Neoadjuvant chemoradiotherapy for esophageal cancer patients often comes with side effects that affect patients’ appetite and body weight and lead to a marked decrease in physical activity that may reduce strength, muscle mass, and functional walking capacity. These side effects may be reduced through a walk-and-eat intervention. Nurses can implement this intervention in radiation oncology departments, provided physician and nursing leadership cooperate to achieve compliance with the protocols.
Keywords: Randomized controlled trial, Esophageal cancer, Neoadjuvant therapy, Exercise, Nutrition
Abstract
Background.
Preserving functional walking capacity and nutritional status is important for patients with esophageal cancer, but no effective intervention is available, particularly during active treatment.
Methods.
This pilot randomized controlled trial tested the effects of a walk-and-eat intervention for patients with esophageal cancer undergoing neoadjuvant chemoradiotherapy. Participants with locally advanced esophageal cancer stage IIB or higher (n = 59) were randomly assigned to receive the walk-and-eat intervention (n = 30; nurse-supervised walking three times per week and weekly nutritional advice) or usual care (n = 29; control group) during 4–5 weeks of chemoradiotherapy. Primary endpoints were changes in distance on the 6-minute walk test, hand-grip strength, lean muscle mass, and body weight between initiation and completion of intervention.
Results.
Participants (mean age: 59.6 years) were mostly male (92.9%) with squamous cell carcinoma (96.4%). During chemoradiotherapy, participants who received the walk-and-eat intervention had 100-m less decline than controls in walk distance (adjusted p = .012), 3-kg less decrease in hand-grip strength (adjusted p = .002), and 2.7-kg less reduction in body weight (adjusted p < .001), regardless of age. The intervention group also had significantly lower rates of need for intravenous nutritional support and wheelchair use.
Conclusion.
The nurse-led walk-and-eat intervention is feasible and effective to preserve functional walking capacity and nutritional status for patients with esophageal cancer undergoing neoadjuvant chemoradiotherapy.
Implications for Practice:
Neoadjuvant chemoradiotherapy for esophageal cancer patients often comes with side effects that affect patients’ appetite and body weight and lead to a marked decrease in physical activity that may reduce strength, muscle mass, and functional walking capacity. This study shows that these side effects may be reduced in esophageal cancer patients who participate in a walk-and-eat intervention. Nurses can implement this intervention in radiation oncology departments, provided physician and nursing leadership cooperate to achieve compliance with the protocols.
Abstract
摘要
背景. 保存功能性步行能力及营养状态对食道癌患者而言非常重要, 但目前尚无有效干预措施, 尤其是在积极治疗期间。
方法. 本项初步随机对照临床试验检验了步行-饮食干预对接受新辅助放化疗的食道癌患者的作用。在为期 4 ∼ 5 周的放化疗期间, 将高于或等于 IIB 期的局部晚期食道癌患者 (n = 59) 随机分配至步行-饮食干预组 (n = 30, 在护士指导下每周步行 3 次, 同时每周给予营养咨询建议) 或常规护理 (n = 29, 对照组)。主要终点为干预开始和结束时六分钟步行距离试验、手握力、肌肉量和体重的变化。
结果. 参与者 (平均年龄为 59.6 岁) 中多数为男性 (92.9%), 患有鳞状细胞癌 (96.4%)。在放化疗期间, 接受步行-饮食干预的受试者步行距离的降幅比对照组少 100 m (校正后P = 0.012), 握力降幅比对照组少 3 kg (校正后P = 0.002), 体重降幅比对照组少 2.7 kg (校正后P < 0.001), 且不受年龄影响。干预组需要静脉营养支持和使用轮椅的频率显著低于对照组。
结论. 对于接受新辅助放化疗的食道癌患者, 在护士指导下接受步行-饮食干预具有可行性, 并且能有效保存功能性步行能力和营养状态。The Oncologist 2015;20:1216–1222
对临床实践的提示: 对食道癌患者而言, 新辅助放化疗常伴有影响食欲和体重的副作用, 并且可导致体力活动明显下降, 继而削弱力量、肌肉量和功能性步行能力。本研究提示参加步行-饮食干预可能减少食道癌患者中的这些副作用。如医生和护理领导能合作促进患者遵循干预方案, 那么护士可以在肿瘤放疗科实施这些干预。
Background
Esophageal carcinoma affects more than 450,000 people worldwide and its prevalence is expected to increase 140% by 2025 [1, 2]. Unfortunately, esophageal cancer is often diagnosed at an advanced stage, and curative surgery can be considered in less than one-third of patients at diagnosis [3]. Although patients’ chances of curative surgery [4] and overall survival [4, 5] can be improved by neoadjuvant chemoradiotherapy, such treatment often comes with side effects that affect patients’ appetite and body weight and lead to a marked decrease in physical activity that may reduce strength, muscle mass, and functional walking capacity [1, 6].
Interventions to increase patients’ physical activity are likely to benefit cancer treatment [7], including treatment completion rate, decisions on treatment reduction [8], and treatment response [9]. Breast cancer patients receiving an exercise intervention in the Supervised Trial of Aerobic versus Resistance Training (START), for example, completed more of their chemotherapy than patients receiving usual care [8]. Similarly, patients with lymphoma who participated in the exercise arm of the Healthy Exercise for Lymphoma Patients (HELP) trial completed 94% of their prescribed maximum number of chemotherapy cycles compared with 89% of patients in the usual care group [9]. Moreover, 46.4% of patients who exercised during treatment had a complete treatment response, with only 30.8% having a complete response in the usual care group [9].
Preserving esophageal cancer patients’ functional walking capacity [10–12], lean muscle mass [13], and body weight [14] has helped enhance treatment tolerance [15], reduce surgical complications [16, 17], reduce length of stay and medical cost [18, 19], and improve quality of life [20] and overall survival [21, 22]. Clinically, these results mirror our observation that the inability of esophageal cancer patients to walk and eat indicates treatment failure. Although interventions have been developed to preserve cancer patients’ functional walking capacity and/or nutritional status, most are single-component approaches with inconsistent benefits [23, 24]. In theory, multifaceted interventions simultaneously targeting two or more functions (i.e., walking and eating) that are most relevant to cancer patients should be more effective than single-component interventions [25]. As a first attempt at this two-pronged approach, we developed the walk-and-eat intervention and tested its effects in esophageal cancer patients because they would have the greatest need for better care during active cancer treatment.
The objectives of this pilot randomized controlled trial (RCT) was to test the effects of a walk-and-eat intervention provided in parallel with 4–5 weeks of neoadjuvant chemoradiotherapy for patients with esophageal cancer. Patients receiving a walk-and-eat intervention or usual care were compared before and after the intervention in terms of changes in distance walked in the 6-minute walk test (6MWT), grip strength, body weight, and lean muscle mass. Treatment tolerance was also evaluated as the secondary endpoint.
Materials and Methods
Sample and Setting
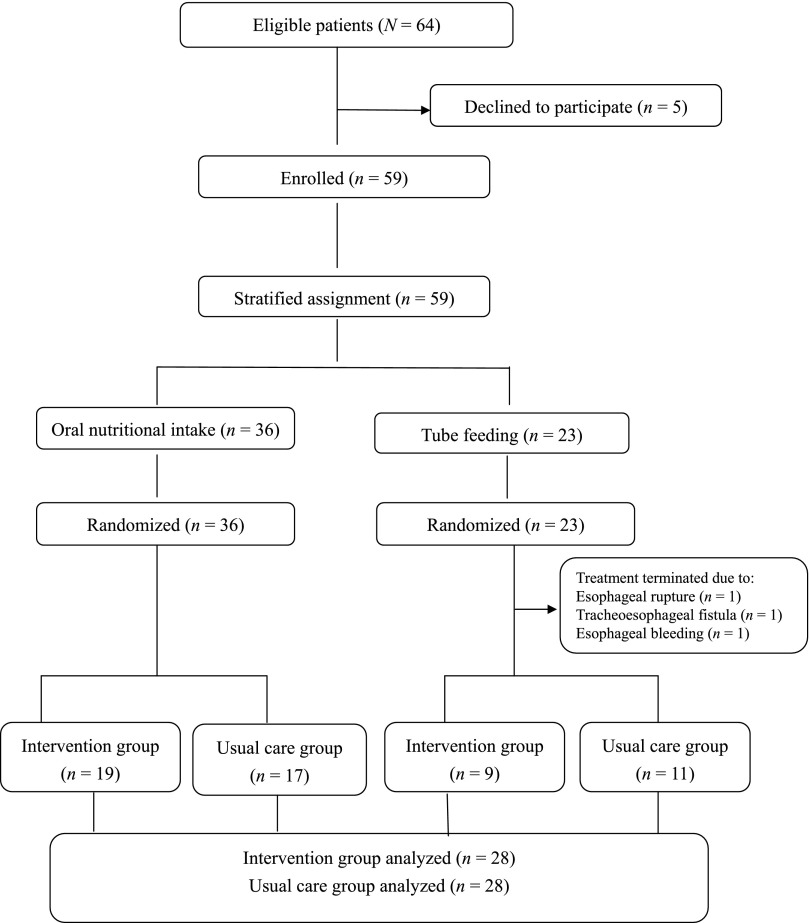
This prospective randomized controlled pilot trial was approved by the human research ethics committee of National Taiwan University Hospital and registered at ClinicalTrials.gov (identifier NCT01952210). Patients were eligible for the study if they had histologically documented, locally advanced tumors of the esophagus, defined as American Joint Committee on Cancer (AJCC) stage IIB or higher, were scheduled for neoadjuvant chemoradiotherapy, and had no contraindication precluding walking. Every patient fulfilling these inclusion criteria from May 2012 through May 2013 was invited to participate. Of 64 eligible patients, 59 (92%) were enrolled. Written informed consent was obtained for every participant.
Participants were first stratified by oral nutritional intake (n = 36) or tube feeding (n = 23) and then randomized separately into the intervention or usual care group, according to computer-generated randomization tables (Fig. 1).
Figure 1.
Flowchart of participants.
Chemoradiotherapy
For patients with esophageal cancer in the study hospital, the standardized treatment protocol when esophagectomy was feasible was to provide neoadjuvant radiotherapy up to a total dose of 40 Gy. For patients with inoperable cancer, radiation was continued to a total dose of 50–55 Gy. Neoadjuvant chemotherapy most commonly used was paclitaxel (35 mg/m2 on days 1 and 4 weekly) plus cisplatin (15 mg/m2 on days 2 and 5 weekly) for 4 cycles [26]. An alternative chemotherapy regimen was cisplatin (75 mg/m2) plus 5-fluorouracil (2,000 mg/m2 on days 1–3 in week 1 and 5-fluorouracil 2,000 mg/m2 on days 1–3 in week 4).
Intervention and Usual Care
Participants in the intervention group received both usual care and the walk-and-eat intervention. The intervention, which was provided by the same trained nurse, consisted of nurse-supervised walking three times a week and weekly nutrition advice. The walking protocol included a 5-minute warm-up (e.g., ankle circles, leg swings, pelvic loops, arm circles) and 20 minutes of hallway ambulation at each patient’s own pace before or after radiotherapy. Patients’ target heart rate during hallway ambulation was determined by the maximal heart rate formula: ([220 − age] × desired intensity of 60%). Weekly nutritional advice followed the Medical Nutrition Therapy (Cancer/Radiation Oncology) protocol of the American Dietetic Association [27] and consisted of weight and intake evaluation, advice on eating and feeding difficulties, food or formula selection, skills for modifying food texture, and oral care before and after eating.
The usual care group received conventional medical care and completed outcome measures in parallel with the intervention group. Before initiation of radiotherapy, all patients at the study setting routinely received nutritional and self-care advice from staff nurses. Participants assigned to the usual care group did not receive care from the trained nurse, but participants in both groups received care from the same radiation oncologists, medical oncologists, staff nurses, and radiation therapists.
Outcome Measures
The primary study endpoints, functional walking capacity and nutritional status, were evaluated by two trained outcome assessors. All participants were assessed before and after the intervention and before and after chemoradiotherapy. Specifically, functional walking capacity was measured using the 6MWT, and strength was measured as hand-grip strength (HGS). The 6MWT, a well-established tool for quantifying functional capacity [28, 29], was performed according to a standardized protocol in the corridor of the department of radiation oncology, with a 30-m walking course [30]. The distance walked was recorded in meters. HGS was measured in kilograms of pressure using a digital handheld dynamometer (T.K.K.5401; Takei Scientific Instruments Co., Ltd, Niigata, Japan, http://www.takei-si.co.jp/en/). Participants were instructed to stand with the shoulder slightly abducted, the elbow fully extended, and the forearm in a neutral position. Measurements were taken from the mean of two trials, as suggested by the NHANES Muscle Strength/Grip Test Procedure Manual [31].
Nutritional status was determined by body weight, measured using a digital scale (Tanita Corp., Tokyo, Japan, http://www.tanita.com/en/), and fat-free lean muscle mass, measured by bioelectrical impedance analysis (BIA; InBody 3.0; InBody Co., Ltd., Seoul, Republic of Korea, http://www.inbody.com/eng/). BIA is a noninvasive method that uses segmental multifrequency bioelectrical impedance analysis to measure body composition, including skeletal muscle mass [32]. Participants were instructed to take off their shoes, stand upright on the scale platform with embedded electrodes, and grasp the analyzer handles for 5–10 seconds.
Treatment tolerance, as the secondary endpoint, was measured by seven indices: interruptions (i.e., discontinuation, reduction) in chemotherapy or radiotherapy, unplanned hospital admission, grade >2 neutropenia, fever >38.5°C, intravenous nutritional support (Aminofluid [Otsuka Pharmaceutical, Takushima, Japan, http://www.otsukakj.jp/en/]; 1,000 mL 4-hour i.v. infusion 1–5 days/week during chemoradiotherapy as a study setting protocol), and wheelchair use. All indices were coded as “yes” or “no.” Data were obtained from the medical record and confirmed by weekly patient interviews.
Statistical Analysis
All analyses were performed on a per-protocol basis because of the exploratory nature of the study. Study variables were analyzed by descriptive statistics (frequencies, means, and standard deviations) and compared by treatment group at baseline. Outcomes were recoded as “post-treatment/pretreatment changes in functional walking capacity and nutritional status.” Because age might be an important covariate, analysis of covariance was used when comparing outcomes by group. All analyses were performed using SPSS version 18 (IBM Corp., Armonk, NY, http://www.ibm.com). Significance level was set at p < .05.
Results
Of the 59 participants, 56 completed follow-up (28 in the intervention group and 28 in the usual care group), with an attrition rate of 5.1% (n = 3). Attrition was due to termination of chemoradiotherapy because of esophageal rupture (n = 1), tracheoesophageal fistula (n = 1), and esophageal bleeding (n = 1). Participant flow is shown in Figure 1.
Patient and Treatment Characteristics
Participants’ mean ages for the intervention and usual care groups were 58.1 ± 9.6 years and 61.1 ± 9.0 years, respectively (Table 1). Most participants were male (92.9%) and had an average 10 ± 3.9 years of education. For both groups, the most common pathology was squamous cell carcinoma, but the intervention group also had one case each of adenocarcinoma and neuroendocrine carcinoma.
Table 1.
Participant baseline characteristics (n = 56)

As shown in Table 1, most participants (85.7%) were scheduled for 40-Gy radiotherapy (daily fraction of 2 Gy; 5 times per week) along with chemotherapy regimens of paclitaxel/cisplatin (58.9%) or 5-fluorouracil/cisplatin (35.7%). Two participants’ chemotherapy was canceled due to severe thrombocytopenia and poor healing of jejunostomy, but their radiotherapy (40 and 55 Gy, respectively) continued as scheduled.
Participants in the intervention and usual care groups did not differ on other baseline characteristics, including AJCC stage, functional status (Eastern Cooperative Oncology Group performance status, hand-grip strength, and distance on the 6MWT), body weight, and current nutritional intake status (severity of dysphagia and route of intake) (Table 1).
Intervention Adherence
Participants positively perceived the walk-and-eat intervention. Attendance at the nutritional advice sessions was 100%. Adherence to the walking protocol was good; participants in the intervention group completed a mean of 8.4 ± 3.6 walking sessions during 4–5 weeks of chemoradiotherapy. For the sessions in which participants walked the most (generally session 12 or 15), the average adherence rate was 68% (range: 32%–100%). The desired maximal heart rate was achieved in 71% of the walking sessions. The average time and distance walked per session were 22.4 ± 4.6 minutes and 1,467.8 ± 159.6 m, respectively.
Walk Distance, Hand-Grip Strength, Body Weight, and Lean Muscle Mass
Analysis of pretreatment to post-treatment changes in outcomes indicated that, regardless of age, the intervention group had significantly less decline in 6MWT walk distance than the usual care group (18 vs. 118 m; group difference: 100, adjusted p = .012) during chemoradiotherapy (Table 2). Likewise, the intervention group had significantly less decline in hand-grip strength than the usual care group (1.1 vs. 4.1 kg, respectively, group difference: 3.0; adjusted p = .002). Weight loss for the intervention group (0.8 kg) was less than one-fourth of the weight loss in the usual care group (3.5 kg), resulting in a 2.7-kg group difference (adjusted p < .001). In addition, the intervention group had minimal loss of lean muscle mass (0.7 kg), whereas the usual care group had considerable loss (2.0 kg) (group difference: 1.3; adjusted p = .057).
Table 2.
Mean pre- to post-treatment changes in functional walking capacity and nutritional status by group

Treatment Tolerance
Participants who received the walk-and-eat intervention showed a decreasing trend in five treatment tolerance indices (Table 3) but did not differ significantly from the usual care group in rates of chemotherapy interruption (28.6% for intervention vs. 63.6% for usual care, p = .10), radiotherapy interruptions (0% vs. 10.7%, p = .24), unplanned hospital admissions (7.1% vs. 21.4%, p = .25), neutropenia (10.7% vs. 25%, p = .30), or fever (3.6% vs. 14.3%, p = .35). However, participants in the intervention group had significantly lower rates of prescribed intravenous nutritional support (3.6% vs. 50%, p < .001) and wheelchair use (0% vs. 32.1%, p < .01) than participants in the usual care group (Table 3).
Table 3.
Comparison of treatment tolerance by group

Discussion
The most noteworthy finding of this randomized controlled pilot study is that the 4- to 5-week walk-and-eat intervention effectively preserved functional walking capacity and nutritional status between initiation and completion of neoadjuvant chemoradiotherapy for patients with newly diagnosed, locally advanced esophageal cancer. The benefits were clinically meaningful; patients who received the walk-and-eat intervention had 100-m less decline in walking distance, 3-kg less decline in grip strength, and 2.7-kg less weight loss. Participants in the intervention also had significantly reduced rates of intravenous nutritional support and wheelchair use during chemoradiotherapy.
We need to emphasize three points. First, our findings indicate that esophageal cancer patients suffer substantial declines during chemoradiotherapy. Patients in the usual care group had considerable declines in walking distance (118 m), hand-grip strength (4.1 kg), and weight loss (3.5 kg), indicating the strong need for interventions. Because chemoradiotherapy alone is insufficient to treat most of these patients, those with better functional walking capacity and nutritional status can receive curative surgery and remain in active treatment as long as the benefits exceed the risks.
Second, the benefits of this 4- to 5-week walk-and-eat intervention are evident for patients with esophageal cancer, but our results cannot be compared with those of other studies because this population is understudied. The effect of preserving 100 m in walking distance is consistent with the effects of an RCT on colorectal cancer patients awaiting surgery with no chemoradiotherapy [34]. In that study, a 4-week intervention of nutritional counseling and supplements with walking or aerobic exercise and relaxation resulted in a 41.6-m group difference [34]. Our results on preserving body weight are not as robust as those of two nonrandomized trials of an 8-week cancer nutrition rehabilitation program for patients with various cancer diagnoses [35, 36]. In one trial of 58 cancer patients with primarily lung or gastrointestinal cancer, 25 who completed the program had a weight gain of 0.7 kg. The high dropout rate (43%) in that study may have been related to 67% of participants concurrently receiving chemotherapy [35]. In the second trial, 27 of 31 head-and-neck cancer patients who completed the intervention after treatment maintained or gained weight [36]. Nevertheless, studies are urgently needed to develop effective and feasible interventions, particularly for esophageal cancer patients.
Third, our study is the first to report this newly developed walk-and-eat intervention and demonstrated that combining walking with nutritional advice works well for esophageal cancer patients. Nutritional advice alone is increasingly recognized as insufficient to combat the complex problem of cancer-related malnutrition [37]. We chose a walking protocol based on an RCT finding that a presurgical walking and breathing intervention outperformed presurgical aerobic and strengthening exercise (biking or strength training) in a sample of colorectal cancer patients before surgery [38]. In that study, the authors cited poor adherence in the aerobic exercise group (only 16%) to explain its lack of effects and hypothesized that the older participants (mean age: 60 ± 16 years) were intimidated by exercise intensity, and some were too deconditioned to follow the protocol. We accounted for adherence and feasibility by intentionally designing our walking protocol to have minimal intensity (20-minute walk at 60% of maximal heart rate), easy access (walk indoors in the department hallway before or after radiotherapy), and secured safety (walk with a nurse). Consequently, despite progressive fatigue and weakness, 54% (n = 15) of our participants completed more than 80% of walking sessions during their neoadjuvant chemoradiotherapy.
Moreover, nutritional interventions have predominantly focused on providing adequate calories and nutrients (i.e., extra supplements) [15, 39] or educating patients about selecting a protein- and calorie-rich diet [23, 40]. Although these aspects of nutrition information are important, our experience indicated that most participants were concerned about practical nutritional issues, such as how to prepare their oral condition to make foods appealing and less painful to swallow. Patients and caregivers wondered what outcomes they could expect (i.e., changes in energy level, nutritional intake status) and whether they had followed advice accurately. Our participants appeared to benefit from our assessment of their weight and intake history, answers to their questions about eating difficulties, and weekly positive reinforcement.
The study had limitations and strengths. First, the sample was small, and most participants were male with squamous cell carcinoma, in line with the Asian population with esophageal cancer patients [41] but limiting generalization of our results directly to other cancer populations. Patients with esophageal adenocarcinoma, for example, might have different patterns of decline in nutritional status and functional walking capacity, and thus not be as responsive to this intervention as patients with squamous cell carcinoma. Conversely, studies are warranted to test whether our intervention can benefit other populations such as head-and-neck cancer patients who also experience significant declines during neoadjuvant chemoradiotherapy. Second, in line with a small sample, post hoc analysis indicated that our study was powered at 83% for 6MWT, 91% for HGS, 100% for body weight, and 48% for lean muscle mass to detect group differences in the mean changes for these outcomes. In other words, with a sample size of 56, we had sufficient power to assess 6MWT, HGS, and body weight with confidence. Future studies with larger samples are needed to verify the intervention effects on lean muscle mass. Third, despite randomization, the baseline 6MWT distance walked was slightly lower for the control group (437 m) than the intervention group (471.4 m, p = .16) due to two outliers in the group (< 300 m walked) that might have biased the results. Fourth, we acknowledge that the extra hydration with cisplatin may have been a source of error with the BIA; however, hydration was completed on average 3.8 days before BIA, and treatment regimens did not differ between the intervention and usual care groups. Consequently, any effect of extra hydration with cisplatin on BIA measurements was likely minimal and random. Last, outcomes were measured on completion of the intervention, limiting understanding of long-term benefits, which remain to be tested. Nevertheless, this study examined an understudied population of esophageal cancer patients and used a rigorous RCT design. The walk-and-eat intervention appears to be feasible, safe, and beneficial, thus augmenting its clinical implications. More large-scale studies are indicated to verify the findings.
Conclusion
This nurse-supervised walk-and-eat intervention was associated with less reduction in walking distance, grip strength, and body weight for esophageal cancer patients, along with lower rates of receiving intravenous nutritional support and using a wheelchair during neoadjuvant chemoradiotherapy. The intervention was feasible and can be implemented successfully in radiation oncology departments but required ongoing cooperation between physician and nursing leadership to achieve compliance with the protocols.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgment
This study was partly supported by the Taiwan Ministry of Science and Technology (Grants 101-2627-B-002-003 and 103-2627-E-002-001).
Author Contributions
Conception/Design: Yu-Juan Xu, Jason Chia-Hsien Cheng, Jang-Ming Lee, Pei-Ming Huang, Cheryl Chia-Hui Chen
Provision of study material or patients: Yu-Juan Xu, Jason Chia-Hsien Cheng, Jang-Ming Lee, Pei-Ming Huang, Cheryl Chia-Hui Chen
Collection and/or assembly of data: Yu-Juan Xu, Jason Chia-Hsien Cheng, Guan-Hua Huang, Cheryl Chia-Hui Chen
Data analysis and interpretation: Yu-Juan Xu, Jason Chia-Hsien Cheng, Jang-Ming Lee, Pei-Ming Huang, Guan-Hua Huang, Cheryl Chia-Hui Chen
Manuscript writing: Yu-Juan Xu, Jason Chia-Hsien Cheng, Jang-Ming Lee, Pei-Ming Huang, Guan-Hua Huang, Cheryl Chia-Hui Chen
Final approval of manuscript: Yu-Juan Xu, Jason Chia-Hsien Cheng, Jang-Ming Lee, Pei-Ming Huang, Guan-Hua Huang, Cheryl Chia-Hui Chen
Disclosures
The authors indicated no financial relationships.
References
- 1.Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 2.Lambert R, Hainaut P. The multidisciplinary management of gastrointestinal cancer. Epidemiology of oesophagogastric cancer. Best Pract Res Clin Gastroenterol. 2007;21:921–945. doi: 10.1016/j.bpg.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 4.Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: An updated meta-analysis. Lancet Oncol. 2011;12:681–692. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 5.Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: A meta-analysis. Lancet Oncol. 2007;8:226–234. doi: 10.1016/S1470-2045(07)70039-6. [DOI] [PubMed] [Google Scholar]
- 6.Capra S, Ferguson M, Ried K. Cancer: Impact of nutrition intervention outcome--nutrition issues for patients. Nutrition. 2001;17:769–772. doi: 10.1016/s0899-9007(01)00632-3. [DOI] [PubMed] [Google Scholar]
- 7.Courneya KS, Rogers LQ, Campbell KL, et al. Top 10 research questions related to physical activity and cancer survivorship. Res Q Exerc Sport. 2015;86:107–116. doi: 10.1080/02701367.2015.991265. [DOI] [PubMed] [Google Scholar]
- 8.Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 9.Courneya KS, Sellar CM, Stevinson C, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. 2009;27:4605–4612. doi: 10.1200/JCO.2008.20.0634. [DOI] [PubMed] [Google Scholar]
- 10.Tatematsu N, Hasegawa S, Tanaka E, et al. Impact of oesophagectomy on physical fitness and health-related quality of life in patients with oesophageal cancer. Eur J Cancer Care (Engl) 2013;22:308–313. doi: 10.1111/ecc.12030. [DOI] [PubMed] [Google Scholar]
- 11.Feeney C, Reynolds JV, Hussey J. Preoperative physical activity levels and postoperative pulmonary complications post-esophagectomy. Dis Esophagus. 2011;24:489–494. doi: 10.1111/j.1442-2050.2010.01171.x. [DOI] [PubMed] [Google Scholar]
- 12.Ferriolli E, Skipworth RJ, Hendry P, et al. Physical activity monitoring: A responsive and meaningful patient-centered outcome for surgery, chemotherapy, or radiotherapy? J Pain Symptom Manage. 2012;43:1025–1035. doi: 10.1016/j.jpainsymman.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Ida S, Watanabe M, Karashima R, et al. Changes in body composition secondary to neoadjuvant chemotherapy for advanced esophageal cancer are related to the occurrence of postoperative complications after esophagectomy. Ann Surg Oncol. 2014;21:3675–3679. doi: 10.1245/s10434-014-3737-z. [DOI] [PubMed] [Google Scholar]
- 14.Riedel B, Ismail H, Findlay M, et al. Nutritional status and fitness in neoadjuvant chemoradiation for oesophagogastric cancer. Cancer Forum. 2011;35:234–237. [Google Scholar]
- 15.Odelli C, Burgess D, Bateman L, et al. Nutrition support improves patient outcomes, treatment tolerance and admission characteristics in oesophageal cancer. Clin Oncol (R Coll Radiol) 2005;17:639–645. doi: 10.1016/j.clon.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Grotenhuis BA, Wijnhoven BP, Grüne F, et al. Preoperative risk assessment and prevention of complications in patients with esophageal cancer. J Surg Oncol. 2010;101:270–278. doi: 10.1002/jso.21471. [DOI] [PubMed] [Google Scholar]
- 17.Nozoe T, Kimura Y, Ishida M, et al. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28:396–400. doi: 10.1053/ejso.2002.1257. [DOI] [PubMed] [Google Scholar]
- 18.Sungurtekin H, Sungurtekin U, Balci C, et al. The influence of nutritional status on complications after major intraabdominal surgery. J Am Coll Nutr. 2004;23:227–232. doi: 10.1080/07315724.2004.10719365. [DOI] [PubMed] [Google Scholar]
- 19.Bozzetti F, Gianotti L, Braga M, et al. Postoperative complications in gastrointestinal cancer patients: The joint role of the nutritional status and the nutritional support. Clin Nutr. 2007;26:698–709. doi: 10.1016/j.clnu.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Isenring EA, Capra S, Bauer JD. Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. Br J Cancer. 2004;91:447–452. doi: 10.1038/sj.bjc.6601962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Fiore F, Lecleire S, Pop D, et al. Baseline nutritional status is predictive of response to treatment and survival in patients treated by definitive chemoradiotherapy for a locally advanced esophageal cancer. Am J Gastroenterol. 2007;102:2557–2563. doi: 10.1111/j.1572-0241.2007.01437.x. [DOI] [PubMed] [Google Scholar]
- 22.Lecleire S, Di Fiore F, Antonietti M, et al. Undernutrition is predictive of early mortality after palliative self-expanding metal stent insertion in patients with inoperable or recurrent esophageal cancer. Gastrointest Endosc. 2006;64:479–484. doi: 10.1016/j.gie.2006.03.930. [DOI] [PubMed] [Google Scholar]
- 23.van den Berg MG, Rasmussen-Conrad EL, Wei KH, et al. Comparison of the effect of individual dietary counselling and of standard nutritional care on weight loss in patients with head and neck cancer undergoing radiotherapy. Br J Nutr. 2010;104:872–877. doi: 10.1017/S0007114510001315. [DOI] [PubMed] [Google Scholar]
- 24.Paccagnella A, Morassutti I, Rosti G. Nutritional intervention for improving treatment tolerance in cancer patients. Curr Opin Oncol. 2011;23:322–330. doi: 10.1097/CCO.0b013e3283479c66. [DOI] [PubMed] [Google Scholar]
- 25.Clinical practice guidelines we can trust. Available at http://www.ncbi.nlm.nih.gov/books/NBK209539/. Accessed February 20, 2015.
- 26.Lin CC, Hsu CH, Cheng JC, et al. Concurrent chemoradiotherapy with twice weekly paclitaxel and cisplatin followed by esophagectomy for locally advanced esophageal cancer. Ann Oncol. 2007;18:93–98. doi: 10.1093/annonc/mdl339. [DOI] [PubMed] [Google Scholar]
- 27.Gillbreath J, Inman-Felton A, Johnson E, et al. Medical Nutrition Therapy Across the Continuum of Care – Client Protocols. Chicago, IL: American Dietetic Association; 1998. [Google Scholar]
- 28.Rasekaba T, Lee AL, Naughton MT, et al. The six-minute walk test: A useful metric for the cardiopulmonary patient. Intern Med J. 2009;39:495–501. doi: 10.1111/j.1445-5994.2008.01880.x. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt K, Vogt L, Thiel C, et al. Validity of the six-minute walk test in cancer patients. Int J Sports Med. 2013;34:631–636. doi: 10.1055/s-0032-1323746. [DOI] [PubMed] [Google Scholar]
- 30.ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 31.NHANES Muscle Strength/Grip Test Procedure Manual. Available at: http://www.cdc.gov/nchs/nhanes/nhanes2011-2012/current_nhanes_11_12.htm. Accessed August 27, 2015.
- 32.Kim M, Kim H. Accuracy of segmental multi-frequency bioelectrical impedance analysis for assessing whole-body and appendicular fat mass and lean soft tissue mass in frail women aged 75 years and older. Eur J Clin Nutr. 2013;67:395–400. doi: 10.1038/ejcn.2013.9. [DOI] [PubMed] [Google Scholar]
- 33.Peters JH, Meester TR, Stein HJ. Surgical therapy for cancer of the esophagus and cardia. In: Castell DO, ed. The Esophagus. 2nd ed. New York, NY: Little, Brown. 1995:293–335. [Google Scholar]
- 34.Gillis C, Li C, Lee L, et al. Prehabilitation versus rehabilitation: A randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121:937–947. doi: 10.1097/ALN.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 35.Glare P, Jongs W, Zafiropoulos B. Establishing a cancer nutrition rehabilitation program (CNRP) for ambulatory patients attending an Australian cancer center. Support Care Cancer. 2011;19:445–454. doi: 10.1007/s00520-010-0834-9. [DOI] [PubMed] [Google Scholar]
- 36.Eades M, Murphy J, Carney S, et al. Effect of an interdisciplinary rehabilitation program on quality of life in patients with head and neck cancer: Review of clinical experience. Head Neck. 2013;35:343–349. doi: 10.1002/hed.22972. [DOI] [PubMed] [Google Scholar]
- 37.Fouladiun M, Körner U, Gunnebo L, et al. Daily physical-rest activities in relation to nutritional state, metabolism, and quality of life in cancer patients with progressive cachexia. Clin Cancer Res. 2007;13:6379–6385. doi: 10.1158/1078-0432.CCR-07-1147. [DOI] [PubMed] [Google Scholar]
- 38.Carli F, Charlebois P, Stein B, et al. Randomized clinical trial of prehabilitation in colorectal surgery. Br J Surg. 2010;97:1187–1197. doi: 10.1002/bjs.7102. [DOI] [PubMed] [Google Scholar]
- 39.Mariette C, De Botton ML, Piessen G. Surgery in esophageal and gastric cancer patients: What is the role for nutrition support in your daily practice? Ann Surg Oncol. 2012;19:2128–2134. doi: 10.1245/s10434-012-2225-6. [DOI] [PubMed] [Google Scholar]
- 40.Isenring EA, Bauer JD, Capra S. Nutrition support using the American Dietetic Association medical nutrition therapy protocol for radiation oncology patients improves dietary intake compared with standard practice. J Am Diet Assoc. 2007;107:404–412. doi: 10.1016/j.jada.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Napier KJ, Scheerer M, Misra S. Esophageal cancer: A review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol. 2014;6:112–120. doi: 10.4251/wjgo.v6.i5.112. [DOI] [PMC free article] [PubMed] [Google Scholar]