Sunitinib benefit was seen in 21 patients (38%; 8 papillary, 4 chromophobe, 3 unclassified, 3 other, 2 collecting duct/renal medullary, and 1 sarcomatoid RCC). No difference was seen in the treatment response between the patients with different nccRCC subtypes (supplemental online Table 1).
Changes in Cardiovascular Parameters Were not Associated With Clinical Outcomes
To investigate whether sunitinib-induced cardiovascular toxic effects are associated with clinical outcomes in nccRCC patients, we evaluated on-treatment changes in cardiovascular parameters. The patients in our study were primarily men, and most had no personal or family history of coronary artery disease, dyslipidemia, diabetes mellitus, or smoking (supplemental online Table 2). Thirty-two patients (56%) had a history of hypertension.
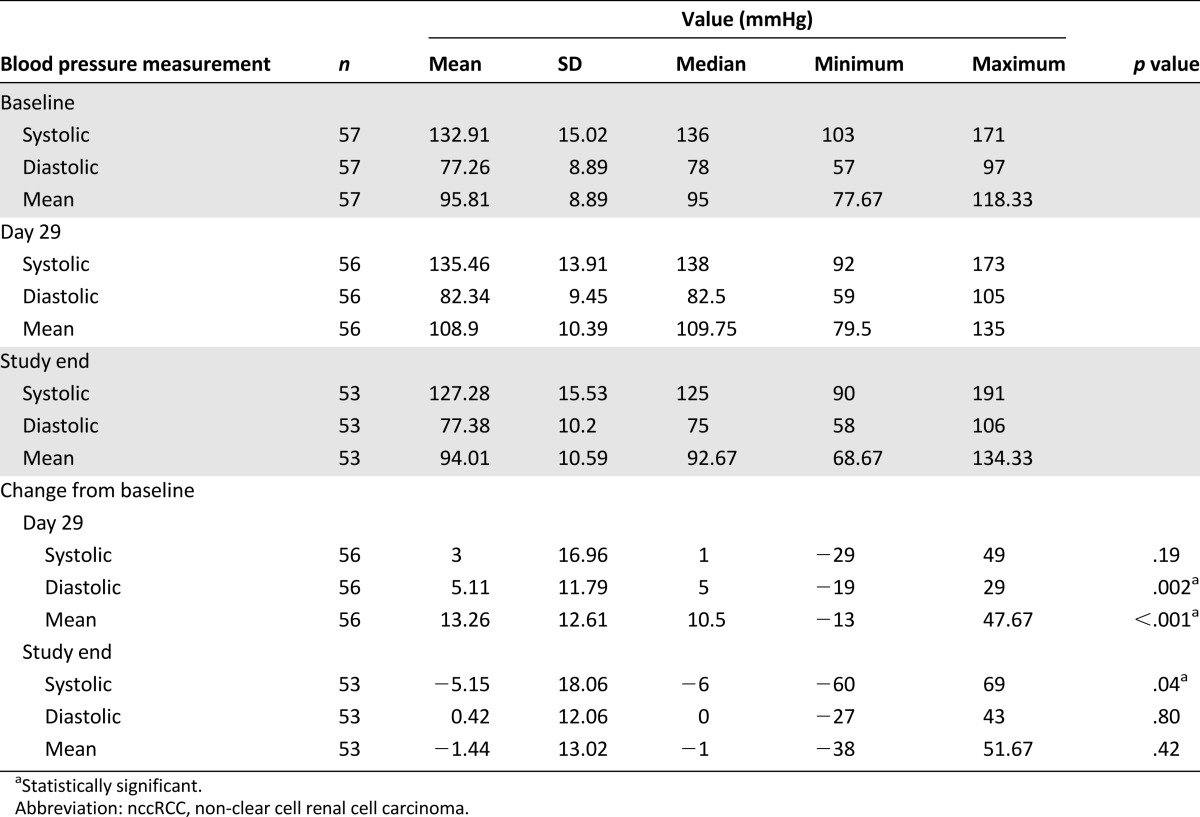
Data on LVEF at baseline and the development of hypertension were available for 37 (65%) and 55 (96%) patients, respectively. The mean LVEF did not change between the baseline and study end assessments (median, 61% for both; range, 52%–76% and 45%–76%, respectively; p = .65). However, 29 patients (53%) developed hypertension during the first treatment cycle, and 4 more (total, 60%) did so during subsequent cycles. The BP measurements at baseline, day 29, and study end are listed in Table 1. The most important changes occurred in DBP (p = .002) and mean arterial pressure (p < .001) between baseline and day 29. However, we found no association between the development of hypertension during treatment and PFS, OS, or sunitinib benefit (p = .25, p = .09, and p = .15, respectively). We also considered the relation between changes in the NO concentrations and the development of hypertension during treatment and found that patients who developed hypertension had a greater median reduction in circulating NO compared with those who did not (median change, −2.45 vs. −0.98, respectively), but the difference was not significant (p = .24). Supplemental online Tables 3 and 4 show the association between CAFs or NO at baseline or on-treatment changes and changes in LVEF.
Table 1.
Blood pressure measures in patients with nccRCC treated with sunitinib
Pretreatment CAF Levels Associated With Clinical Outcomes
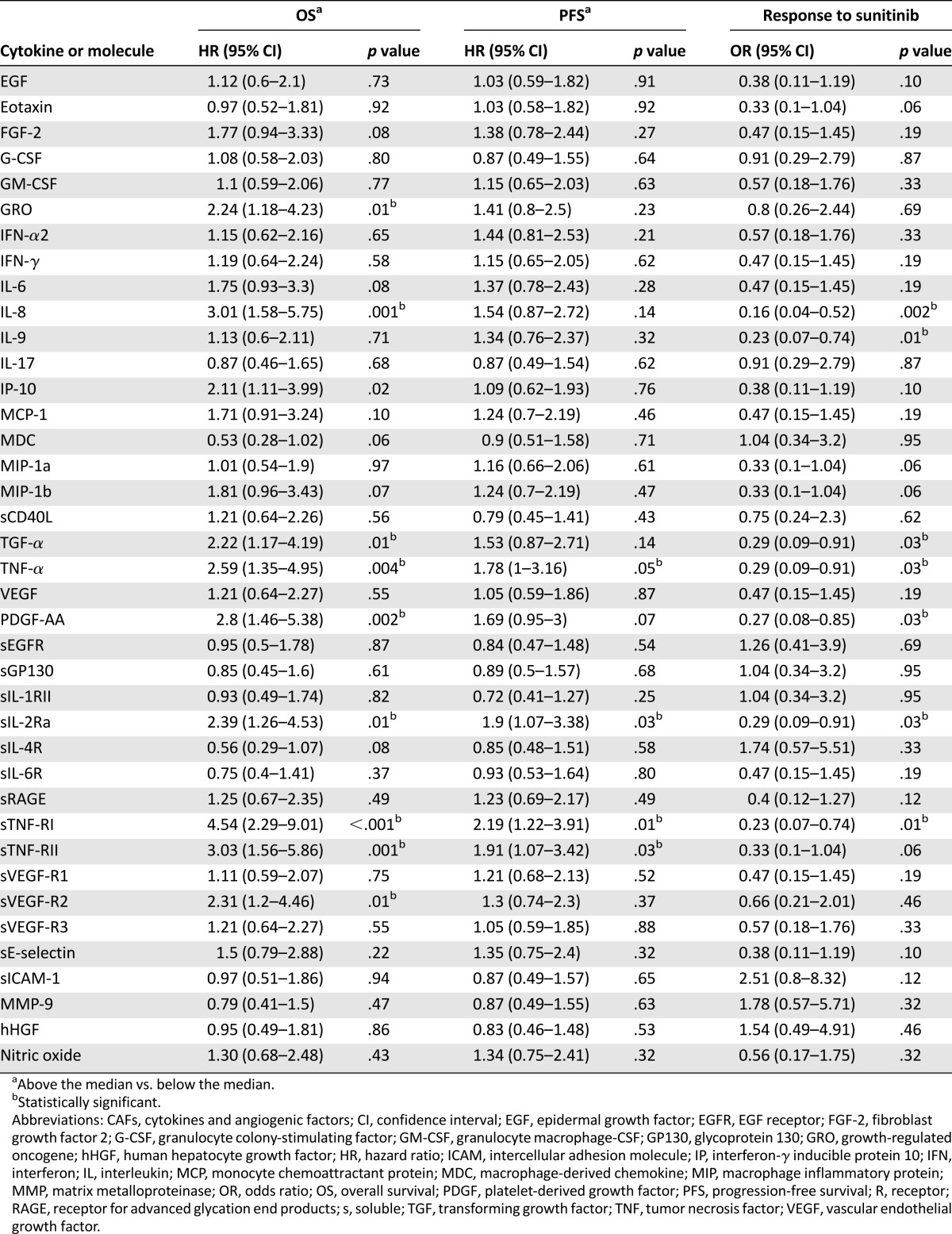
CAF determinations were available for 53 of the 57 patients (93%). The CAF and NO levels at baseline and day 29 of treatment and the differences between the two time points are listed in supplemental online Table 5. PDGF-AA was the only CAF different in the papillary (median, 352 pg/mL) versus nonpapillary (median, 148 pg/mL) subtypes at baseline (p = .02; supplemental online Table 6). Using the median pretreatment CAF levels as binary cutoffs, we found that high levels of soluble tumor necrosis factor receptor I (sTNF-RI), sTNF-RII, TNF-α, and sIL-2Ra were associated with reduced PFS and OS and that high levels of IL-8, PDGF-AA, growth-regulated oncogene (GRO), transforming growth factor-α (TGF-α), sVEGFR-2, and interferon-γ inducible protein 10 associated with reduced OS only (highest hazard ratio, 4.54 for sTNF-RI; 95% CI, 2.29–9.01; p < .001; Table 2). High concentrations of sTNF-RI, TNF-α, sIL-2Ra, IL-8, IL-9, PDGF-AA, and TGF-α were also associated with a low response to sunitinib (Table 2).
Table 2.
Univariate Cox modeling for OS and PFS and univariate logistic modeling for sunitinib benefit, using dichotomized baseline levels of CAFs and nitric oxide as covariates
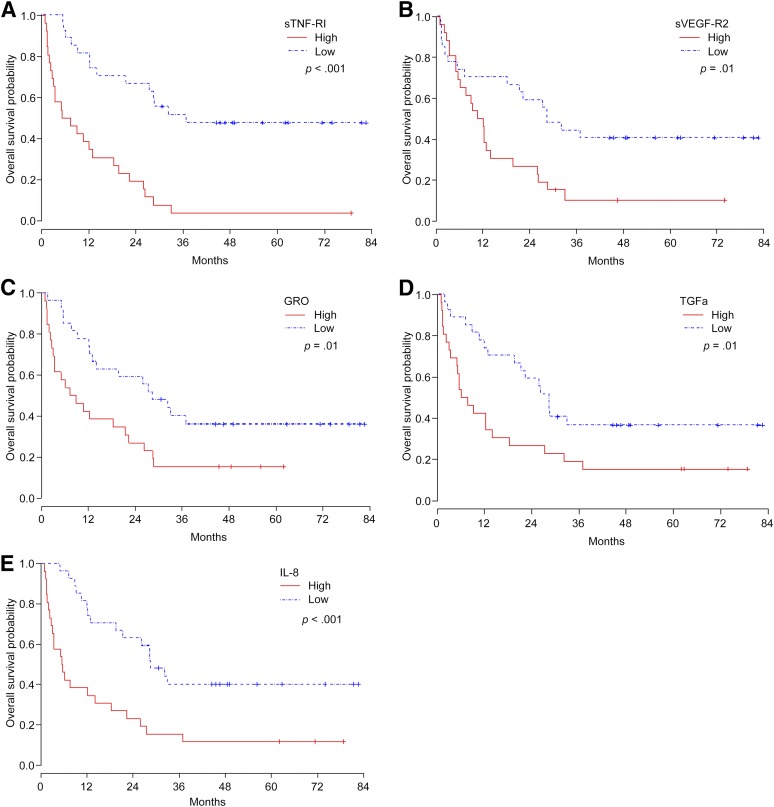
In a multivariate Cox model for OS, we determined that patients with high baseline levels of sTNF-RI, IL-8, GRO, TGF-α, and sVEGFR-2 had a higher risk of death (Table 3; Fig. 1). Using the same modeling for PFS, only sTNF-RI was significant; patients with high sTNF-RI were at a higher risk of disease progression and/or death (Table 3). Soluble TNF-RI and IL-9 were the only pretreatment CAFs associated with sunitinib benefit on multivariate logistic regression analysis (Table 3); patients with high sTNF-RI and IL-9 levels were less likely to benefit from the drug.
Table 3.
Multivariate Cox modeling for OS and PFS and multivariate logistic modeling for sunitinib benefit, using dichotomized baseline levels of CAFs as covariates

Figure 1.
Kaplan-Meier estimates for overall survival in patients with metastatic non-clear cell renal cell carcinoma treated with sunitinib, according to baseline levels of sTNF-RI (A), sVEGF-R2 (B), GRO (C), TGF-α (D), and IL-8 (E).
Abbreviations: GRO, growth-regulated oncogene; IL-8, interleukin-8; sTNF-RI, soluble tumor necrosis factor receptor I; sVEGF-R2, soluble vascular endothelial growth factor receptor 2; TGF-α, tumor necrosis factor-α.
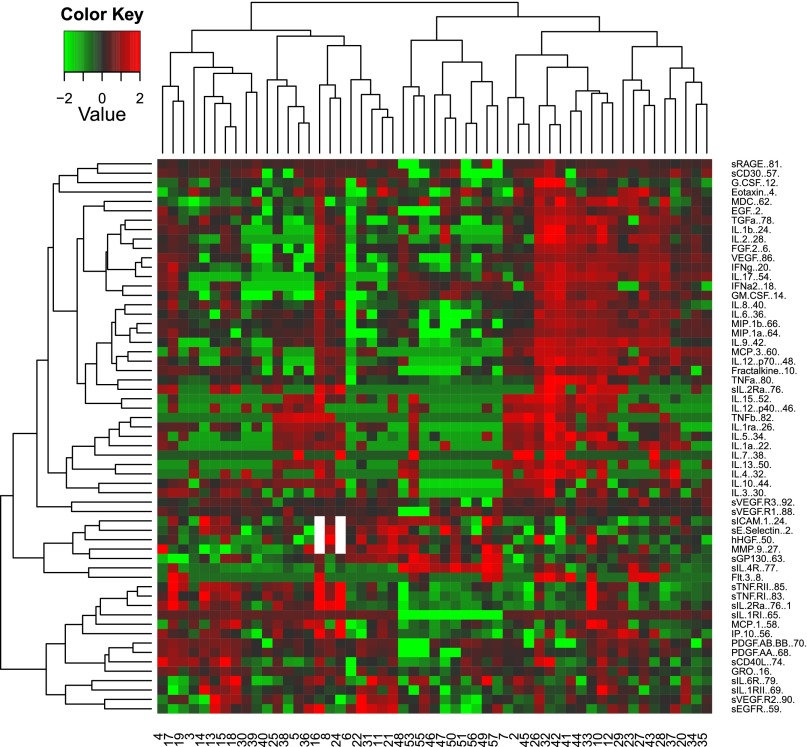
To gain insight into the associations between CAFs in treatment-naïve patients with metastatic nccRCC, we conducted an unsupervised hierarchical clustering analysis to group patients according to CAF levels (Fig. 2). Two main groups of patients were identified: one with relatively high concentrations of proangiogenic and inflammatory tumorigenic mediators, such as VEGF, fibroblast growth factor 2, TGF-α, IL-6, and IL-8, and a second with generally high levels of sTNF receptors. A small cluster of patients had relatively low concentrations of the CAFs defining the other two groups. No histological subtype was predominant in any of these patient groups.
Figure 2.
Unsupervised cluster analysis of cytokines and angiogenic factors in patients with metastatic non-clear cell renal cell carcinoma.
Discussion
The present study is, to the best of our knowledge, the first analysis of the significance of treatment-induced hypertension and circulating CAFs in patients with advanced nccRCC receiving targeted therapy, in this case, sunitinib. We found no significant link between the development of hypertension with sunitinib and PFS, OS, or response benefit in nccRCC. We also analyzed the concentrations of 38 angiogenic and immunomodulatory cytokines for prognostic and treatment benefit and found that elevated baseline levels of sTNF-RI, IL-8, GRO, sVEGFR-2, and TGF-α were associated with a higher risk of death.
Sunitinib treatment causes hypertension in 15%–34% of cancer patients through activation of the endothelin system and suppression of NO production by endothelial cells in the host [15]. Rini et al. reported that the development of hypertension in response to sunitinib is a biomarker of antitumor efficacy in RCC; patients developing hypertension had a fourfold increase in median PFS and OS [13, 16]. However, the applicability of those results to nccRCC is unclear, because >85% of the participants in that study had clear cell histology [13]. In ours, >50% patients developed hypertension in response to sunitinib therapy, and we observed a greater, although nonsignificant, reduction in circulating NO in those with hypertension compared with those who did not. However, sunitinib-induced hypertension was not associated with better outcomes. We used a more rigorous definition of hypertension, because all BP measurements documented in the medical records at any time point during the study were independently considered. Still, although our relatively small sample size precludes any firm conclusion, our data point to limited or nonexistent clinical significance for hypertension emerging during treatment as a biomarker of VEGFR-TK inhibitor treatment benefit in nccRCC.
With the exception of PDGF-AA, which was higher in papillaries, we found no differences in the levels of any of the CAFs in the papillary versus nonpapillary tumors. To gain insight into the soluble drivers of the biology of nccRCC and their classification, we conducted a nonsupervised clustering analysis that uncovered relatively high levels of the sTNF-R in approximately one third of the cases. High pretreatment levels of TNF-α and the soluble forms of its receptors, TNF-RI and TNF-RII, were associated with shorter PFS and OS in the univariate models, and high sTNF-RI predicted for shorter PFS and OS and poorer sunitinib benefit on multivariate analysis. The roles TNF and sTNF-RI play in cancer progression and their prognostic significance are not fully understood. An extensive array of early reports indicated that TNF had oncolytic and antiproliferative effects in RCC [17–19]. However, later studies have cast this cytokine, which is secreted by immune and tumor cells, as an immunosuppressive mediator that promotes RCC progression [20–22]. TNF has thus been reported to kill T cells isolated from patients with metastatic RCC but not T cells from healthy donors and to inhibit CD8+ T cells through the induction of programmed death ligand 1 on RCC cells [23, 24]. Similarly, sTNF-RI has been linked to downregulation of cell surface adhesion molecules and reduction of lymphocyte infiltration into tissues [25, 26]. Moreover, circulating TNF has been shown to be elevated in nonresponders to sunitinib [27]; however, research on the clinical significance of the sTNF-R is very limited [28]. Our finding linking sTNF-RI to a poor prognosis and low response to sunitinib in nccRCC is novel and suggests that, despite the biological heterogeneity of nccRCC, TNF signaling has relevance to the clinical outcomes in this group of diseases.
The other CAFs identified in association with outcomes in our study are angiogenic and inflammatory mediators, which we found in relatively high levels in a substantial number of patients. Of those, IL-8 is a proangiogenic chemokine implicated in invasiveness, antiangiogenic treatment resistance, and immune modulation. In animal models of RCC, neutralization of IL-8 with a monoclonal antibody sensitized RCC tumors to sunitinib treatment [29], and in metastatic RCC patients, we and others found IL-8 to be prognostic for PFS and OS [10, 11, 30]. Our results therefore are consistent with previous studies relevant predominantly to the clear cell type and extend the prognostic significance of IL-8 to nccRCC treated with sunitinib. GRO, like IL-8, activates the CXCR1 and -R2 receptors [31] and plays a role in immune cell recruitment and angiogenesis [32–34] and in predicting metastatic seeding in breast cancer [35]. In line with these multifaceted protumorigenic influences, we also found that high levels of GRO were associated with shorter OS in the present study. That both GRO and IL-8 were identified as prognosticators in the multivariate model attests to the credible importance of CXCR2 signaling in nccRCC biology.
Similarly, TGF-α has been associated with prognosis or a poor response to treatment in a number of cancers, but no data were previously available for RCC [36–38]. A member of the epidermal growth factor (EGF) family and a ligand of the EGF receptor, TGF-α is upregulated by hypoxia-inducible factor in RCC and was found sufficient to promote the growth of ccRCC tumor cells [39]. VEGFR-2, another angiogenesis mediator, is the most important molecular target of sunitinib and other VEGFR-TK inhibitors. sVEGFR-2 has been investigated as a biomarker for antiangiogenic therapies [10]. Although early studies of RCC patients treated with sunitinib suggested that on-treatment reduction in sVEGFR-2 (and sVEGFR-3) is associated with clinical benefit [9], the significance of pretreatment sVEGFR2 in RCC and other tumor types is far less clear. Because sVEGFR-2 might serve as a surrogate biomarker for VEGF-dependent tumor growth [40], it might be that some of the more angiogenic nccRCCs would have a worse prognosis. Our findings support the notion that TGF-α is a marker of poor prognosis in nccRCC as in other cancers.
Conclusion
Our results are exploratory because of the limited number of patient samples (total and for each of the different types of nccRCC) and the absence of validation in an independent data set. Still, the lack of association between sunitinib-related hypertension and patient outcomes and the candidate biological and clinical significance of the CAFs identified, in particular, those associated with TNF and CXCR1/2 signaling, are novel findings worth further evaluation in nccRCC.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672. We thank Arthur Gelmis at the Department of Scientific Publications, The University of Texas MD Anderson Cancer Center, for editing the manuscript.
Footnotes
For Further Reading: Sumanta K. Pal, Naomi B. Haas. Adjuvant Therapy for Renal Cell Carcinoma: Past, Present, and Future. The Oncologist 2014;19:851–859.
Implications for Practice: This work provides an overview of current and ongoing trials of adjuvant (postoperative) therapy for localized renal cell carcinoma. Currently, the gold standard in this disease setting is observation with serial radiographs. The studies we highlight may lead to a dramatic paradigm shift for this disease.
Author Contributions
Conception/Design: Mehmet Asim Bilen, Amado J. Zurita, Nasreen A. Ilias-Khan, Hsiang-Chun Chen, Xuemei Wang, Alper Y. Kearney, Sherie Hodges, Eric Jonasch, Shixia Huang, Aarif Yusuf Khakoo, Nizar M. Tannir
Provision of study material or patients: Mehmet Asim Bilen, Amado J. Zurita, Nasreen A. Ilias-Khan, Hsiang-Chun Chen, Xuemei Wang, Alper Y. Kearney, Sherie Hodges, Eric Jonasch, Shixia Huang, Aarif Yusuf Khakoo, Nizar M. Tannir
Collection and/or assembly of data: Mehmet Asim Bilen, Amado J. Zurita, Nasreen A. Ilias-Khan, Hsiang-Chun Chen, Xuemei Wang, Alper Y. Kearney, Sherie Hodges, Eric Jonasch, Shixia Huang, Aarif Yusuf Khakoo, Nizar M. Tannir
Data analysis and interpretation: Mehmet Asim Bilen, Amado J. Zurita, Nasreen A. Ilias-Khan, Hsiang-Chun Chen, Xuemei Wang, Alper Y. Kearney, Sherie Hodges, Eric Jonasch, Shixia Huang, Aarif Yusuf Khakoo, Nizar M. Tannir
Manuscript writing: Mehmet Asim Bilen, Amado J. Zurita, Nasreen A. Ilias-Khan, Hsiang-Chun Chen, Xuemei Wang, Alper Y. Kearney, Sherie Hodges, Eric Jonasch, Shixia Huang, Aarif Yusuf Khakoo, Nizar M. Tannir
Final approval of manuscript: Mehmet Asim Bilen, Amado J. Zurita, Nasreen A. Ilias-Khan, Hsiang-Chun Chen, Xuemei Wang, Alper Y. Kearney, Sherie Hodges, Eric Jonasch, Shixia Huang, Aarif Yusuf Khakoo, Nizar M. Tannir
Disclosures
Eric Jonasch: Pfizer, Novartis, GlaxoSmithKline (C/A), Exelixis, Pfizer, GlaxoSmithKline, Onyx (RF); Aarif Yusuf Khakoo: Amgen, Inc. (E). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Klatte T, Pantuck AJ, Kleid MD, et al. Understanding the natural biology of kidney cancer: Implications for targeted cancer therapy. Rev Urol. 2007;9:47–56. [PMC free article] [PubMed] [Google Scholar]
- 2.Linehan WM, Srinivasan R, Garcia JA. Non-clear cell renal cancer: Disease-based management and opportunities for targeted therapeutic approaches. Semin Oncol. 2013;40:511–520. doi: 10.1053/j.seminoncol.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tannir NM, Plimack E, Ng C, et al. A phase 2 trial of sunitinib in patients with advanced non-clear cell renal cell carcinoma. Eur Urol. 2012;62:1013–1019. doi: 10.1016/j.eururo.2012.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vera-Badillo FE, Templeton AJ, Duran I, et al. Systemic therapy for non-clear cell renal cell carcinomas: A systematic review and meta-analysis. Eur Urol. 2015;67:740–749. doi: 10.1016/j.eururo.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Moore LE, Nickerson ML, Brennan P, et al. Von Hippel-Lindau (VHL) inactivation in sporadic clear cell renal cancer: Associations with germline VHL polymorphisms and etiologic risk factors. PLoS Genet. 2011;7:e1002312. doi: 10.1371/journal.pgen.1002312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ljungberg BJ, Jacobsen J, Rudolfsson SH, et al. Different vascular endothelial growth factor (VEGF), VEGF-receptor 1 and -2 mRNA expression profiles between clear cell and papillary renal cell carcinoma. BJU Int. 2006;98:661–667. doi: 10.1111/j.1464-410X.2006.06387.x. [DOI] [PubMed] [Google Scholar]
- 7.Yamazaki K, Sakamoto M, Ohta T, et al. Overexpression of KIT in chromophobe renal cell carcinoma. Oncogene. 2003;22:847–852. doi: 10.1038/sj.onc.1206153. [DOI] [PubMed] [Google Scholar]
- 8.Leppert JT, Lam JS, Yu H, et al. Targeting the vascular endothelial growth factor pathway in renal cell carcinoma: A tissue array based analysis. J Clin Oncol (Meeting Abstracts) 2005;23(suppl):4536a. [Google Scholar]
- 9.Deprimo SE, Bello CL, Smeraglia J, et al. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: Modulation of VEGF and VEGF-related proteins. J Transl Med. 2007;5:32. doi: 10.1186/1479-5876-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zurita AJ, Jonasch E, Wang X, et al. A cytokine and angiogenic factor (CAF) analysis in plasma for selection of sorafenib therapy in patients with metastatic renal cell carcinoma. Ann Oncol. 2012;23:46–52. doi: 10.1093/annonc/mdr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran HT, Liu Y, Zurita AJ, et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: A retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol. 2012;13:827–837. doi: 10.1016/S1470-2045(12)70241-3. [DOI] [PubMed] [Google Scholar]
- 12.Le Tourneau C, Raymond E, Faivre S. Sunitinib: A novel tyrosine kinase inhibitor. A brief review of its therapeutic potential in the treatment of renal carcinoma and gastrointestinal stromal tumors (GIST) Ther Clin Risk Manag. 2007;3:341–348. doi: 10.2147/tcrm.2007.3.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rini BI, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103:763–773. doi: 10.1093/jnci/djr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lankhorst S, Kappers MH, van Esch JH, et al. Treatment of hypertension and renal injury induced by the angiogenesis inhibitor sunitinib: Preclinical study. Hypertension. 2014;64:1282–1289. doi: 10.1161/HYPERTENSIONAHA.114.04187. [DOI] [PubMed] [Google Scholar]
- 15.Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol. 2009;6:465–477. doi: 10.1038/nrclinonc.2009.94. [DOI] [PubMed] [Google Scholar]
- 16.Sunshine SB, Dallabrida SM, Durand E, et al. Endostatin lowers blood pressure via nitric oxide and prevents hypertension associated with VEGF inhibition. Proc Natl Acad Sci USA. 2012;109:11306–11311. doi: 10.1073/pnas.1203275109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakashima J. In vitro induction of endogenous tumor necrosis factor (TNF) and its cytotoxicity against renal cell carcinoma (RCC) Keio J Med. 1988;37:72–84. doi: 10.2302/kjm.37.72. [DOI] [PubMed] [Google Scholar]
- 18.van Moorselaar RJ, Beniers AJ, Hendriks BT, et al. In vivo antiproliferative effects of gamma-interferon and tumor necrosis factor alpha in a rat renal cell carcinoma model system. J Urol. 1990;143:1247–1251. doi: 10.1016/s0022-5347(17)40247-3. [DOI] [PubMed] [Google Scholar]
- 19.Baisch H, Otto U, Klöppel G. Antiproliferative and cytotoxic effects of single and combined treatment with tumor necrosis factor alpha and/or alpha interferon on a human renal cell carcinoma xenotransplanted into nu/nu mice: Cell kinetic studies. Cancer Res. 1990;50:6389–6395. [PubMed] [Google Scholar]
- 20.Ikemoto S, Sugimura K, Yoshida N, et al. TNF alpha, IL-1 beta and IL-6 production by peripheral blood monocytes in patients with renal cell carcinoma. Anticancer Res. 2000;20:317–321. [PubMed] [Google Scholar]
- 21.Chuang MJ, Sun KH, Tang SJ, et al. Tumor-derived tumor necrosis factor-alpha promotes progression and epithelial-mesenchymal transition in renal cell carcinoma cells. Cancer Sci. 2008;99:905–913. doi: 10.1111/j.1349-7006.2008.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu ST, Sun GH, Hsu CY, et al. Tumor necrosis factor-α induces epithelial-mesenchymal transition of renal cell carcinoma cells via a nuclear factor kappa B-independent mechanism. Exp Biol Med (Maywood) 2011;236:1022–1029. doi: 10.1258/ebm.2011.011058. [DOI] [PubMed] [Google Scholar]
- 23.Das T, Sa G, Hilston C, et al. GM1 and tumor necrosis factor-alpha, overexpressed in renal cell carcinoma, synergize to induce T-cell apoptosis. Cancer Res. 2008;68:2014–2023. doi: 10.1158/0008-5472.CAN-07-6037. [DOI] [PubMed] [Google Scholar]
- 24.Quandt D, Jasinski-Bergner S, Müller U, et al. Synergistic effects of IL-4 and TNFα on the induction of B7-H1 in renal cell carcinoma cells inhibiting allogeneic T cell proliferation. J Transl Med. 2014;12:151. doi: 10.1186/1479-5876-12-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quinn TD, Miller FN, Wilson MA, et al. Interleukin-2-induced lymphocyte infiltration of multiple organs is differentially suppressed by soluble tumor necrosis factor receptor. J Surg Res. 1994;56:117–122. doi: 10.1006/jsre.1994.1020. [DOI] [PubMed] [Google Scholar]
- 26.Chen TC, Hinton DR, Sippy BD, et al. Soluble TNF-alpha receptors are constitutively shed and downregulate adhesion molecule expression in malignant gliomas. J Neuropathol Exp Neurol. 1997;56:541–550. doi: 10.1097/00005072-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Gracia JL, Prior C, Guillén-Grima F, et al. Identification of TNF-alpha and MMP-9 as potential baseline predictive serum markers of sunitinib activity in patients with renal cell carcinoma using a human cytokine array. Br J Cancer. 2009;101:1876–1883. doi: 10.1038/sj.bjc.6605409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlsson AC, Juhlin CC, Larsson TE, et al. Soluble tumor necrosis factor receptor 1 (sTNFR1) is associated with increased total mortality due to cancer and cardiovascular causes—Findings from two community based cohorts of elderly. Atherosclerosis. 2014;237:236–242. doi: 10.1016/j.atherosclerosis.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Huang D, Ding Y, Zhou M, et al. Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res. 2010;70:1063–1071. doi: 10.1158/0008-5472.CAN-09-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harmon CS, DePrimo SE, Figlin RA, et al. Circulating proteins as potential biomarkers of sunitinib and interferon-α efficacy in treatment-naïve patients with metastatic renal cell carcinoma. Cancer Chemother Pharmacol. 2014;73:151–161. doi: 10.1007/s00280-013-2333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravindran A, Sawant KV, Sarmiento J, et al. Chemokine CXCL1 dimer is a potent agonist for the CXCR2 receptor. J Biol Chem. 2013;288:12244–12252. doi: 10.1074/jbc.M112.443762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bechara C, Chai H, Lin PH, et al. Growth related oncogene-alpha (GRO-alpha): Roles in atherosclerosis, angiogenesis and other inflammatory conditions. Med Sci Monit. 2007;13:RA87–RA90. [PubMed] [Google Scholar]
- 33.Wang D, Wang H, Brown J, et al. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J Exp Med. 2006;203:941–951. doi: 10.1084/jem.20052124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scapini P, Morini M, Tecchio C, et al. CXCL1/macrophage inflammatory protein-2-induced angiogenesis in vivo is mediated by neutrophil-derived vascular endothelial growth factor-A. J Immunol. 2004;172:5034–5040. doi: 10.4049/jimmunol.172.8.5034. [DOI] [PubMed] [Google Scholar]
- 35.Divella R, Daniele A, Savino E, et al. Circulating levels of transforming growth factor-βeta (TGF-β) and chemokine (C-X-C motif) ligand-1 (CXCL1) as predictors of distant seeding of circulating tumor cells in patients with metastatic breast cancer. Anticancer Res. 2013;33:1491–1497. [PubMed] [Google Scholar]
- 36.Tarhini AA, Lin Y, Yeku O, et al. A four-marker signature of TNF-RII, TGF-α, TIMP-1 and CRP is prognostic of worse survival in high-risk surgically resected melanoma. J Transl Med. 2014;12:19. doi: 10.1186/1479-5876-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhee J, Han SW, Cha Y, et al. High serum TGF-α predicts poor response to lapatinib and capecitabine in HER2-positive breast cancer. Breast Cancer Res Treat. 2011;125:107–114. doi: 10.1007/s10549-010-1200-9. [DOI] [PubMed] [Google Scholar]
- 38.Masago K, Fujita S, Hatachi Y, et al. Clinical significance of pretreatment serum amphiregulin and transforming growth factor-alpha, and an epidermal growth factor receptor somatic mutation in patients with advanced non-squamous, non-small cell lung cancer. Cancer Sci. 2008;99:2295–2301. doi: 10.1111/j.1349-7006.2008.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gunaratnam L, Morley M, Franovic A, et al. Hypoxia inducible factor activates the transforming growth factor-alpha/epidermal growth factor receptor growth stimulatory pathway in VHL(−/−) renal cell carcinoma cells. J Biol Chem. 2003;278:44966–44974. doi: 10.1074/jbc.M305502200. [DOI] [PubMed] [Google Scholar]
- 40.Ebos JM, Lee CR, Bogdanovic E, et al. Vascular endothelial growth factor-mediated decrease in plasma soluble vascular endothelial growth factor receptor-2 levels as a surrogate biomarker for tumor growth. Cancer Res. 2008;68:521–529. doi: 10.1158/0008-5472.CAN-07-3217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.