This multicenter, international study sought to characterize the clinical characteristics, treatment, and natural history of salivary gland MALT lymphoma in 247 patients. There was no difference in the outcomes between patients receiving local or systemic therapy in first-line management. On multivariate analysis, age less than 60 years and low to intermediate international prognostic index were associated with improved overall survival and progression-free survival; Sjögren disease was associated with improved overall survival.
Keywords: Lymphoma, B-cell, Marginal zone, Lymphoid tissue, Salivary glands, Sjögren's disease
Abstract
Background.
The salivary gland is one of the most common sites involved by nongastric, extranodal marginal zone lymphomas of mucosa-associated lymphoid tissue (MALT). A large series of patients with long-term follow-up has not been documented. This multicenter, international study sought to characterize the clinical characteristics, treatment, and natural history of salivary gland MALT lymphoma.
Methods.
Patients with biopsy-confirmed salivary gland MALT lymphoma were identified from multiple international sites. Risk factors, treatment, and long-term outcomes were evaluated.
Results.
A total of 247 patients were evaluated; 76% presented with limited-stage disease. There was a history of autoimmune disorder in 41%, with Sjögren disease being the most common (83%). Fifty-seven percent of patients were initially treated with local therapy with surgery, radiation, or both; 37 of patients were treated with systemic therapy initially, with 47% of those receiving rituximab; and 6% of patients were observed. The median overall survival (OS) was 18.3 years. The median progression-free survival (PFS) following primary therapy was 9.3 years. There was no difference in the outcomes between patients receiving local or systemic therapy in first-line management. On multivariate analysis, age <60 years and low to intermediate international prognostic index were associated with improved OS and PFS; Sjögren disease was associated with improved OS.
Conclusion.
Salivary gland MALT lymphoma has an excellent prognosis regardless of initial treatment, and patients with Sjögren disease have improved survival. Risks for long-term complications must be weighed when determining initial therapy.
Implications for Practice:
Patients with salivary gland extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) have an excellent prognosis, particularly those with associated Sjögren's disease. A wide range of available therapies may provide similar durable rates of disease control and survival. Therefore, an important goal in selection of therapy should be to minimize morbidity from treatment. When determining initial therapy for these patients, practitioners should consider the potential side effects and long-term toxicities of treatment.
Introduction
Mucosa-associated lymphoid tissue (MALT) lymphomas are a subtype of low-grade non-Hodgkin lymphoma (NHL) that often arise in the setting of chronic autoimmune or antigenic stimulation. The salivary gland is the most frequently affected nongastric site of MALT lymphoma, representing 14% of cases [1]. While lymphomas represent only a small fraction (5%–10%) of all salivary gland tumors, MALT lymphomas are the most common subtype, accounting for 50% of cases [2].
The disease is clinically indolent, with a 10-year overall survival (OS) rate of 85%. Even those with disseminated disease (stage IIIE/IV) do well, with a 9-year OS rate of 79% [3]. A history of Sjögren disease or sialadenitis is common [4, 5].
Although the salivary gland is the most frequently affected nongastric site of MALT lymphoma, literature focusing specifically on this entity is sparse. Much of what is known of salivary MALT lymphomas has been gleaned from case series including MALT lymphomas of other nongastric sites [6], or other types of NHL involving the head and neck or salivary glands [7, 8]. Given the heterogeneity of tissues affected by MALT, it is to be expected that disease associations, clinical outcomes, and data regarding treatment-related toxicities and morbidities may vary considerably by site. There have been only two large studies focused specifically on salivary MALT lymphomas [3, 4]. The larger of these was a population-based study using the Surveillance, Epidemiology and End Results (SEER) database and included 500 patients with salivary MALT lymphoma. Unfortunately, the SEER database has several limitations that limit the scope of the study: Information on staging is imprecise, and there is no expert pathology review of diagnostic specimens. Further, because the database does not include information regarding chemotherapy or biologic therapy, response data, or data on progression, analysis of treatment efficacy cannot be ascertained. The second study enrolled only 63 patients. Because the number of patients in each treatment arm was small (range: 4–23), analysis of the efficacy of different treatment modalities was limited. Thus, more information regarding risk factors, management strategies, and outcomes in salivary gland MALT lymphomas is warranted to determine the natural history and optimal treatment.
This is a report of 248 patients with salivary MALT treated at multiple international sites. The focus was to describe the clinical characteristics, association with other conditions, and prognosis, and, importantly, to better define treatment outcomes.
Methods
Patients
Patients were identified from multiple international sites, including the International External Lymphoma Study Group (IELSG) (n = 138), the Mayo Clinic Lymphoma Database (n = 89), and the University of Athens (n = 21). The IELSG cohort included patients from three studies: a multicenter, randomized trial of chlorambucil versus chlorambucil plus rituximab versus rituximab alone in extranodal MALT lymphoma (IELSG 19) [9]; a retrospective evaluation of primary head and neck lymphoma (IELSG 23); and a retrospective study of patients with low-grade MALT lymphoma primarily arising at nongastric sites (IELSG 1) [8]. The Mayo Clinic Lymphoma Database is a prospective database of patients who have been followed at the institution since 1987. The clinical characteristics and outcomes of 20 patients from the University of Athens cohort have been previously described [7].
All patients had a biopsy-confirmed diagnosis of MALT lymphoma of the salivary gland. Central review of pathology specimens was performed in 221 patients from the Mayo Clinic, University of Athens, and IELSG 1 and 19 cohorts (89%). Patients with other low-grade histologies or transformation to diffuse large B-cell lymphoma at diagnosis were excluded (n = 3). Clinical characteristics, treatment, recurrence, and survival were extracted from patient databases. Clinical staging was based on the Ann Arbor classification with the following exception [10]: Bilateral involvement of the same salivary gland was considered stage I in the absence of lymph node or other sites of extranodal involvement, as per Union for International Cancer Control guidelines [11]. Response assessment was by standard criteria [12].
Statistical Analyses
The endpoints of interest for statistical analysis were progression-free survival (PFS) and OS. Survival probability was estimated using the Kaplan-Meier method and compared between groups with the log-rank test. The Cox proportional hazards model was used to assess the impact of variables on survival. Two-sided Fisher exact tests were used to test for differences between categorical variables. Two-sided Wilcoxon rank sum tests were used to compare continuous variables. Data were analyzed using JMP Pro 9.0.1 (SAS, Inc., Cary, NC; http://www.sas.com).
Results
Patients and Characteristics
From 1983 to 2012, 247 patients with MALT lymphoma were identified. The clinical characteristics are presented in Table 1. The median age at diagnosis was 59 years. The ratio of female patients to male patients was 3:1. The majority of patients presented with localized (59%) or locoregional (17%) disease. The parotid gland was the most common site of involvement (78%). Twenty-one patients (8.5%) had bilateral salivary gland involvement. Of these, four had disease confined to the parotids, eight had locoregional disease, and the remainder had disseminated disease involving multiple sites. Fifty-one percent of patients had a low international prognostic index (IPI) score at the time of presentation, and only one patient presented with an Eastern Cooperative Oncology Group performance status >1. The median follow-up time was 55 months (range: 1–276; interquartile range: 32–94).
Table 1.
Characteristics of 247 patients with salivary mucosa-associated lymphoid tissue lymphoma
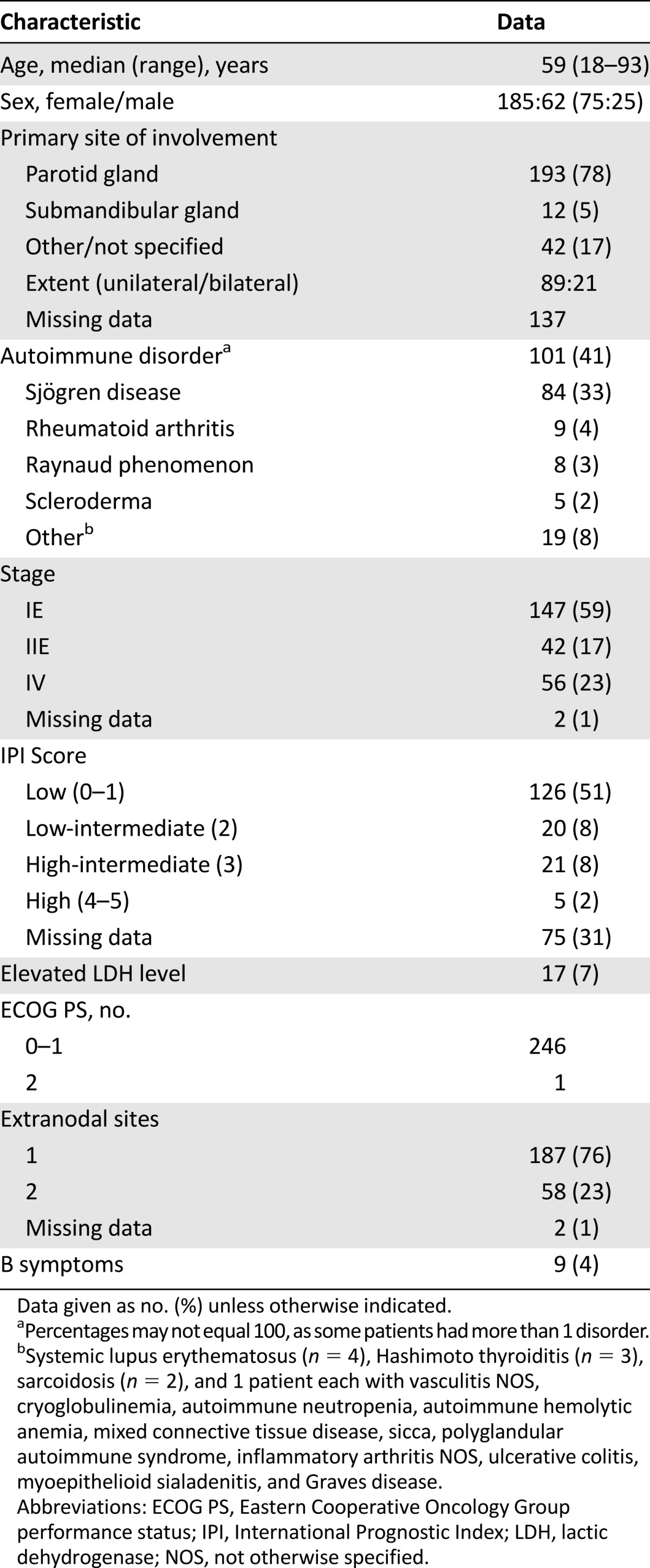
Forty-one percent of patients (n = 101) had a history of an antecedent autoimmune disorder, and one-fifth of these (n = 20) had multiple autoimmune conditions. Sjögren disease was the most frequently reported (33% of all patients). Rheumatoid arthritis was the next most common association (4%). Other less frequently reported conditions are outlined in Table 1.
Initial Treatment
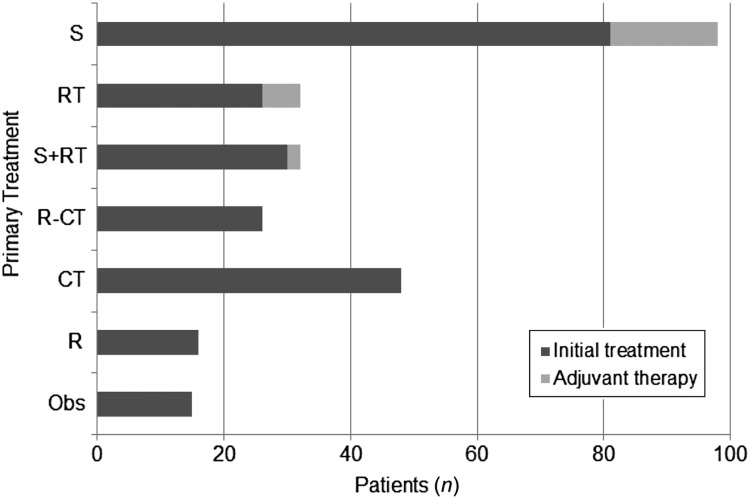
Information on initial therapeutic intervention was available on 242 of 247 patients (98%) (Fig. 1). Of 242 patients, 137 (57%) initially received local therapy consisting of surgery (n = 81), radiation (n = 26), or both (n = 30). Adjuvant chemotherapy was administered in 18% (n = 25). Rituximab was added in six patients.
Figure 1.
Initial treatment of patients with mucosa-associated lymphoid tissue lymphoma.
Abbreviations: CT, chemotherapy; Obs, observation; R, rituximab; RT, radiation; S, surgery.
Ninety of 242 patients (37%) were initially treated with systemic therapy, including 54% with localized and 46% with stage IV disease. The regimens used were heterogeneous. An alkylator as monotherapy (n = 30) or in combination with rituximab (n = 11) were the most common regimens (45%). Treatment was anthracycline based in 17 patients (19%). Rituximab monotherapy (n = 16) or immunochemotherapy (n = 26) was incorporated in 42 of the 90 (47%). Fifteen patients (6%) were initially observed.
Survival
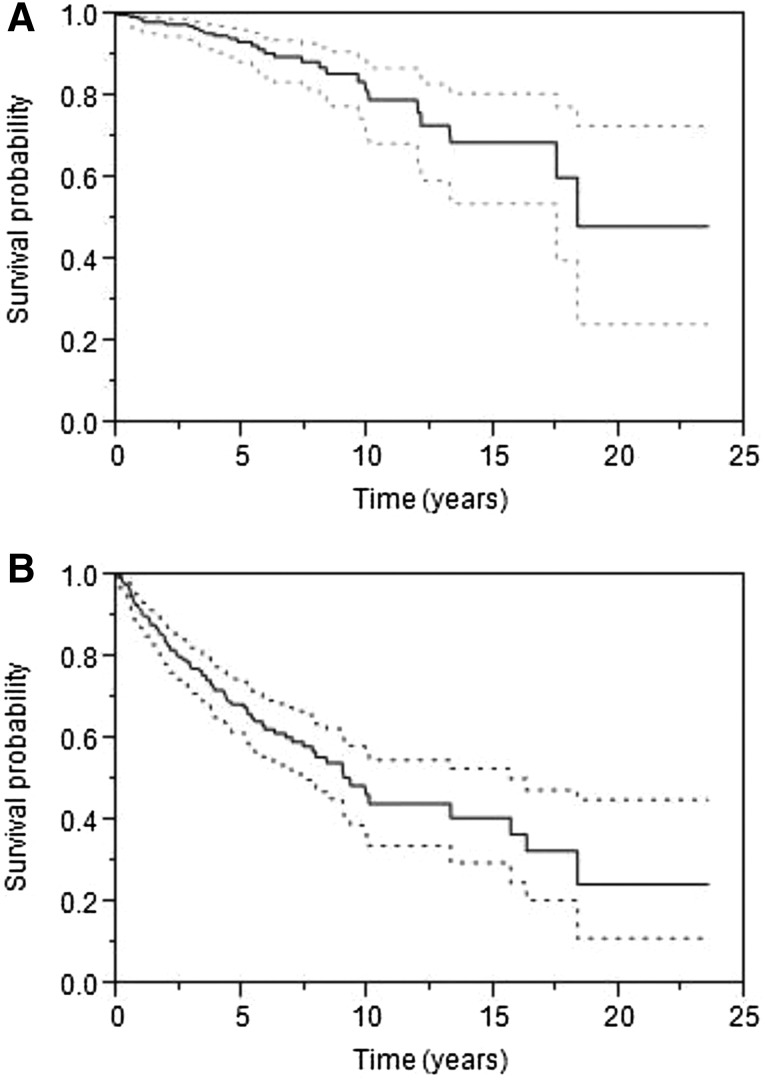
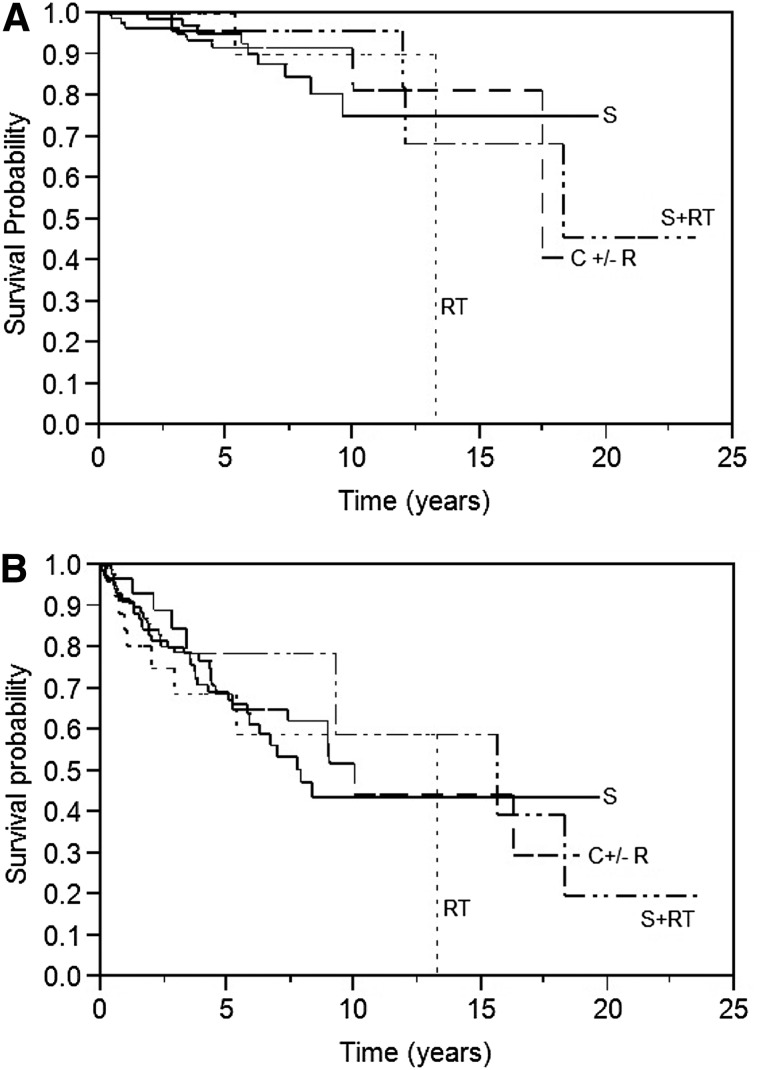
The median OS for all patients was 18.3 years, and PFS following initial therapeutic intervention was 9.3 years (Fig. 2). There was no difference in the PFS or OS in patients receiving surgery, radiation, or systemic therapy as first-line therapy (Fig. 3). However, patients whose initial treatment included rituximab had an increased OS (median not yet reached, p = .03). Survival in patients with stage I or II disease was not impacted by initial therapy. Among patients who underwent surgery, the median PFS was 8.3 years and the median OS was not reached. There was no difference in OS or PFS of those who underwent complete excision of the involved gland (n = 59) versus those who had a partial resection (n = 33). The type of surgery was not recorded in 19 patients.
Figure 2.
Survival for all patients. (A): Median overall survival was 18.3 years (95% confidence interval [CI]: 17.4–not reached). (B): Median progression-free survival was 9.2 years (95% CI: 7.4–15.6 years).
Figure 3.
Survival by initial treatment. (A): Overall survival, (B): Progression-free survival.
Abbreviations: C, chemotherapy; R, rituximab; RT, radiation; S, surgery.
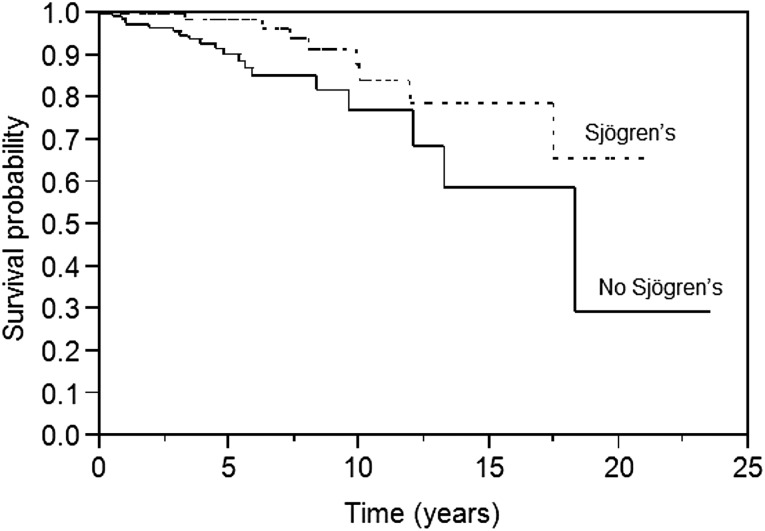
The presence of Sjögren disease, but not all autoimmune conditions, was associated with an improvement in OS (p = .03) (Fig. 4). Other factors that were associated with an improved OS included age <60 years, stage, IPI, and normal lactate dehydrogenase (LDH) level. On multivariate analysis, age <60 years, IPI, and Sjögren disease remained significant. Age <60 years, low to intermediate IPI, and normal LDH level were associated with improved PFS, while age <60 years and IPI retained significance on multivariate analysis.
Figure 4.
Overall survival in patients with and without Sjögren disease (p = .03).
Relapse/Progression
A total of 76 patients (31%) had progressive disease after a median follow-up of 42 months (range: 4–204 months). The site of progression was reported in 51 of 76 patients (67%). Fifteen patients (29%) progressed in the same salivary gland or regional nodes, 16 recurrences (21%) involved the contralateral salivary gland, and 20 patients (31%) had distant progression. Relapse histology was identified in 30 patients (39%). Twenty-eight of 30 patients (93%) relapsed with MALT, 1 patient progressed to diffuse large B-cell lymphoma, and a second patient developed a mantle cell lymphoma. In this case, the original MALT lymphoma was λ light-chain restricted, while the mantle cell lymphoma was κ light-chain restricted, consistent with an independent primary lymphoma.
Discussion
This international series confirms that salivary gland MALT lymphomas have a favorable clinical course independent of the type of initial therapy used. This large experience demonstrates that there was no difference in PFS or OS among patients treated initially with surgery, radiation, chemotherapy, or chemoimmunotherapy. Further, in the subsets of patients with stage I or stage II disease, there was no benefit observed for local therapy with either radiation or surgery over systemic therapy. OS was improved in patients with Sjögren disease and in patients who were treated with rituximab.
Because patients have excellent outcomes regardless of initial treatment strategy, the side-effect profile and potential long-term complications of therapy must be considered when individualizing treatment to a particular patient. Parotidectomy results in scarring and long-term alterations in appearance. Other recognized potential risks include temporary or permanent injury to the facial nerve, sensory loss, auriculotemporal nerve syndrome, wound infection, sialocele, and fistula [13, 14]. Radiation therapy may result in xerostomia and accompanying secondary effects such as altered appetite, impaired swallowing, and increased dental caries [15]. In one study of radiotherapy in MALT lymphoma patients, all of those with salivary gland MALT had symptomatic xerostomia, which required permanent dietary modification, including frequent sips of water [16]. The risk for potential long-term local toxicities must be balanced against the side effects of cytotoxic therapy, as the majority of these patients will survive many years.
Interestingly, an improvement in OS was observed in patients who received rituximab as part of their initial therapy, either alone in combination with chemotherapy or in an adjuvant fashion following surgery or radiation. However, there was no observed benefit in PFS. The reason for this finding is not clear. Given that patients receiving rituximab would have been treated more recently, part of the difference could be reflective of better supportive care in the modern era. Further prospective studies including rituximab are necessary to clarify its role in the treatment of patients with salivary MALT lymphomas.
Sjögren disease is a well-established risk factor for the development of salivary gland MALT lymphomas [4, 5]. In the present study, patients with Sjögren disease had an improved OS (p = .03) when compared with patients without a history of this disease. Presumably, lymphomagenesis in these patients is related to chronic immune stimulation and dysregulation. The difference in outcomes of patients without underlying autoimmune dysfunction may be reflective of differing biology leading to the development of MALT lymphomas in this population.
Conclusion
The present study represents the largest international study of patients with salivary gland MALT lymphomas and provides important information on the outcomes and natural history of the disease. The long life expectancy and favorable outcomes regardless of initial therapeutic interventions suggest that anticipated long-term complications of treatment should be the primary consideration in determining initial approaches in salivary gland MALT lymphoma. Incorporation of rituximab into initial treatment may be beneficial, and its use in the treatment of salivary gland MALT lymphoma should be further explored in prospective studies.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
This study was partly supported by the Swiss Cancer League/Oncosuisse (grants SKL 652-2-1998, ICP OCS-01356-03-2003 and ICP OCS-02062-03-200) and the Mayo Clinic Division of Hematology. We thank the following IELSG investigators who enrolled patients into the IELSG 1, 19, and/or 23 studies: A. Van Hoof (Brugge, Belgium); A. Kentos (Bruxelles, Belgium); C. Haioun (Creteil, France); R. Boubdallah (Marseille, France); B. Coiffier (Lyon, France); O. Casasnovas (Dijon, France); B. Mahe (Nantes, France); M. Sertoli (Genova, Italy); C. Stelitano (Reggio Calabria, Italy); U. Vitolo (Torino, Italy); G. Rossi and A. Tucci (Brescia, Italy); D. Caballero (Salamanca, Spain); A. McMillan (Nottingham, U.K.); M. Pocock (Devon, U.K.); S. Sadullah (Norfolk, U.K.); A. Kruger (Cornwall, U.K.); K. Ardeshna (Middlesex, U.K.); R. Pettengell (London, U.K.); B.W. Hancock (Sheffield, U.K.); A.J.M. Ferreri (Milan, Italy); L. Baldini (Milan, Italy); L. Devizzi and C.A. Tondini (Milan, Italy); G. Pinotti (Varese, Italy); M. Magagnoli (Bologna, Italy); M. Gospodarowicz (Toronto, Canada); E. Cabrera (Santiago, Chile); M. Busetto (Mestre, Italy); E.M. Pogliani (Monza, Italy).
The data from this study were presented in abstract form at the December 2014 American Society of Hematology annual meeting in San Francisco, CA (Abstract ID #1637).
Author Contributions
Conception/Design: Amie E. Jackson, Anastasios Stathis, Annarita Conconi, Sergio Cortelazzo, Gianluca Gaidano, Armando Lopez Guillermo, Peter W. Johnson, Maurizio Martelli, Giovanni Martinelli, Catherine Thieblemont, Ellen D. McPhail, Thomas M. Habermann, Franco Cavalli, Grzegorz Nowakowski, Emanuele Zucca
Collection and/or assembly of data: Michael Mian, Christina Kalpadakis, Gerassimos A. Pangalis, Anastasios Stathis, Elena Porro, Annarita Conconi, Sergio Cortelazzo, Gianluca Gaidano, Armando Lopez Guillermo, Peter W. Johnson, Maurizio Martelli, Giovanni Martinelli, Catherine Thieblemont, Ellen D. McPhail, Christiane Copie-Bergman, Stefano A. Pileri, Andrew Jack, Elias Campo, Luca Mazzucchelli, Kay Ristow, Thomas M. Habermann, Franco Cavalli, Grzegorz Nowakowski, Emanuele Zucca
Data analysis and interpretation: Amie E. Jackson, Anastasios Stathis, Annarita Conconi, Sergio Cortelazzo, Gianluca Gaidano, Armando Lopez Guillermo, Peter W. Johnson, Maurizio Martelli, Giovanni Martinelli, Catherine Thieblemont, Ellen D. McPhail, Thomas M. Habermann, Franco Cavalli, Grzegorz Nowakowski, Emanuele Zucca
Manuscript Writing: Amie E. Jackson
Final approval of manuscript: Amie E. Jackson, Michael Mian, Christina Kalpadakis, Gerassimos A. Pangalis, Anastasios Stathis, Elena Porro, Annarita Conconi, Sergio Cortelazzo, Gianluca Gaidano, Armando Lopez Guillermo, Peter W. Johnson, Maurizio Martelli, Giovanni Martinelli, Catherine Thieblemont, Ellen D. McPhail, Christiane Copie-Bergman, Stefano A. Pileri, Andrew Jack, Elias Campo, Luca Mazzucchelli, Kay Ristow, Thomas M. Habermann, Franco Cavalli, Grzegorz Nowakowski, Emanuele Zucca
Disclosures
Gianluca Gaidano: Roche, Novartis, Janssen (C/A), Pfizer (RF); Stefano A. Pileri: Takeda Millennium (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Swerdlow SHCE, Harris NL, Jaffe ES et al., eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Systems. 4th ed. Lyon, France: IARC, 2008.
- 2.Zucca E, Roggero E, Bertoni F, et al. Primary extranodal non-Hodgkin’s lymphomas. Part 2: Head and neck, central nervous system and other less common sites. Ann Oncol. 1999;10:1023–1033. doi: 10.1023/a:1008313229892. [DOI] [PubMed] [Google Scholar]
- 3.Vazquez A, Khan MN, Sanghvi S, et al. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue of the salivary glands: A population-based study from 1994 to 2009. Head Neck. 2015;37:18–22. doi: 10.1002/hed.23543. [DOI] [PubMed] [Google Scholar]
- 4.Anacak Y, Miller RC, Constantinou N, et al. Primary mucosa-associated lymphoid tissue lymphoma of the salivary glands: A multicenter Rare Cancer Network study. Int J Radiat Oncol Biol Phys. 2012;82:315–320. doi: 10.1016/j.ijrobp.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 5.Royer B, Cazals-Hatem D, Sibilia J, et al. Lymphomas in patients with Sjogren’s syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses. Blood. 1997;90:766–775. [PubMed] [Google Scholar]
- 6.Thieblemont C, Berger F, Dumontet C, et al. Mucosa-associated lymphoid tissue lymphoma is a disseminated disease in one third of 158 patients analyzed. Blood. 2000;95:802–806. [PubMed] [Google Scholar]
- 7.Kalpadakis C, Pangalis GA, Vassilakopoulos TP, et al. Non-gastric extra-nodal marginal zone lymphomas—a single centre experience on 76 patients. Leuk Lymphoma. 2008;49:2308–2315. doi: 10.1080/10428190802510331. [DOI] [PubMed] [Google Scholar]
- 8.Zucca E, Conconi A, Pedrinis E, et al. Nongastric marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Blood. 2003;101:2489–2495. doi: 10.1182/blood-2002-04-1279. [DOI] [PubMed] [Google Scholar]
- 9.Zucca E, Conconi A, Laszlo D, et al. Addition of rituximab to chlorambucil produces superior event-free survival in the treatment of patients with extranodal marginal-zone B-cell lymphoma: 5-year analysis of the IELSG-19 Randomized Study. J Clin Oncol. 2013;31:565–572. doi: 10.1200/JCO.2011.40.6272. [DOI] [PubMed] [Google Scholar]
- 10.Moormeier JA, Williams SF, Golomb HM. The staging of non-Hodgkin’s lymphomas. Semin Oncol. 1990;17:43–50. [PubMed] [Google Scholar]
- 11.Wittekind CH, Henson DE, Hutter RPV et al., eds. UICC-TNM Supplement. A Commentary on Uniform Use. 2nd ed. New York, NY: Wiley-Liss Publishers; 2001.
- 12.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 13.Upton DC, McNamar JP, Connor NP, et al. Parotidectomy: Ten-year review of 237 cases at a single institution. Otolaryngol Head Neck Surg. 2007;136:788–792. doi: 10.1016/j.otohns.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 14.Nitzan D, Kronenberg J, Horowitz Z, et al. Quality of life following parotidectomy for malignant and benign disease. Plast Reconstr Surg. 2004;114:1060–1067. doi: 10.1097/01.prs.0000135326.50939.c1. [DOI] [PubMed] [Google Scholar]
- 15.Eneroth C-M, Henrikson CO, Jakobsson PA. Effect of fractionated radiotherapy on salivary gland function. Cancer. 1972;30:1147–1153. doi: 10.1002/1097-0142(197211)30:5<1147::aid-cncr2820300502>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Tsang RW, Gospodarowicz MK, Pintilie M, et al. Stage I and II MALT lymphoma: results of treatment with radiotherapy. Int J Radiat Oncol Biol Phys. 2001;50:1258–1264. doi: 10.1016/s0360-3016(01)01549-8. [DOI] [PubMed] [Google Scholar]