In premenopausal women affected by early breast cancer who need adjuvant cytotoxic regimens, the proposed nomogram can assess the personal probability of maintaining ovarian activity at 1 year from the end of chemotherapy. The ongoing validation process is evaluating pretreatment age and anti-Müllerian hormone serum levels and other key factors contributing to post-treatment ovarian activity and will confirm the nomogram’s reliability and clinical utility.
Keywords: Anti-Müllerian hormone, Ovarian reserve, Breast cancer, Ovarian toxicity, Predictive factors
Abstract
Background.
The assessment of ovarian reserve in premenopausal women requiring anticancer gonadotoxic therapy can help clinicians address some challenging issues, including the probability of future pregnancies after the end of treatment. Anti-Müllerian hormone (AMH) and age can reliably estimate ovarian reserve. A limited number of studies have evaluated AMH and age as predictors of residual ovarian reserve following cytotoxic chemotherapy in breast cancer patients.
Materials and Methods.
To conduct a meta-analysis of published data on this topic, we searched the medical literature using the key MeSH terms “amenorrhea/chemically induced,” “ovarian reserve,” “anti-Mullerian hormone/blood,” and “breast neoplasms/drug therapy.” Preferred Reporting Items for Systematic Reviews and Meta-Analyses statements guided the search strategy. U.K. National Health Service guidelines were used in abstracting data and assessing data quality and validity. Area under the receiver operating characteristic curve (ROC/AUC) analysis was used to evaluate the predictive utility of baseline AMH and age model.
Results.
The meta-analysis of data pooled from the selected studies showed that both age and serum AMH are reliable predictors of post-treatment ovarian activity in breast cancer patients. Importantly, ROC/AUC analysis indicated AMH was a more reliable predictor of post-treatment ovarian activity in patients aged younger than 40 years (0.753; 95% confidence interval [CI]: 0.602–0.904) compared with those older than 40 years (0.678; 95% CI: 0.491–0.866). We generated a nomogram describing the correlations among age, pretreatment AMH serum levels, and ovarian activity at 1 year from the end of chemotherapy.
Conclusion.
After the ongoing validation process, the proposed nomogram may help clinicians discern premenopausal women requiring cytotoxic chemotherapy who should be considered high priority for fertility preservation counseling and procedures.
Implications for Practice:
In general, a nomogram helps clinicians better visualize a specific risk for a single patient. In premenopausal women affected by early breast cancer who need adjuvant cytotoxic regimens, the proposed nomogram—based on the assessment of pretreatment age and anti-Müllerian hormone serum levels—can assess the personal probability of maintaining ovarian activity at 1 year from the end of chemotherapy. The ongoing validation process is also evaluating other key factors contributing to post-treatment ovarian activity (i.e., type of cytotoxic regimen) and will confirm the nomogram’s reliability and clinical utility.
Background
Breast cancer (BC) is the most common malignancy in premenopausal women [1, 2]. Due to the efficacy of anticancer treatments and early diagnosis, there are currently several millions of BC survivors worldwide [3]. In premenopausal BC patients, however, cytotoxic anticancer agents may cause various side effects including ovarian dysfunction, a condition that can manifest as oligomenorrhea, transient amenorrhea, and even early menopause [4]. Damaged fertility, sexual dysfunction, and bone loss are among the most significant consequences of ovarian toxicity that can impair the physical and psychosocial well-being of these women [5]. Residual ovarian activity (OA) following anticancer treatments may be affected by patient-related factors, such as patient age and actual ovarian reserve at the beginning of treatment, and treatment-related factors, such as treatment gonadotoxicity (i.e., type of cytotoxics and drug cumulative dose). Genetic and environmental factors and BC itself are also suggested to influence ovarian reserve [6, 7]. The assessment of ovarian reserve in premenopausal women who need gonadotoxic agents as anticancer treatment can help clinicians address some challenging issues, including the probability of future pregnancy [8]. In patients affected by endocrine-responsive early breast cancer, reliable markers of ovarian reserve might help discriminate transient amenorrhea occurring after cytotoxic chemotherapy (CT) from the onset of menopause [9]. This also might avoid the inappropriate prescription of aromatase inhibitors that are contraindicated in BC patients with residual OA [10, 11]. Radiological and biochemical assessments currently provide an estimate of ovarian reserve [12]. Ovary ultrasound examination, the most commonly used imaging assessment, measures ovarian volume and other parameters and counts primordial ovarian follicles [13]. Biochemical estimation of ovarian reserve is based on the assessment of ovarian endocrine activity provided by serum levels of anti-Müllerian hormone (AMH), follicle-stimulating hormone (FSH), 17-β-estradiol (E2), and inhibin-B [14]. Such hormonal assessment can currently estimate residual ovarian reserve in healthy women and their fertility potential and age at menopause [15–21]. In recent years, AMH has emerged as the most reliable marker of ovarian reserve in healthy women and in cancer patients [22, 23]. In patients affected by early BC (EBC), a limited number of studies evaluated serum markers of ovarian endocrine activity as predictive factors of residual ovarian reserve after CT [24, 25]. In this paper, we report the results of a meta-analysis of data pooled from studies selected from a systematic review of the literature. The analysis was aimed at defining whether pretreatment age and serum levels of AMH can predict future OA in patients who are treated with CT for the cure of EBC. Finally, a nomogram has been proposed as a useful tool in predicting the probability of maintaining OA at 1 year from the end of CT (EoC) in this subgroup of patients.
Methods
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements guided the search strategy for identifying studies of interest [26]. Trials were considered valid and included in the analysis when the effect of CT on OA, defined as post-CT amenorrhea (chemotherapy-related amenorrhea [CRA]) and/or recovery of OA, was clearly reported and correlated with serum levels of endocrine serum markers of ovarian reserve (at least AMH) in patients who received CT for EBC. The literature search was conducted by using PubMed, limiting the search to articles in English. The key MeSH terms were (“amenorrhea/chemically induced”) or (“ovarian reserve”) or (“anti-Mullerian hormone/blood” or “inhibins/blood” or “ovary/physiology”) and (“breast neoplasms/drug therapy”) and (“English” [language]). The article eligibility was assessed by full-text reading. Reviews were excluded, but reference lists of articles were screened for additional articles. The process was carried out in December 2014 by two independent researchers, and a third researcher settled disputes, as suggested by a U.K. National Health Service report on systematic reviews [27]. Disagreements were resolved by discussion with the other authors, and judgment was based on consensus.
Type of Studies, Participants, Outcome Measures, and Reference Variables
Prospective and retrospective observational studies were considered eligible. Studies were also considered eligible only when all variables of interest (age, CT regimen, pre-CT serum AMH values, CRA, or OA) were reported and data could be extracted from tables or figures. Participants were premenopausal women affected by EBC who underwent adjuvant or neoadjuvant CT followed or not by endocrine therapy. Concomitant CT/endocrine treatment was not permitted. In the analysis, CRA or OA (i.e., recovered or ongoing menstrual activity, or estradiol >10 pg/mL) were the alternative outcome measures. Pretreatment age and AMH serum levels were the reference variables.
Data Collection
Data were extracted from the selected trials and registered, including design, sample size, methods of assessment of serum markers, characteristics of participants, interventions, and outcome measures. For each patient, age and values of endocrine serum markers before starting CT were collected with the outcome measures CRA and/or OA at 1 year from EoC (supplemental online Tables 1, 2). Individual patient data of interest were extracted directly from tables or by using WinDig software (http://www.unige.ch/sciences/chifi/cpb/windig.html) when data were reported in a figure.
Data Analysis
For each patient, the baseline serum AMH value was correlated with age and 1-year OA or 1-year CRA. Moreover, to assess the strength of a predictive approach based on AMH serum levels and the heterogeneity observed in the selected studies, box plots of baseline AMH values were generated by grouping patients by outcome, by author, and by two age subgroups (≥40 vs. <40 years), according to other reports [28–33].
The 1-year OA was used as the gold standard for nonparametric clustered receiver operating characteristic (ROC) analysis to evaluate the predictive utility of the baseline AMH and age model. By comparing observed and calculated OA, sensitivity and specificity were plotted in ROC form [34]. When a perfect correlation of predicted versus observed OA was found, the area under curve (AUC) was equal to 1, whereas random assignment of outcome led to a ROC/AUC of 0.5 [35]. For ROC analysis, 1,000 validation data sets were generated adopting the bootstrap approach. Multivariate analysis of the predictive factors was performed using the logistic regression model including the covariates (i.e., baseline AMH and age) as continuous variables. A linear regression model was used to identify predictors of OA and was presented as a nomogram. Differences between groups were calculated by using a two-tailed t test. The data analysis was performed with R version 2.15 [36].
Results
Description of Studies
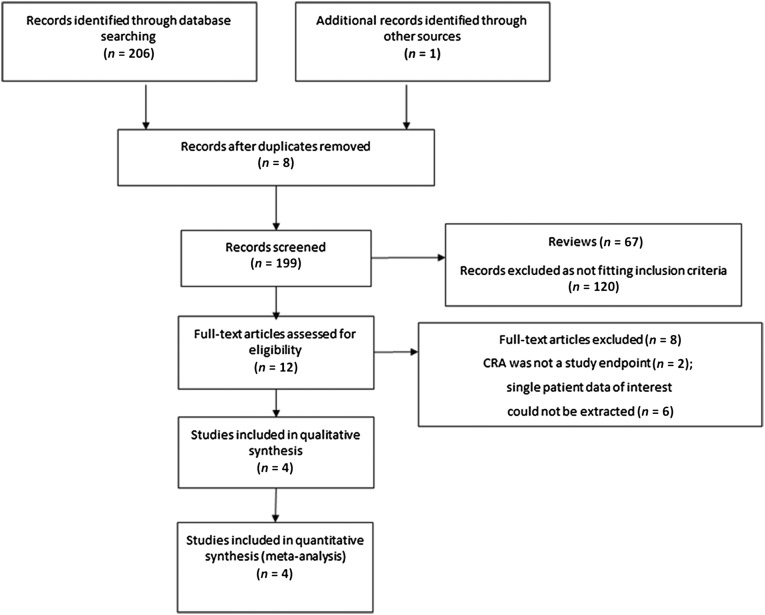
Among 207 articles identified through the database search, 8 were excluded as duplicates. Of the 199 articles screened, 187 were excluded because they were reviews (n = 67) or did not fit the inclusion criteria (n = 120). Of the 12 eligible articles, 8 were excluded because CRA was not the study endpoint (n = 2) or single-patient data of interest could not be extracted (n = 6). Consequently, data from four studies were included in the meta-analysis [32, 37–39]. Reasons for excluding studies were reported in the PRISMA diagram workflow (Fig. 1). Evaluations did not differ between reviewers. The characteristics of the studies that fit the inclusion criteria are summarized in supplemental online Table 1. In the four selected studies, 218 patients were evaluable. All patients were premenopausal, received adjuvant CT due to EBC (followed by endocrine therapy, in the case of endocrine-responsive disease), and were observed for 1–2 years. Only one of these studies presented values of all serum markers of ovarian reserve (i.e., FSH, AMH, inhibin-B, estradiol) as extractable data. In the others, only pre-CT AMH serum levels, age, and 1-year OA were reported in detail; therefore, we limited correlative evaluations to AMH serum levels and the aforementioned parameters. In the selected studies, CRA was recorded at 1 and 1.5 or 2 years after EoC; however, because differences in CRA rate at 1 year compared with 1.5 or 2 years were negligible, 1-year CRA and 1-year OA were used as outcome measures.
Figure 1.
The meta-analysis flow diagram (according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses, PRISMA 2009) [26].
Abbreviation: CRA, chemotherapy-related amenorrhea.
Characteristics of the Study Population
Individual data from 176 of 218 patients (81.2%) were extracted from the selected trials. The frequency of patients among different age subgroups was calculated and reported in Table 1. In this new large virtual cohort of patients, median age was 44 years (range: 23–55 years), and the 25th–75th percentiles were 39–48 years.
Table 1.
Characteristics of patients included in the meta-analysis

Data regarding the type or duration of CT delivered to each patient were not available. The majority (175 of 223, 78.5%) of patients received a 3-drug CT-regimen including cyclophosphamide, anthracyclines, and taxanes. Only 10 patients (4.5%) received cyclophosphamide and taxane-containing regimens (supplemental online Table 2). At 1 year after EoC, CRA was recorded in 125 patients (79.6%), whereas OA was observed in 32 patients (20.4%). Tamoxifen was used by 118 patients (67%), with the others presumably having estrogen/progesterone receptor-negative BC (supplemental online Table 1).
In three of four trials [32, 38, 39] AMH serum levels were assessed using a second-generation enzyme-linked immunosorbent assay. AMH was measured in nanograms per milliliter in three of four trials [32, 37, 39] and in picomoles per liter in another [38], but data were appropriately converted (1.1 pmol/L = 0.16 ng/mL) [40].
Correlative Findings
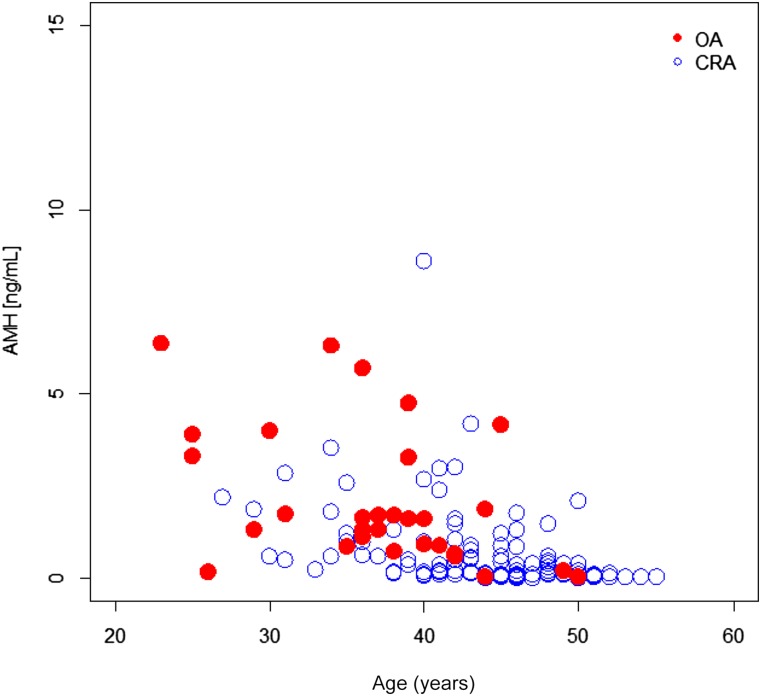
In one study [32], patient age at the beginning of CT (n = 19) was not reported; therefore, correlations including age were based on data from 157 patients. In this cohort, baseline AMH values decreased with age independently of 1-year OA (Fig. 2). Overall, baseline AMH values were higher in patients who showed recovery of OA or whose menses never stopped compared with those who did not recover or whose menses stopped (p = .00017). Notably, dispersion of AMH values appeared to be dependent on age (Fig. 2; supplemental online Figs. 1, 2). With the exception of the study by Rosendahl et al. [37] (only three patients eligible for the analysis), median and range values of AMH were higher in patients who showed 1-year OA compared with values found in the CRA group (supplemental online Fig. 1).
Figure 2.
Baseline AMH values against patients age based on ovarian activity or chemotherapy-related amenorrhea.
Abbreviations: AMH, anti-Müllerian hormone; CRA, chemotherapy-related amenorrhea; OA, ovarian activity.
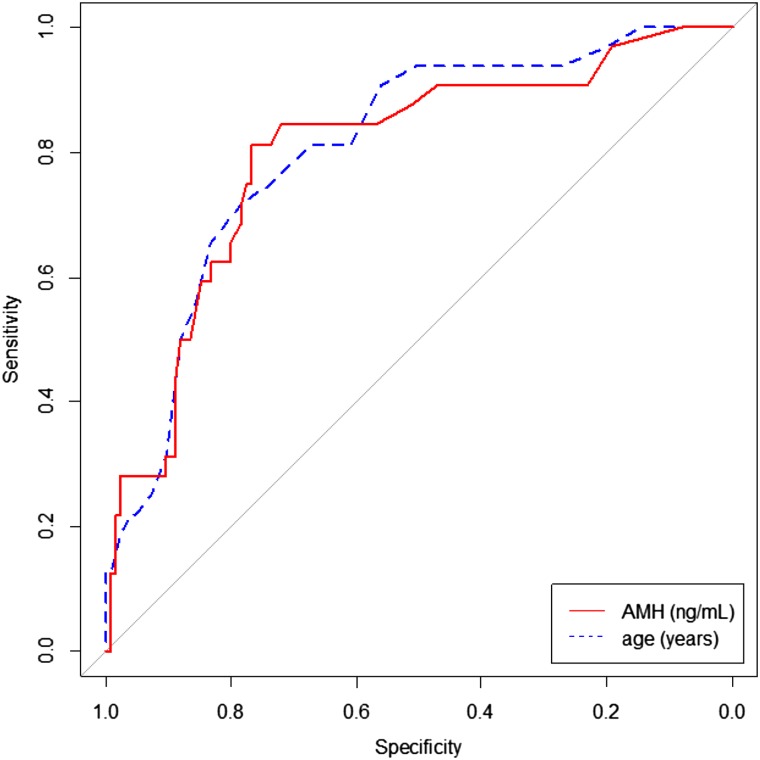
AUC was calculated for the whole group and for the two age subgroups of patients (<40 vs. ≥40 years). The AUC for the whole group was 0.797 (95% confidence interval [CI]: 0.707–0.887) [35]. The AUC for the group younger than 40 years was 0.753 (95% CI: 0.602–0.904), but the AUC for the group older than 40 years was 0.678 (95% CI: 0.491–0.866) (Fig. 3). These results suggest that AMH is a more reliable predictor of 1-year OA in patients younger than 40 years than in older patients.
Figure 3.
Received operating characteristic curves testing AMH and age as predictors of ovarian activity at 1 year from the end of chemotherapy.
Abbreviation: AMH, anti-Müllerian hormone.
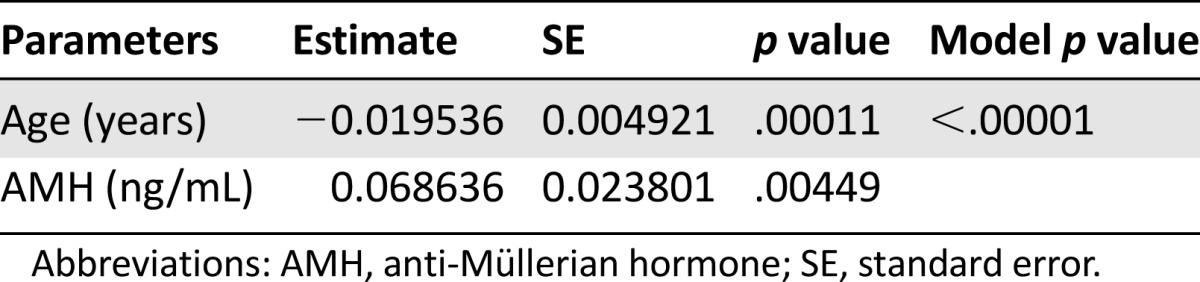
Multivariate logistic regression analysis confirmed that for the whole group (n = 157), both AMH and age were significantly and independently related to post-treatment 1-year OA (Table 2).
Table 2.
Multivariate logistic regression of the probability of maintaining ovarian activity at 1 year from the end of chemotherapy in the whole group (n = 157)
Finally, results of multivariate analysis are presented as a nomogram (Fig. 4) in which AMH values (in nanograms per milliliter) and patient age are input data to assess a score that could be used in predicting the probability of maintaining OA at 1 year after EoC. Higher scores were obtained in patients with higher AMH values and at younger ages.
Figure 4.
Nomogram model developed to predict current ovarian activity at 1 year from the end of chemotherapy (EoC) in premenopausal patients with early breast cancer. The result for each variable (anti-Müllerian hormone, age at the beginning of chemotherapy) has a corresponding point score (top scale). The score for each variable is determined by summing the total points for a given patient. This value is located on the total point scale (second from the bottom). The predicted probability of having current ovarian activity at 1 year from EoC is obtained by drawing a vertical line down from the total points to the probability scale below.
Abbreviation: AMH, anti-Müllerian hormone.
Discussion
The present study is based on a meta-analysis of data available from a selection of studies that prospectively evaluated baseline AMH and age as predictors of post-CT OA in premenopausal women with EBC. In the newly generated cohort of patients (n = 176), pre-CT serum AMH levels were higher in younger patients and in those showing 1-year OA (i.e., who never stopped or who recovered menses or who had E2 >10 pg/mL) compared with those with CRA (p = .00017). These results are consistent with those reported in the source studies and in studies of patients with EBC and other malignancies [22, 25].
AMH is now considered the most reliable measure of ovarian reserve in healthy women and in patients affected by various conditions, including those with anticancer agent-induced ovarian toxicity [23]. In BC patients, a rapid decrease in AMH levels following CT is invariably seen [37, 41–46]. In the pivotal study by Anderson et al. [41], AMH serum levels rapidly and markedly fell after the first cycles of CT and during the treatment, becoming undetectable in many patients. AMH serum levels may even increase again over time, but return to pre-CT levels appeared unlikely [37, 47–49]. In large case-control studies, BC survivors who underwent CT had lower AMH serum levels than age-matched controls [45, 46]. In the majority of studies, high pretreatment AMH levels were associated with higher post-treatment levels [37, 41, 44]. Similarly, pre-CT AMH was lower among women showing CRA at 1–2 years [38] and at later time points during the follow-up period [50]. Recent studies have shown that pre-CT AMH serum levels predict recovery of OA in reproductive-aged BC patients [32], independent of age [33]. Ruddy et al. [39] calculated that for every 1-year increase in age, there was a 20% increase in the odds of developing 12-month CRA and an 18% increase in the odds of developing 18-month CRA. Moreover, for every 1-ng/mL increase in AMH, there was a 59% decrease in the odds of developing 18-month CRA.
In the present study, we used 40 years as a “cut-off” age, based on recent studies aimed at evaluating predictive tools of post-CT OA [33]. ROC/AUC indicated that AMH was a more reliable predictor of OA in patients that were aged <40 years (0.753; 95% CI: 0.602–0.904) compared with those aged ≥40 years (0.678; 95% CI: 0.491–0.866) (Fig. 3). Given the pooled population, a larger number of patients who experienced return of menses was available. This enabled better investigation of the roles of age and AMH serum levels in predicting 1-year OA. Consequently, in the whole group (n = 157), both AMH and age were significantly and independently related to post-treatment OA at multivariate logistic regression analysis (Table 2). Interestingly, for the first time, baseline AMH serum levels showed a different strength in predicting post-treatment OA according to patient age.
These findings are consistent with current knowledge about the pathophysiology of ovarian reserve. In women, AMH is secreted by the granulosa cells in ovarian follicles during early stages of follicle development. In healthy women, AMH serum levels increase until early adulthood, decrease with increasing age, and become undetectable approximately 5 years before menopause, the time when the pool of primordial follicles is considered exhausted [51]. At each specific age, individual AMH serum concentration reflects the size of the antral follicle pool, representing the quantity of the remaining primordial follicles. This, in turn, provides a reliable estimation of the actual ovarian reserve and allows prediction of the residual length of a woman’s reproductive potential [51]. Various factors affect this estimation, including body mass index, smoking habits, endocrine disruptor exposure, and specific gene variants [52, 53]. Our results confirm that pre-CT AMH serum levels provide an accurate estimation of the post-CT ovarian reserve in patients with EBC. Its greater reliability in predicting post-treatment OA in younger women (i.e., aged <40 years) might be related to the fact that younger women usually have larger ovarian reserves than older women. Conversely, the lower ability of AMH in predicting residual OA in older women may be due to lower AMH serum levels at baseline.
In opposition to our findings, Anderson et al. showed that only AMH was a strong predictor of early and late ovarian function compared with age, FSH or luteinizing hormone, E2, and inhibin-B [38, 50]. The relatively low number of patients evaluated might have influenced results obtained in these trials. Alternatively, Ruddy et al. [39] showed that at 18 months from EoC, lower pre-CT AMH and older age were both statistically significant predictors of CRA. In a study by Henry et al. [32], both age and detectable levels of pre-CT AMH predicted recovery of 1-year OA at univariate but not multivariate analysis, presumably due to the limited number of enrolled patients. In our opinion, inconsistencies among studies may be resolved by looking at the predominant age of patients in each trial; however, because the complex links between age and decline in AMH in cancer patients are not yet fully elucidated, this hypothesis needs to be tested in large prospective trials.
Another key aim of our study was to create a valuable tool for predicting the individual risk of CRA, an emerging endeavor in oncology [33, 38]. For this aim, based on the available variables (i.e., age and AMH serum levels at the beginning of CT and 1-year OA), we initially evaluated different methods that have been used previously, including a mosaic chart, as suggested by Anderson et al. [38], and a nomogram. Because our study showed that age had a linear impact on the hazard of the event (CRA or recovery of OA), a nomogram appeared more appropriate in describing the correlation between the variables of interest. Nomograms are an established model-visualization technique that can graphically encode the complete model. The dimensional visualization of the model does not depend on the number of input variables but rather on the properties of the investigated model. Our nomogram model is the first analytical tool developed to predict OA at 1 year from completion of CT using a log-odds ratio scale in premenopausal patients who received adjuvant CT for EBC. A similar correlation between post-treatment ovarian function and AMH or age was suggested by Anderson et al. [38] and presented as a mosaic chart clustering patients in four groups. Su et al. [33] recently proposed a prognostic score based on three cut-off rates for pre-CT age (40 years), AMH (0.7 ng/mL), and body mass index (25 kg/m2) to estimate the time to recovery of menses after chemotherapy in BC patients [33]. Notably, our model presents the probability of maintaining ovarian function following CT using continuous-value scales and not age-discrete steps. At the multivariate logistic regression analysis, both age and AMH were predictive factors. Indeed, at the same AMH serum level, the probability of maintaining 1-year OA decreased as age increased. Moreover, at the same age, the probability of maintaining 1-year OA increased according to baseline AMH serum level. By following an appropriate validation process, the nomogram could be used to discern patients who should be considered high priority for fertility preservation counseling and procedures [54, 55].
Our study presents some limitations. The selected trials were heterogeneous in terms of patient ages, CT regimens, outcome measures (CRA, return of menses, estradiol >10 pg/mL), time when outcome measures were determined, and assessment method of AMH serum levels. Difference in patient age among the selected trials was rendered negligible by pooling data into a new cohort. Studies included in the meta-analysis evaluated OA at 1–2 years after EoC. We preferred 1-year OA because the larger study [39] and another [32] reported extractable data for 1-year OA. In the study by Anderson et al. [38], extractable data were referred at 2 years; however, the authors highlighted that the proportions of patients showing CRA or OA were very similar at 1 and 2 years. These approximations might allow extension of the predictive potential of our nomogram to 2 years, but a cautious approach was preferred. The impact of using different ELISA methods to assess AMH serum levels appeared minimal because extracted data were properly converted [40]. More important, we were able to correlate only age and AMH serum level at starting CT with 1-year OA for each patient. No information could be extracted from the selected trials on the CT regimen received by each patient, the cumulative dose of cytotoxic drugs, use of tamoxifen, body mass index, smoking habits, or any other relevant variables. All of these factors are known to affect residual ovarian reserve and the rate and duration of CRA [23]. Moreover, the reliability of 1-year OA as outcome measure may raise concerns. In cancer patients, 1-year CRA is widely used as a predictive factor of exhausted ovarian reserve. Accordingly, in the selected studies generating the new cohort of patients, a limited number of patients recovered menses during the follow-up period (i.e., 18–24 months). Although in the majority of studies recovery of menses occurs within 1 year, some patients may recover their menses within 3 years from EoC. Notably, whether taxanes increase CRA rates when added to anthracycline and cyclophosphamide-based regimens is still uncertain [56–58]. In terms of fertility, 1-year CRA and return of menses unreliably predict post-CT residual fertility potential [59]; however, pretreatment estimation of individual risk of residual ovarian reserve at the EoC could help tailor fertility preservation options for patients who require them. Another limitation may regard the data extraction process. The study data set was constructed based on an indirect extraction process. This approach may have reduced the level of accuracy of extracted values compared with values available as raw data; however, in a simulation test, differences in approximation of extracted values and actual data appeared negligible. Finally, overall results of meta-analysis may be biased by the fact that the majority of data came from a single large study (Ruddy et al. [39]). In contrast, the addition of data from smaller studies, which enrolled younger patients, to the larger one increased the number of events useful for the univariate and multivariate analysis, and this allowed better description of correlations between age and AMH values and 1-year OA. In particular, AMH values were statistically lower in the cohort of Ruddy et al. than in the remaining studies (0.82 vs. 1.53; p = .007, t test), whereas median age was similar among different cohorts. The ROC analysis and the nomogram plot for the two populations (Ruddy et al. vs. others) led to similar results (data not shown). Despite the large cohort obtained by pooling data, a still limited number of events (i.e., return of menses) were recorded. Consequently, our nomogram model needs to be validated prospectively in a larger data set of patients throughout an appropriate follow-up period (at least 3 years). Finally, in the pooled cohort, data on the CT regimen and the cumulative dose of gonadotoxic agents received by each patient are missing. In addition, the post-CT use of tamoxifen in this study was not available. All of these lacking data may have altered the power of the model and are being carefully considered in the ongoing validation process together with somatic (i.e., obesity), behavioral (i.e., smoke habits), and genetic factors influencing not only age at menopause but also metabolism of gonadotoxic drugs.
Conclusion
In this study, we generated the first nomogram model to predict current 1-year OA in premenopausal patients affected by EBC. The nomogram was derived from a meta-analysis process and is being prospectively validated. The nomogram could be a valuable tool for improving the care of premenopausal women needing CT for EBC by providing information on the personal risk of gonad toxicity and on the requirement of fertility-preservation procedures. Future studies will explore potential applications of this model as a tool that might help clinicians prescribe appropriate hormone agents for specific subgroups of patients, such as those with endocrine-responsive EBC and CRA.
See http://www.TheOncologist.com for supplemental material available online.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Supplementary Material
Author Contributions
Conception/Design: Agnese Barnabei, Lidia Strigari, Francesco Torino
Collection and/or assembly of data: Agnese Barnabei, Lidia Strigari, Valentina Sini, Francesco Torino
Data analysis and interpretation: Agnese Barnabei, Lidia Strigari, Paolo Marchetti, Liana De Vecchis, Salvatore Maria Corsello, Francesco Torino
Manuscript writing: Agnese Barnabei, Lidia Strigari, Salvatore Maria Corsello, Francesco Torino
Final approval of manuscript: Agnese Barnabei, Lidia Strigari, Paolo Marchetti, Valentina Sini, Liana De Vecchis, Salvatore Maria Corsello, Francesco Torino
Disclosures
Paolo Marchetti: Roche, Novartis, Astra Zeneca, Bristol-Myers Squibb (C/A), Pfizer, Novartis (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Cancer facts & figures 2014. Available at http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014/. Accessed January 21, 2015.
- 3.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Chapman RM. Effect of cytotoxic therapy on sexuality and gonadal function. Semin Oncol. 1982;9:84–94. [PubMed] [Google Scholar]
- 5.Howard-Anderson J, Ganz PA, Bower JE, et al. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: A systematic review. J Natl Cancer Inst. 2012;104:386–405. doi: 10.1093/jnci/djr541. [DOI] [PubMed] [Google Scholar]
- 6.Wood MA, Rajkovic A. Genomic markers of ovarian reserve. Semin Reprod Med. 2013;31:399–415. doi: 10.1055/s-0033-1356476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HR, Jeung EB, Cho MH, et al. Molecular mechanism(s) of endocrine-disrupting chemicals and their potent oestrogenicity in diverse cells and tissues that express oestrogen receptors. J Cell Mol Med. 2013;17:1–11. doi: 10.1111/j.1582-4934.2012.01649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson RA, Wallace WH. Antimüllerian hormone, the assessment of the ovarian reserve, and the reproductive outcome of the young patient with cancer. Fertil Steril. 2013;99:1469–1475. doi: 10.1016/j.fertnstert.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Torino F, Barnabei A, De Vecchis L, et al. Chemotherapy-induced ovarian toxicity in patients affected by endocrine-responsive early breast cancer. Crit Rev Oncol Hematol. 2014;89:27–42. doi: 10.1016/j.critrevonc.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Breast cancer, version 1.2015. Available at http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed January 19, 2015.
- 12.Lambalk CB, van Disseldorp J, de Koning CH, et al. Testing ovarian reserve to predict age at menopause. Maturitas. 2009;63:280–291. doi: 10.1016/j.maturitas.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Wallace WH, Kelsey TW. Ovarian reserve and reproductive age may be determined from measurement of ovarian volume by transvaginal sonography. Hum Reprod. 2004;19:1612–1617. doi: 10.1093/humrep/deh285. [DOI] [PubMed] [Google Scholar]
- 14.Nelson SM. Biomarkers of ovarian response: Current and future applications. Fertil Steril. 2013;99:963–969. doi: 10.1016/j.fertnstert.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 15.Broer SL, Eijkemans MJ, Scheffer GJ, et al. Anti-mullerian hormone predicts menopause: A long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab. 2011;96:2532–2539. doi: 10.1210/jc.2010-2776. [DOI] [PubMed] [Google Scholar]
- 16.Kelsey TW, Wright P, Nelson SM, et al. A validated model of serum anti-müllerian hormone from conception to menopause. PLoS One. 2011;6:e22024. doi: 10.1371/journal.pone.0022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almog B, Shehata F, Suissa S, et al. Age-related normograms of serum antimüllerian hormone levels in a population of infertile women: A multicenter study. Fertil Steril. 2011;95:2359–2363, 2363.e1. doi: 10.1016/j.fertnstert.2011.02.057. [DOI] [PubMed] [Google Scholar]
- 18.La Marca A, Spada E, Grisendi V, et al. Normal serum anti-Müllerian hormone levels in the general female population and the relationship with reproductive history. Eur J Obstet Gynecol Reprod Biol. 2012;163:180–184. doi: 10.1016/j.ejogrb.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Lie Fong S, Visser JA, Welt CK, et al. Serum anti-müllerian hormone levels in healthy females: A nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97:4650–4655. doi: 10.1210/jc.2012-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson SM, Iliodromiti S, Fleming R, et al. Reference range for the antimüllerian hormone Generation II assay: A population study of 10,984 women, with comparison to the established Diagnostics Systems Laboratory nomogram. Fertil Steril. 2014;101:523–529. doi: 10.1016/j.fertnstert.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Tehrani FR, Mansournia MA, Solaymani-Dodaran M, et al. Age-specific serum anti-Müllerian hormone levels: Estimates from a large population-based sample. Climacteric. 014;17:591–597. doi: 10.3109/13697137.2014.912262. [DOI] [PubMed] [Google Scholar]
- 22.Peigné M, Decanter C. Serum AMH level as a marker of acute and long-term effects of chemotherapy on the ovarian follicular content: A systematic review. Reprod Biol Endocrinol. 2014;12:26. doi: 10.1186/1477-7827-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dewailly D, Andersen CY, Balen A, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20:370–385. doi: 10.1093/humupd/dmt062. [DOI] [PubMed] [Google Scholar]
- 24.Torino F, Barnabei A, De Vecchis L, et al. Recognizing menopause in women with amenorrhea induced by cytotoxic chemotherapy for endocrine-responsive early breast cancer. Endocr Relat Cancer. 2012;19:R21–R33. doi: 10.1530/ERC-11-0199. [DOI] [PubMed] [Google Scholar]
- 25.Bozza C, Puglisi F, Lambertini M, et al. Anti-Mullerian hormone: Determination of ovarian reserve in early breast cancer patients. Endocr Relat Cancer. 2014;21:R51–R65. doi: 10.1530/ERC-13-0335. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Undertaking systematic reviews of research on effectiveness: CRD's guidance for carrying out or commissioning reviews. 2nd ed. CRD Report 4. York, U.K.: University of York, 2001.
- 28.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718–1729. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 29.Pagani O, O’Neill A, Castiglione M, et al. Prognostic impact of amenorrhoea after adjuvant chemotherapy in premenopausal breast cancer patients with axillary node involvement: Results of the International Breast Cancer Study Group (IBCSG) Trial VI. Eur J Cancer. 1998;34:632–640. doi: 10.1016/s0959-8049(97)10036-3. [DOI] [PubMed] [Google Scholar]
- 30.Goodwin PJ, Ennis M, Pritchard KI, et al. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999;17:2365–2370. doi: 10.1200/JCO.1999.17.8.2365. [DOI] [PubMed] [Google Scholar]
- 31.Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006;24:5769–5779. doi: 10.1200/JCO.2006.07.2793. [DOI] [PubMed] [Google Scholar]
- 32.Henry NL, Xia R, Schott AF, et al. Prediction of postchemotherapy ovarian function using markers of ovarian reserve. The Oncologist. 2014;19:68–74. doi: 10.1634/theoncologist.2013-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su HC, Haunschild C, Chung K, et al. Prechemotherapy antimullerian hormone, age, and body size predict timing of return of ovarian function in young breast cancer patients. Cancer. 2014;120:3691–3698. doi: 10.1002/cncr.28942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venkatraman ES, Begg C. A distribution-free procedure for comparing receiver operating characteristic curves from a paired experiment. Biometrika. 1996;83:835–848. [Google Scholar]
- 35.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 36.R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2011. Available at http://www.R-project.org.
- 37.Rosendahl M, Andersen CY, la Cour Freiesleben N, et al. Dynamics and mechanisms of chemotherapy-induced ovarian follicular depletion in women of fertile age. Fertil Steril. 2010;94:156–166. doi: 10.1016/j.fertnstert.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 38.Anderson RA, Rosendahl M, Kelsey TW, et al. Pretreatment anti-Müllerian hormone predicts for loss of ovarian function after chemotherapy for early breast cancer. Eur J Cancer. 2013;49:3404–3411. doi: 10.1016/j.ejca.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruddy KJ, O’Neill A, Miller KD, et al. Biomarker prediction of chemotherapy-related amenorrhea in premenopausal women with breast cancer participating in E5103. Breast Cancer Res Treat. 2014;144:591–597. doi: 10.1007/s10549-014-2891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallace AM, Faye SA, Fleming R, et al. A multicentre evaluation of the new Beckman Coulter anti-Mullerian hormone immunoassay (AMH Gen II) Ann Clin Biochem. 2011;48:370–373. doi: 10.1258/acb.2011.010172. [DOI] [PubMed] [Google Scholar]
- 41.Anderson RA, Themmen AP, Al-Qahtani A, et al. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod. 2006;21:2583–2592. doi: 10.1093/humrep/del201. [DOI] [PubMed] [Google Scholar]
- 42.Oktay K, Oktem O, Reh A, et al. Measuring the impact of chemotherapy on fertility in women with breast cancer. J Clin Oncol. 2006;24:4044–4046. doi: 10.1200/JCO.2006.06.9823. [DOI] [PubMed] [Google Scholar]
- 43.Lutchman Singh K, Muttukrishna S, Stein RC, et al. Predictors of ovarian reserve in young women with breast cancer. Br J Cancer. 2007;96:1808–1816. doi: 10.1038/sj.bjc.6603814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anders C, Marcom PK, Peterson B, et al. A pilot study of predictive markers of chemotherapy-related amenorrhea among premenopausal women with early stage breast cancer [published correction appears in Cancer Invest 2008;26:1068] Cancer Invest. 2008;26:286–295. doi: 10.1080/07357900701829777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Partridge AH, Ruddy KJ, Gelber S, et al. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril. 2010;94:638–644. doi: 10.1016/j.fertnstert.2009.03.045. [DOI] [PubMed] [Google Scholar]
- 46.Su HI, Sammel MD, Green J, et al. Antimullerian hormone and inhibin B are hormone measures of ovarian function in late reproductive-aged breast cancer survivors. Cancer. 2010;116:592–599. doi: 10.1002/cncr.24746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Decanter C, Morschhauser F, Pigny P, et al. Anti-Müllerian hormone follow-up in young women treated by chemotherapy for lymphoma: Preliminary results. Reprod Biomed Online. 2010;20:280–285. doi: 10.1016/j.rbmo.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 48.Brougham MFH, Crofton PM, Johnson EJ, et al. Anti-Müllerian hormone is a marker of gonadotoxicity in pre- and postpubertal girls treated for cancer: A prospective study. J Clin Endocrinol Metab. 2012;97:2059–2067. doi: 10.1210/jc.2011-3180. [DOI] [PubMed] [Google Scholar]
- 49.Hamy AS, Porcher R, Cuvier C, et al. Ovarian reserve in breast cancer: Assessment with anti-Müllerian hormone. Reprod Biomed Online. 2014;29:573–580. doi: 10.1016/j.rbmo.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 50.Anderson RA, Cameron DA. Pretreatment serum anti-müllerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab. 2011;96:1336–1343. doi: 10.1210/jc.2010-2582. [DOI] [PubMed] [Google Scholar]
- 51.Broer SL, Broekmans FJ, Laven JS, et al. Anti-Müllerian hormone: Ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20:688–701. doi: 10.1093/humupd/dmu020. [DOI] [PubMed] [Google Scholar]
- 52.La Marca A, Sighinolfi G, Papaleo E, et al. Prediction of age at menopause from assessment of ovarian reserve may be improved by using body mass index and smoking status. PLoS One. 2013;8:e57005. doi: 10.1371/journal.pone.0057005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carty CL, Spencer KL, Setiawan VW, et al. Replication of genetic loci for ages at menarche and menopause in the multi-ethnic Population Architecture using Genomics and Epidemiology (PAGE) study. Hum Reprod. 2013;28:1695–1706. doi: 10.1093/humrep/det071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peccatori FA, Azim HA, Jr, Orecchia R, et al. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi160–vi170. doi: 10.1093/annonc/mdt199. [DOI] [PubMed] [Google Scholar]
- 55.Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sukumvanich P, Case LD, Van Zee K, et al. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment: A prospective study. Cancer. 2010;116:3102–3111. doi: 10.1002/cncr.25106. [DOI] [PubMed] [Google Scholar]
- 57.Ganz PA, Land SR, Geyer CE, Jr, et al. Menstrual history and quality-of-life outcomes in women with node-positive breast cancer treated with adjuvant therapy on the NSABP B-30 trial. J Clin Oncol. 2011;29:1110–1116. doi: 10.1200/JCO.2010.29.7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao J, Liu J, Chen K, et al. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: A meta-analysis. Breast Cancer Res Treat. 2014;145:113–128. doi: 10.1007/s10549-014-2914-x. [DOI] [PubMed] [Google Scholar]
- 59.Schover LR. Premature ovarian failure and its consequences: Vasomotor symptoms, sexuality, and fertility. J Clin Oncol. 2008;26:753–758. doi: 10.1200/JCO.2007.14.1655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.