Abstract
Background
Hepatorenal syndrome (HRS) is a serious complication of advanced chronic liver disease. Abdominal compartment syndrome (ACS) occurs with dysfunction of multiple organs when abdominal pressure increases. Here, we report on a novel model of ACS with ascites and a model of HRS in rats to observe the urea transporter protein (UT) expression in the 2 models.
Material/Methods
A liver cirrhosis model was induced by CCl4. After changes of liver histopathology were observed, rats were injected intraperitoneally with succinylated gelatin to establish a model of ACS and HRS. Then, changes in BUN, Cr, and renal histopathology were detected. Moreover, the UT in ACS and HRS were also quantified.
Results
The surfaces of liver in the cirrhotic group became coarse, with visible small nodules and became yellow and greasy. The normal structure of the hepatic lobules were destroyed, and hyperplasia of fibrotic tissue and pseudo-lobe was observed. The levels of BUN and Cr were significantly increased in rats suffering from ACS and HRS, respectively, compared to their control groups. In addition, the mRNA levels of UT-A2 and UT-A3 decreased in rats with HRS compared to cirrhotic rats. However, there was no significant difference between the mRNA levels of UT-A2, UT-A3, and UT-B in rats with ACS vs. normal rats.
Conclusions
It is feasible to model ACS in rats by injecting succinylated gelatin into the abdominal cavity. Increasing the intra-abdominal pressure by succinylated gelatin is also a novel approach for modeling HRS in cirrhotic rats. Compared with control rats, there is an abnormal mRNA expression of UT in ACS rats and HRS rats.
MeSH Keywords: Hepatorenal Syndrome, Intra-Abdominal Hypertension, Liver Cirrhosis
Background
Patients with advanced cirrhosis can suffer from hepatorenal syndrome (HRS), a unique form of kidney injury that results from renal vasoconstriction in the setting of systemic and splanchnic arterial vasodilatation, in the absence of intrinsic renal pathological changes [1]. In a small fraction of patients, renal function can be restored following liver transplantation [2]. However, the prognosis of patients with cirrhosis who develop HRS remains poor because of the limited therapies we can provide for end-stage liver disease and because very few patients can get liver transplants. There are several theories about the pathogenesis of HRS [3–6]. Patients with HRS often suffer from ascites. It was found that mice with cirrhosis can be converted to have HRS when injected intraperitoneally with albumin [7]. The urea transporter protein (UT) in the kidneys is involved in the regulation of urea secretion [8]. The relationship between UT and HRS is not clear yet. Therefore, we injected succinylated gelatin into the abdominal cavity of cirrhotic rats to produce an HRS model, then explored the changes of UT expression in this model.
Material and Methods
Animals
The study was implemented strictly according to the institution’s guidelines for experimental animals. The protocol was authorized by the Animal Experimental Ethics Committee of Tongji Hospital, Tongji University. Male SD rats, weighing 200±20 g (n=100) were bred under standard conditions at constant humidity, room temperature, and regular 12 h/12 h light/dark cycles. The rats were fed with standard diet and had free access to water.
Animal model of cirrhosis
Fifty male SD rats were randomly divided into 2 groups: model group rats were subcutaneously injected with a mixture of carbon tetrachloride (30%) and plant oil (70%) according to its weight (initial dosage was 0.5 ml/100 g. Maintenance dose was 0.3 ml/100 g twice a week) in the model group and the control group rats were injected with plant oil (0.3 ml/100 g twice a week). A subset of rats in the model group was sacrificed after 12 weeks and the liver histopathology was examined to ensure the successful establishment of a cirrhotic model.
Animal model of HRS
Twelve rats in the control groups were randomly divided into 2 subgroups: Group A was the new control group and Group B was the abdominal compartment syndrome (ACS) group. Twelve cirrhotic rats were also divided into 2 groups: Group C was the new cirrhotic group and Group D was the HRS group. The rats in Group B and D were anesthetized subcutaneously with 0.03% Na-pentobarbital (0.1 ml/100 g) and fixed on experimental tables. Succinylated gelatin was dripped into the abdominal cavity of rats via an infusion tube. A pressure gauge with a T-shaped 3-way pipe, graduated L-shaped glass tube, and infusion tube was punctured into the lumbar region of midaxillary line on umbilicus level of rats to detect the abdominal pressure. When fluid levels of the L-shaped glass tube raised to 26.7 cm of water column (equivalent to 20 mmHg), abdominal pressure had been maintained at this level for 3 h by adjusting the dripping speed. Except for dripping of succinylated gelatin, the remaining methods were the same as above for the rats in Group A and Group C. Then, serum BUN and Cr were determined from blood samples obtained from rat aortas. In addition, left kidneys were cryopreserved in liquid nitrogen and right kidneys were fixed in neutral buffered formalin. The later was then sectioned in coronal plane (5-μm slice thickness) and prepared with H and E staining for routine light microscopy. Histopathological assessment was performed by 2 experienced pathologists.
RT-PCR
Total RNA was extracted from150 mg of renal medulla into Trizol reagent (Kusatsu, Japan) according to manufacturer’s instructions. For cDNA synthesis, RT reactions were performed with total RNA (2 ug) according to the directions of the Takara RT reagent kit (Kusatsu, Japan). The product was amplified in a reaction volume of 10 ul including 1 ul RT product, 5 ul SYBR Premix Ex Taq II (5×), 0.2 ul 50×ROX Reference Dye II (50×), and 20 pmol of each primer. PCRs were performed for 40 cycles at 94°C for 45 s, 60°C for 30 s, and 72°C for 30 s in a 7900HT Fast RT-PCR System (ABI, Palo Alto, CA, USA). Threshold cycle values of each sample were normalized to b-actin mRNA expression, and the fold change for each mRNA was calculated using the 2−ΔΔCt method.
Western blot analysis
Western blot analysis was performed using protein from renal medulla specimens. UT-A1 (Genetex, USA) and b-actin (Santa Cruz, USA) were used and visualized using the detection system (Odyssey LI-COR 9120, USA) following the procedure provided by the manufacturer.
Statistical analysis
Statistical analysis was performed using SPSS 17.0. All results are expressed as mean ± standard deviation. The unpaired t test was used for comparison of 2 groups. P<0.05 was considered statistically significant.
Results
Animal model of cirrhosis
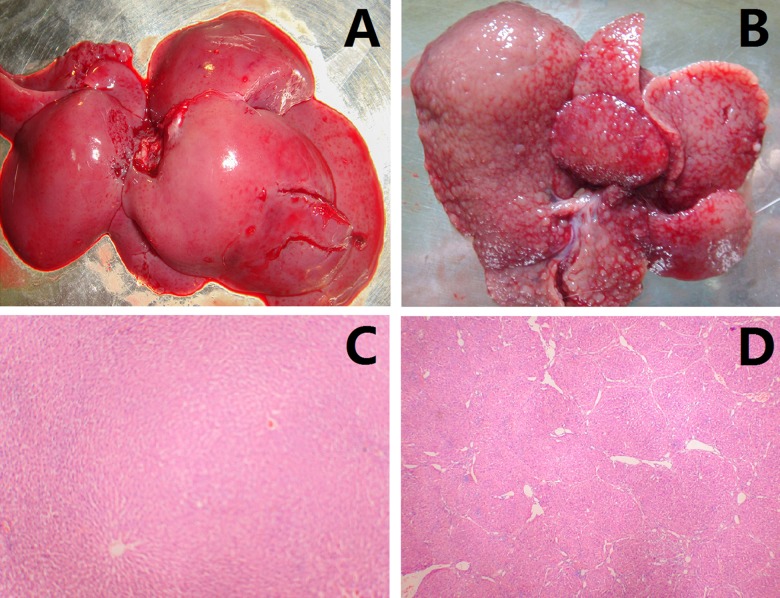
Compared with the control group (Figure 1A), the surfaces of gross liver specimens of the cirrhotic group became coarser. The cirrhotic livers also became yellow and greasy (Figure 1B). In histopathology sections, small nodules could be seen. The normal structures of the hepatic lobules (Figure 1C) were destroyed with hyperplasia of fibrous tissue with formation of pseudo-lobes (Figure 1D).
Figure 1.
Animal model of cirrhosis. (A) Liver specimen of control group; (B) liver specimen of cirrhotic group; (C) normal hepatic lobule of control group; (D) pseudo-lobule of cirrhotic group.
Animal model of HRS
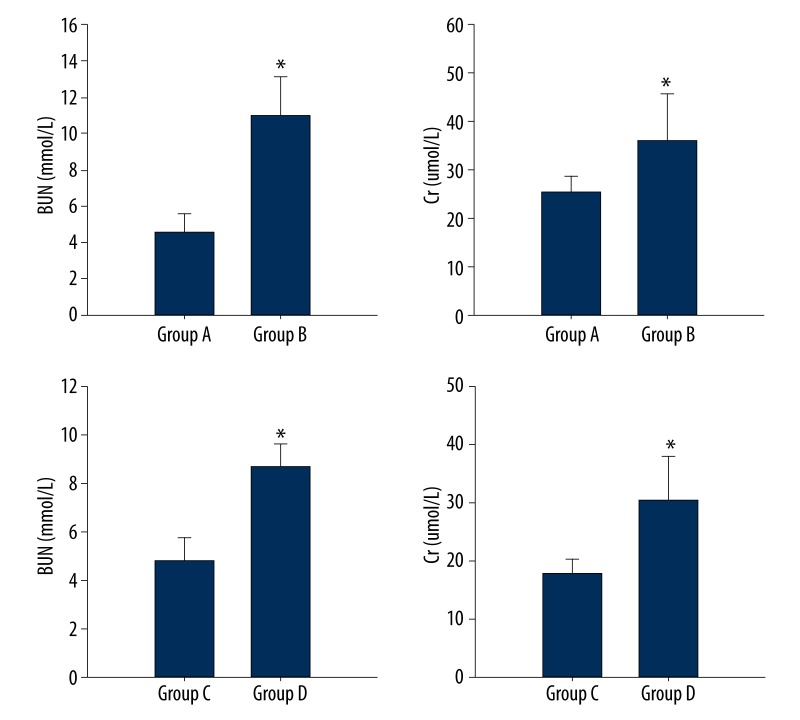
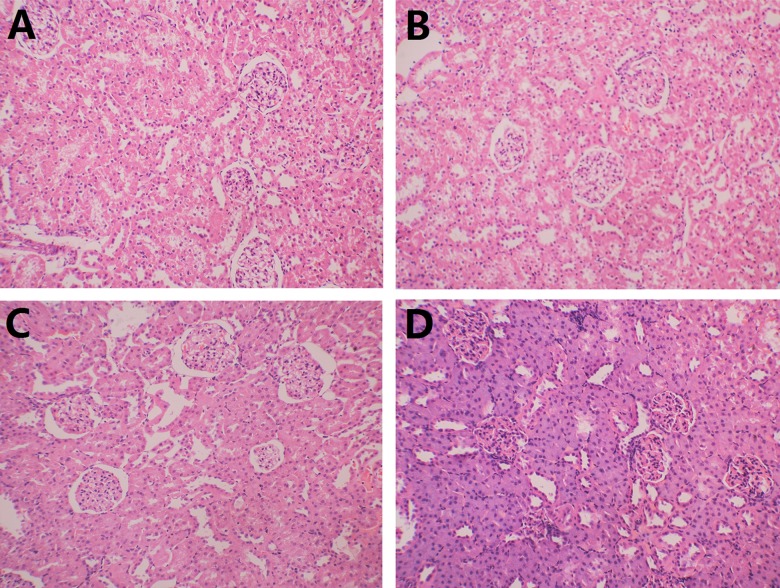
During the experiment, the respiratory rate of rats increased with increased intra-abdominal pressure and abdominal distension. BUN and Cr levels of Group B were significantly higher than those of Group A, respectively (10.98±2.14 mmol/L vs. 4.54±1.05 mmol/L, 36±9.64 umol/L vs. 25.4±3.29 umol/L, P<0.05). In addition, BUN and Cr levels of Group D were also higher than those of Group C (8.7±0.96 mmol/L vs. 4.84±0.93 mmol/L, 30.3±7.81 umol/L vs. 17.8±2.68 umol/L, P<0.05) (Figure 2). There were no significant morphological differences among the 4 groups of rat kidneys in pathological sections. Loops of glomerular vasculature of the 4 groups were thin and clear. Endothelial cells, mesangial cells, and renal tubules were also normal (Figure 3).
Figure 2.
BUN and Cr levels. * p<0.05.
Figure 3.
Pathological manifestations of rat kidneys. (A) Control group; (B) abdominal compartment syndrome group; (C) cirrhotic group; (D) hepatorenal syndrome group.
RT-PCR and western blot
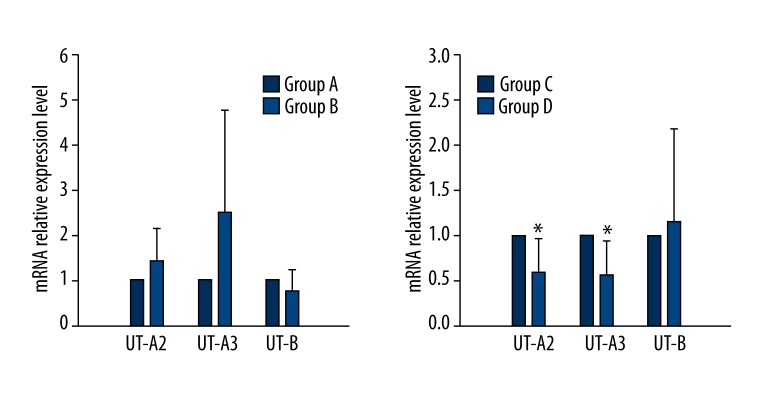
The mRNA expression of UT-A2 and UT-A3 in Group D decreased markedly compared with that of Group C (P<0.05) (Figure 4). However, no significant difference in mRNA level of UT-B was detected. Moreover, there was also no significant difference in UT-A2, UT-A3, and UT-B between Group A and Group B (P<0.05) (Figure 4).
Figure 4.
mRNA expression levels of UT-A2, UT-A3, and UT-B. * p<0.05.
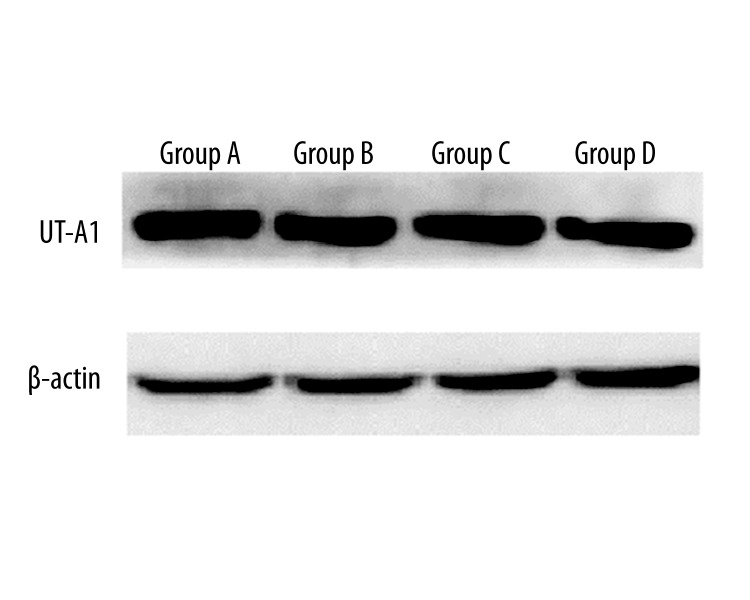
In terms of protein expression, no significant difference in UT-A1was detected among the 4 groups by Western blotting (Figure 5).
Figure 5.
Protein expression level of UT-A1.
Discussion
Some commonly used animal models of cirrhosis are induced by CCl4, dimethylnitrosamine, thioacetamide, and bile duct ligation [9–12]. The model induced by CCl4 was used in the present study. According to the changes in gross liver specimen morphology and histopathology, the rat model of cirrhosis was prepared successfully. Then, we established the rat model of ACS. In the clinic, the definition of ACS is an intra-abdominal pressure ≥20 mmHg with abdominal organ dysfunction. It can occur in conditions such as abdominal surgery, mechanical ventilation, ileus, and acute pancreatitis [13–16]. For further study of the pathogenesis, prevention, and therapy of this disease, several animal models of ACS have been reported [17,18]. In these studies, gas, liquid, or solid were used to maintain the intra-abdominal pressure. For example, Kaussen et al. used CO2 to establish the ACS model [19]. However, metabolic changes and inflammatory response were found in peritoneal cells exposed to high CO2 concentrations. Therefore, the impact of CO2 for the model was not restricted to increasing intra-abdominal pressure [20]. Benninger et al. used a crystal solution that was easily absorbed by the peritoneum [21]. Intraperitoneal tamponade was used with cotton dressing by Lima et al. [22]. However, intra-abdominal pressure was not optimally controlled and the tamponade was tedious and inaccurate. The ascites that results in intra-abdominal hypertension is a colloid suspension that contains albumin. However, the cost of injecting albumin intraperitoneally is too high. Succinylated gelatin was injected by Christoph et al. to establish models of ACS [23].
In the present study, succinylated gelatin was used to establish a model of ASC and HRS. Because of leakage from the junction of the hose and abdominal cavity, succinylated gelatin needs to be replenished. Cyanoacrylate glue was used to adhere to the junction between tubes and the skin of the rat to prevent leakage of succinylated gelatin. In our study, rats survived for 3 h when intra-abdominal pressure reached 20 mmHg. Afterward, they gradually died due to cardiopulmonary failure induced by thoracic cavity oppression. However, we admit that 3 h may not ne enough time to cause the change of urea transporter protein by Western Blot. The respiration rate was returned to normal by alleviating the abdominal pressure. We successfully established the ACS model and confirmed that BUN and Cr levels in Group B increased. To the best of our knowledge, the present study is the first to show that the model can be used in cirrhotic animal to mimic HRS. In addition, the 20 mmHg level in the ACS definition pertains to humans, not rats. This ACS model in rats can be further improved through studying changes of renal function at different intra-abdominal pressures. As a result, the pressure of ACS in rats could be obtained and the prolonged survival will be beneficial to detect the changes of urea transporter protein. Moreover, the phosphorylation of urea transporter protein could be also examined, although there is no change in total protein. HRS contains Type 1 and Type 2. Type 2 HRS is usually concomitant with ascites. Renal insufficiency has been induced by arterial blood supply deficiency rather than renal organic injury [24,25]. Because it usually occurs at the last stage of cirrhosis, animal models of HRS have been difficult to establish. Pereira et al. used bile duct ligation, but found it was complex and had an unpredictable success rate [26]. Chang et al. dripped albumin into rat abdominal cavities to model ACS [7]; however, they used the method of measuring body weight instead of measuring pressure directly, which fails to ascertain the pressure. The rat model of HRS in this study accorded with the cirrhosis with ascites and increased level of Cr without renal organic injury. Taken together, these results indicate that dripping succinylated gelatin into the abdominal cavities of cirrhotic rats is a feasible method to produce a HRS model. ACS participates in occurrence and development of HRS.
As nitrogen metabolites, BUN is involved in urine concentration in the renal medulla and is excreted. A theory of passive urine concentration in the renal medulla was proposed in 1972 by Koko et al. [27]. In the process of renal water metabolism, the gradient osmotic pressure constituted by a great quantity of BUN in renal medulla facilitates reabsorption of initial urine and excretion of BUN without loss of much water [28]. Studies showed that accumulation of BUN and concentration of urine was regulated by UT-A1 and UT-A3 in the inner medullary collecting duct [29]. In addition, UT-A2 distributed in descending thin limb of Helen’s loop was found to be involved in countercurrent exchange located in the ascending limb and thick descending limb, which likewise enhances the effect of gradient osmotic pressure in the renal papilla [30]. UT-B protein supplies the lost urea in the inner medulla by enhancing the countercurrent exchange in the vasa recta [31]. Through researching UT expression in the renal medulla in a rat model of ACS and HRS, we found that compared with the normal group (Group A), there was no significant difference in expression of UT-A2, UT-A3, and UT-B mRNA in the ACS group (Group B), and that compared with the cirrhotic group (Group C), the level of UT-A2 and UT-A3 mRNA was significantly decreased in the HRS group (Group D). However, the difference in UT-B mRNA was without statistical significance. In addition, there was no significant difference in UT-A1among the 4 groups. Although ascites can induce both ACS and HRS, the expression of UTs in the 2 formations was different.
Conclusions
It is feasible create a model of ACS in rats by injecting succinylated gelatin into the abdominal cavity. Increasing the intra-abdominal pressure by succinylated gelatin is also a novel approach for modeling HRS in cirrhotic rats. Compared with control rats, there is an abnormal mRNA expression of UT in ACS rats and HRS rats.
Abbreviations
- ACS
abdominal compartment syndrome
- HRS
hepatorenal syndrome
- UT
urea transporter protein
Footnotes
Source of support: National Natural Science Foundation of China (No. 81370559); Shanghai Innovation Program (12431901002); Shanghai Major Joint Project for Important Diseases (2014ZYJB0201); Shanghai Joint Project with Advanced Technology (SHDC 12014122)
Conflicts of interest
The authors have declared no conflicts of interest.
References
- 1.Nadim MK, Kellum JA, Davenport A, et al. ADQI Workgroup. Hepatorenal syndrome: the 8th International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2012;16( 1):R23. doi: 10.1186/cc11188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sourianarayanane A, Raina R, Garg G, et al. Management and outcome in hepatorenal syndrome: need for renal replacement therapy in non-transplanted patients. Int Urol Nephrol. 2014;46(4):793–800. doi: 10.1007/s11255-013-0527-7. [DOI] [PubMed] [Google Scholar]
- 3.Sacerdoti D, Bolognesi M, Merkel C, et al. Renal vasoconstriction in cirrhosis evaluated by duplex Doppler ultrasonography. Hepatology. 1993;17(2):219–24. [PubMed] [Google Scholar]
- 4.Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8(5):1151–57. doi: 10.1002/hep.1840080532. [DOI] [PubMed] [Google Scholar]
- 5.Moreau R, Lebrec D. Transduction of antinatriuretic signals in renal proximal tubular cells in cirrhosis: introduction to novel approaches to the treatment of sodium retention. J Hepatol. 1998;28(6):1064–69. doi: 10.1016/s0168-8278(98)80358-9. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-del-Arbol L, Monescillo A, Arocena C, et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42(2):439–47. doi: 10.1002/hep.20766. [DOI] [PubMed] [Google Scholar]
- 7.Chang Y, Qi X, Li Z, et al. Hepatorenal syndrome: insights into the mechanisms of intra-abdominal hypertension. Int J Clin Exp Pathol. 2013;6(11):2523–28. [PMC free article] [PubMed] [Google Scholar]
- 8.Sands JM, Nonoguchi H, Knepper MA. Vasopressin effects on urea and H2O transport in inner medullary collecting duct subsegments. Am J Physiol. 1987;253(5):F823–32. doi: 10.1152/ajprenal.1987.253.5.F823. [DOI] [PubMed] [Google Scholar]
- 9.Muñoz L, José Borrero M, Ubeda M, et al. Interaction between intestinal dendritic cells and bacteria translocated from the gut in rats with cirrhosis. Hepatology. 2012;56(5):1861–69. doi: 10.1002/hep.25854. [DOI] [PubMed] [Google Scholar]
- 10.de Gouville AC, Boullay V, Krysa G, et al. Inhibition of TGF-beta signaling by an ALK5 inhibitor protects rats from dimethylnitrosamine-induced liver fibrosis. Br J Pharmacol. 2005;145(2):166–77. doi: 10.1038/sj.bjp.0706172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abramovitch S, Dahan-Bachar L, Sharvit E, et al. Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats. Gut. 2011;60(12):1728–37. doi: 10.1136/gut.2010.234666. [DOI] [PubMed] [Google Scholar]
- 12.Steib CJ, Hennenberg M, Beitinger F, et al. Amiloride reduces portal hypertension in rat liver cirrhosis. Gut. 2010;59(6):827–36. doi: 10.1136/gut.2009.197756. [DOI] [PubMed] [Google Scholar]
- 13.Malbrain ML, Cheatham ML, Kirkpatrick A, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006;32(11):1722–32. doi: 10.1007/s00134-006-0349-5. [DOI] [PubMed] [Google Scholar]
- 14.Reintam Blaser A, Parm P, Kitus R, Starkopf J. Risk factors for intra-abdominal hypertension in mechanically ventilated patients. Acta Anaesthesiol Scand. 2011;55(5):607–14. doi: 10.1111/j.1399-6576.2011.02415.x. [DOI] [PubMed] [Google Scholar]
- 15.Vidal MG, Ruiz Weisser J, Gonzalez F, et al. Incidence and clinical effects of intra-abdominal hypertension in critically ill patients. Crit Care Med. 2008;36(6):1823–31. doi: 10.1097/CCM.0b013e31817c7a4d. [DOI] [PubMed] [Google Scholar]
- 16.Ke L, Ni HB, Sun JK, et al. Risk factors and outcome of intra-abdominal hypertension in patients with severe acute pancreatitis. World J Surg. 2012;36(1):171–78. doi: 10.1007/s00268-011-1295-0. [DOI] [PubMed] [Google Scholar]
- 17.Chen CH, Tsai PS, Huang CJ. Minocycline ameliorates lung and liver dysfunction in a rodent model of hemorrhagic shock/resuscitation plus abdominal compartment syndrome. J Surg Res. 2013;180(2):301–9. doi: 10.1016/j.jss.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 18.Cortes-Puentes GA, Gard KE, Adams AB, et al. Value and limitations of transpulmonary pressure calculations during intra-abdominal hypertension. Crit Care Med. 2013;41(8):1870–77. doi: 10.1097/CCM.0b013e31828a3bea. [DOI] [PubMed] [Google Scholar]
- 19.Kaussen T, Srinivasan PK, Afify M, et al. Influence of two different levels of intra-abdominal hypertension on bacterial translocation in a porcine model. Ann Intensive Care. 2012;2(Suppl 1):S17. doi: 10.1186/2110-5820-2-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kopernik G, Avinoach E, Grossman Y, et al. The effect of a high partial pressure of carbon dioxide environment on metabolism and immune functions of human peritoneal cells-relevance to carbon dioxide pneumoperitoneum. Am J Obstet Gynecol. 1998;179(6 Pt 1):1503–10. doi: 10.1016/s0002-9378(98)70016-x. [DOI] [PubMed] [Google Scholar]
- 21.Benninger E, Laschke MW, Cardell M, et al. Intra-abdominal pressure development after different temporary abdominal closure techniques in a porcine model. J Trauma. 2009;66(4):1118–24. doi: 10.1097/TA.0b013e3181820d94. [DOI] [PubMed] [Google Scholar]
- 22.Lima RA, Schanaider A, Santana MC, et al. Developing a new experimental model of abdominal compartment syndrome. Rev Col Bras Cir. 2011;38(6):417–21. doi: 10.1590/s0100-69912011000600009. [DOI] [PubMed] [Google Scholar]
- 23.Meier C, Contaldo C, Schramm R, et al. A new model for the study of the abdominal compartment syndrome in rats. J Surg Res. 2007;139(2):209–16. doi: 10.1016/j.jss.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Arroyo V, Guevara M, Ginès P, et al. Hepatorenal syndrome in cirrhosis: pathogenesis and treatment. Gastroenterology. 2002;122(6):1658–76. doi: 10.1053/gast.2002.33575. [DOI] [PubMed] [Google Scholar]
- 25.Ginés P, Arroyo V, Quintero E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93(2):234–41. doi: 10.1016/0016-5085(87)91007-9. [DOI] [PubMed] [Google Scholar]
- 26.Pereira RM, dos Santos RA, Oliveira EA, et al. Development of hepatorenal syndrome in bile duct ligated rats. World J Gastroenterol. 2008;14(28):4505–11. doi: 10.3748/wjg.14.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imai M, Kokko JP. Effect of peritubular protein concentration on reabsorption of sodium and water in isolated perfused proxmal tubules. J Clin Invest. 1972;51(2):314–25. doi: 10.1172/JCI106816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fenton RA, Chou CL, Sowersby H, et al. Gamble’s “economy of water” revisited: studies in urea transporter knockout mice. Am J Physiol Renal Physiol. 2006;291(1):F148–54. doi: 10.1152/ajprenal.00348.2005. [DOI] [PubMed] [Google Scholar]
- 29.Fenton RA, Chou CL, Stewart GS, et al. Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc Natl Acad Sci USA. 2004;101(19):7469–74. doi: 10.1073/pnas.0401704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lei T, Zhou L, Layton AT, et al. Role of thin descending limb urea transport in renal urea handling and the urine concentrating mechanism. Am J Physiol Renal Physiol. 2011;301(6):F1251–59. doi: 10.1152/ajprenal.00404.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang B, Bankir L, Gillespie A, et al. Urea-selective concentrating defect in transgenic mice lacking urea transporter UT-B. J Biol Chem. 2002;277(12):10633–37. doi: 10.1074/jbc.M200207200. [DOI] [PubMed] [Google Scholar]