Abstract
[Pasteurella] pneumotropica biotypes Jawetz and Heyl and [Actinobacillus] muris are the most prevalent Pasteurellaceae species isolated from laboratory mouse. However, mechanisms contributing to their high prevalence such as the ability to form biofilms have not been studied yet. In the present investigation we analyze if these bacterial species can produce biofilms in vitro and investigate whether proteins, extracellular DNA and polysaccharides are involved in the biofilm formation and structure by inhibition and dispersal assays using proteinase K, DNase I and sodium periodate. Finally, the capacity of the biofilms to confer resistance to antibiotics is examined. We demonstrate that both [P.] pneumotropica biotypes but not [A.] muris are able to form robust biofilms in vitro, a phenotype which is widely spread among the field isolates. The biofilm inhibition and dispersal assays by proteinase and DNase lead to a strong inhibition in biofilm formation when added at the initiation of the biofilm formation and dispersed pre-formed [P.] pneumotropica biofilms, revealing thus that proteins and extracellular DNA are essential in biofilm formation and structure. Sodium periodate inhibited the bacterial growth when added at the beginning of the biofilm formation assay, making difficult the assessment of the role of β-1,6-linked polysaccharides in the biofilm formation, and had a biofilm stimulating effect when added on pre-established mature biofilms of [P.] pneumotropica biotype Heyl and a majority of [P.] pneumotropica biotype Jawetz strains, suggesting that the presence of β-1,6-linked polysaccharides on the bacterial surface might attenuate the biofilm production. Conversely, no effect or a decrease in the biofilm quantity was observed by biofilm dispersal using sodium periodate on further biotype Jawetz isolates, suggesting that polysaccharides might be incorporated in the biofilm structure. We additionally show that [P.] pneumotropica cells enclosed in biofilms were less sensitive to treatment with amoxicillin and enrofloxacin than planktonic bacteria. Taken together, these findings provide a first step in understanding of the biofilm mechanisms in [P.] pneumotropica, which might contribute to elucidation of colonization and pathogenesis mechanisms for these obligate inhabitants of the mouse mucosa.
Introduction
Bacterial biofilms are structured aggregations of bacterial cells, encased in a self synthesized extracellular matrix that may consist of proteins, nucleic acids and polysaccharides [1]. Biofilm production occurs in a multi-step process in which the bacteria adhere to a surface and then produce the extracellular matrix which confer them a firmer adherence [2]. The biofilms are recognized as complicating factors in many bacterial infections and one of the reasons why treatment with antibiotics fails [3]. Moreover, the biofilms enhance the resistance of bacteria to host immune defense mechanisms [4].
[Pasteurella] pneumotropica biotypes Jawetz and Heyl and [Actinobacillus] muris are the most prevalent Pasteurellaceae species isolated from laboratory mouse [5]. Although described several decades ago, these species are expected to undergo taxonomic changes soon, since they have not been formally classified under genera due to insufficient knowledge regarding their taxonomy [6]. [P.] pneumotropica, the most well-known member of the rodent Pasteurellaceae, has two biovars, Jawetz and Heyl, which show a few phenotypic differences [7, 8], but are regarded as two distinct species based on the 16S rDNA and gyrB sequences [9–12].
[P.] pneumotropica seems to possess the most pathogenic potential among the rodent Pasteurellaceae and is associated with several pathogenic aspects in laboratory rodents such as inflammation and abscess formation in a majority of tissues [13–16]. In addition, [P.] pneumotropica can occasionally cause disease in immunodeficient [17], as well as in immunocompetent humans [18]. Moreover, subclinical infection with [P.] pneumotropica may trigger immune mechanisms with possible interference on research using infected mice [19]. No pathogenic potential could be attributed to date to [A.] muris [20, 21].
Despite the biological and economic importance of [P.] pneumotropica in relation to experimental animals, the virulence factors and the pathogenesis of [P.] pneumotropica infections are largely unknown. Nevertheless, in the last years these infectious agents received an increased importance and putative virulence factors such as hemolysin-like proteins similar to RTX toxin [22] and their immunogenic role have been considered [23]. The immunogenic and protective role of several outer membrane proteins of [P.] pneumotropica have also been studied recently [24]. The increasing interest in [P.] pneumotropica characterization is also underlined by the report of the first genome sequence for this species [25]. Although [P.] pneumotropica as a pathogen was less investigated, its potential as a model organism in the mouse, to study some pathogenic processes such as periodontal bone resorption [26, 27] or the role of toll-like receptor 4 in the gram-negative bacterial pneumonia [28] has been recognized. The ability of [P.] pneumotropica to evade the human complement was also documented recently [29]. It is known that many pathogenic Pasteurellaceae species of big animals are strictly adapted to their host species. For this reason, we believe that the mouse is not an ideal animal model to study pathogenesis aspects like colonization for big animal Pasteurellaceae and that [P.] pneumotropica can serve as prototype for investigations of other related Pasteurellaceae in vivo, due to its adaptation to the mouse, the animal species most easily and frequently used in experimental studies.
The aim of the present investigation was to analyze if [P.] pneumotropica biotypes Jawetz and Heyl and [A.] muris produce a biofilm and, if so, to look if this phenomenon is prevalent among the field isolates. Moreover, we aimed to characterize the thickness and the chemical composition of the biofilm and to test whether the susceptibility to antibiotics in biofilms was different to that of the planktonic cells for these obligate inhabitants of the mouse mucosa. We demonstrate for the first time that both [P.] pneumotropica biotypes but not [A.] muris are able to form robust biofilms in vitro, which seems to consist predominantly of proteins and extracellular DNA (eDNA). Moreover, we reveal that this phenotype is widely spread among the field isolates and might interfere with the sensitivity to antibiotics.
Materials and Methods
Bacterial strains and culture conditions
A total of 60 mouse Pasteurellaceae strains (Table 1), consisting of 17 isolates of [P.] pneumotropica biotype Jawetz (including the type strain Frederiksen P421T = CCUG12398T), 25 isolates of [P.] pneumotropica biotype Heyl (including the type strain ATCC12555T) and 18 [A.] muris isolates (including the type strain CCUG16938T) were used in this study. The clinical strains were isolated during the periodically health monitoring [30] of the mice from the Animal Research Facility of the Heinrich-Heine-University in Düsseldorf and identified by standard biochemical tests as well as by the 16S–23S rDNA internal spacer profile and PCR as described previously [5, 21, 31]. The isolates were propagated from -80°C stocks onto Columbia blood-agar plates (Biomerieux, Nuertingen, Germany) for approximately 30 h at 37°C under aerobic conditions. Isolated colonies were further inoculated in brain heart infusion (BHI) broth (Sigma-Aldrich, Steinheim, Germany), overnight (approx. 16–18 h) at 37°C. The overnight cultures were used as inoculum for the following experiments.
Table 1. Bacterial strains used in this study.
| Organism | Strain | Reference |
|---|---|---|
| Pasteurellaceae (n = 60) | ||
| [Actinobacillus] muris (n = 18) | CCUG16938T | Christensen et al., 2003 [9] |
| 1596/07, 450/10, 490/11, 622/11, 691/11, 693/11, 694/11, 696/11, 808/11, 1040/11, 1041/11, 1239/11, 1526/12, 1528/12, 1576/12, 1578/12, 143/13 | Benga et al., 2012 [5], Benga et al., 2013 [20] | |
| [P.] pneumotropica biotype Heyl (n = 25) | ATCC12555T | Christensen et al., 2003 [9] |
| 218/08, 314/08, 520/08, 1825/08, 450/10, 256/11, 490/11, 543/11, 566/11, 622/11, 666/11, 693/11, 696/11, 705/11, 1023/11, 1070/11, 1527/12, 1528/12, 1552/12, 526/13, 716/13, 717/13, 1378/13, 1379/13 | Benga et al., 2012 [5], Benga et al., 2013 [30] | |
| [P]. pneumotropica biotype Jawetz (n = 17) | P421T = CCUG12398T | Christensen et al., 2003 [9] |
| 1596/07, 217/08, 607/10, 567/11, 665/11, 691/11, 695/11, 847/11, 1009/11, 1012/11, 1023/11, 394/12, 1526/12, 1528/12, 1550/12, 530/13 | Benga et al., 2012 [5], Benga et al., 2013 [30] |
Biofilm assay in microtiter plates and glass tubes
To quantify biofilm formation in microtiter plates and glass tubes the protocols described previously by [32] and [33] respectively were adapted with some modifications. In brief, the bacterial strains were grown overnight in 10 ml BHI, then 20 μl culture was mixed with 2 ml fresh warm BHI in laboratory glass tubes 16 x 100 mm. Subsequently, 100 μl of the diluted culture was transferred in triplicate to 96-well plates (Rotilabo®-microtest plates, Carl-Roth, Karlsruhe, Germany). The 96-well plates and the glass tubes with the remaining culture were incubated aerobe, under static conditions for 24 h at 37°C, when the culture medium was removed and the recipients were rinsed once with tap water (pH 7.3). Subsequently, 100 μl or 2 ml of 1% crystal violet dye (Merck, Darmstadt, Germany) was added to each well and glass tube respectively for 15 min at room temperature. Following, the plates and the tubes were washed three times with water and the excess water was removed from the plates and glass tubes by tapping the inverted recipients on paper towel. The glass tubes were allowed to dry and then photographed, whereas the biofilm formed in the 96-well plates was quantified by recording the absorbance at 540 nm after addition of 100 μl of 70% ethanol for 15 min at room temperature to solubilize the crystal violet. Wells containing only BHI were used as controls and their OD540 values were subtracted from all the samples. Glass tubes containing sterile BHI were included as well as controls and compared with the inoculated tubes.
Confocal laser scanning microscopy (CLSM)
For microscopic visualization of the biofilms, overnight BHI broth cultures of the type strains of [P.] pneumotropica biotypes Jawetz and Heyl and of [A.] muris were diluted 1:100 in fresh BHI broth. Subsequently, 7 ml of the dilution was placed in Ф 5.5 cm Petri dishes (Greiner, Solingen, Germany) containing glass cover slips for 24 h at 37°C under aerobe conditions, then the culture supernatant was replaced and the biofilms were gently rinsed with distilled water. Each cover slip was then treated with 4% formalin for 5 min at room temperature and the excess formalin was rinsed with water. The biofilms were then stained with 0.2% water solution of acridine orange (Sigma-Aldrich), which labels double-stranded nucleic acids in green and single-stranded nucleic acids in red, for 5 min as previously described [34]. The excess stain was gently rinsed off and the cover slips were mounted up-side down using fluoromontTM aqueous mounting medium (Sigma-Aldrich). Confocal images were acquired with a Zeiss LSM710 Confocal Microscope (Carl Zeiss, Jena, Germany) using a Plan Apochromat 63X/1.4 objective. Stacks of horizontal plane images with a z-step of 1 μm were acquired. Orthogonal projections of the biofilms were constructed using the Zeiss confocal ZEN software.
Biofilm inhibition and detachment assay
To find out whether proteins, DNA and polysaccharides were involved in the biofilm formation or are incorporated within the mature biofilms of [P.] pneumotropica, we performed biofilm inhibition and detachment assays respectively in the presence of (i) proteinase K (Sigma-Aldrich), (ii) DNase I (Sigma-Aldrich) and (iii) sodium meta-periodate (NaIO4) (Sigma-Aldrich) as major protein, DNA and β-1,6-linked polysaccharide inhibitors as previously described [35, 36] with some modifications. For the inhibition assays, overnight cultures in BHI were diluted 1:100 in BHI containing (i) 100, 50, 25, 12.5 or 6.25 μg/ml proteinase K, (ii) 100, 50, 25, 12.5 or 6.25 μg/ml DNase I, or (iii) 10, 5, 2.5, 1.25 or 0.625 mM sodium periodate and allowed to form biofilms for 24 h at 37°C whereupon the biofilm quantity was recorded by the crystal violet assay described above. Wells Wells containing BHI without any supplements were used as controls. For detachment experiments, the culture supernatant from the wells containing 24 h old biofilms was replaced for 2 h at 37°C by 100 μl (i) proteinase K, (ii) DNase I or (iii) sodium periodate (NaIO4) at the concentrations listed above. Proteinase K was solubilized in a buffer containing 20 mM Tris-HCl (pH 7.5) – 100 mM NaCl, whereas the DNase I and sodium periodate were solubilized in 150 mM NaCl – 1 mM CaCl2 and 50 mM sodium acetate respectively. Wells treated with the corresponding buffers without the active substances were used as controls. Finally, the wells were washed three times with tap water and the biofilm was quantified using the crystal violet assay.
Antibiotic susceptibility of planktonic and biofilm bacteria
The susceptibility of biofilm versus planktonic bacteria to amoxicillin (Sigma-Aldrich) and enrofloxacin (Baytril, Bayer, Leverkusen, Germany) was assessed by recording the minimal inhibitory concentrations (MICs) and by a viability cfu plating assay as previously described [37, 38], using the type strains of the [P.] pneumotropica biotypes Jawetz and Heyl as well as an additional field isolate (394/12 and 1070/11) of each biotype originating from abscesses. Briefly, to determine the MICs for planktonic bacteria, overnight cultures were diluted to OD540 = 0.15 in BHI containing two-fold serial dilutions (200–0.19 μg/ml) of antibiotic and 200 μl of the suspensions were transferred in 96-well plates. For biofilms, the supernatants of 24 h old biofilms of the same strains were replaced by 200 μl BHI containing the same concentrations of antibiotic as for the planktonic cells. After incubation for 24 h at 37°C the growth was recorded by measurement of absorbance at 540 nm. The experiments were repeated at least three times. The MIC was considered the lowest concentration at which no statistically significant growth occurred after 24 h. For the viability cfu plating assays [37], the supernatants of 24 h old biofilms prepared in 96-well microtiter plates as described above were replaced with 200 μl of either fresh BHI broth or of BHI containing one of the antibiotics at a concentration of 100 μg/ml. After 3 h incubation at 37°C, the wells were washed three times with 250 μl sterile 0.9% NaCl. The biofilms were then removed by thoroughly scraping of the wells and suspended in 1 ml 0.9% NaCl. The viable cell number was determined by performing 10-fold serial dilution, plating and cfu enumeration after 24 h incubation at 37°C. To determine the susceptibility of planktonic bacteria to antibiotics, a fivefold dilution of overnight cultures in BHI followed by 3 h incubation at 37°C was performed. Subsequently, 2 ml of this culture were added to 10 ml BHI broth containing each antibiotic at a concentration of 100 μg/ml. A control dilution was obtained by diluting the suspension in BHI without antibiotics. Growth was allowed to occur over 3 h at 37°C when 1 ml culture was harvested and centrifuged for 8 min at 9,000 g. The pellet was then resuspended in 1 ml 0.9% NaCl and washed three times using the same procedure. Finally, the number of viable cells was determined by the same procedure used for the biofilm. These experiments were repeated at least three times, in duplicates.
Statistical analysis
For the inhibition and dispersal assays as well as for the bacterial growth, variance analysis followed by two-tailed Student´s t test was used to determine significance in the differences between the treated versus control groups. Differences were considered significant when confidence levels >95% were achieved.
Results
[P.] pneumotropica biotypes Heyl and Jawetz but not [A.] muris isolates form robust biofilms
To date, it is not known whether the three rodent Pasteurellaceae species included in this study are able to produce biofilms. To address this, biofilm formation was quantified by a standard microtiter plate crystal violet assay as well as by a glass tube assay in a various collection of reference and field isolates of [P.] pneumotropica biotypes Jawetz and Heyl and [A.] muris. Fig 1 shows the amount of biofilm formed after 24 h in BHI as recorded photometrical using the crystal violet technique. There is an obvious difference in the biofilm formation of [P.] pneumotropica species belonging to both biotypes in comparison to the [A.] muris isolates which can be categorized as no biofilm producers. In sharp contrast to [A.] muris, all [P.] pneumotropica strains tested were capable to produce robust biofilms, even though one can differentiate among them strong and weak biofilm producers. The results obtained by the glass tubes biofilm formation assays correlated with the results obtained by the crystal violet microtiter plate assays, showing as well the ability of [P.] pneumotropica biotypes Jawetz and Heyl but not of [A.] muris to form biofilms (Fig 2). Confocal microscopy analyses using the type reference strains of [P.] pneumotropica biotypes Jawetz and Heyl and of [A.] muris showed that indeed only [P.] pneumotropica strains, but not [A.] muris, were able to produce consistent biofilms (Fig 3).
Fig 1. Biofilm formation in different rodent Pasteurellaceae strains.
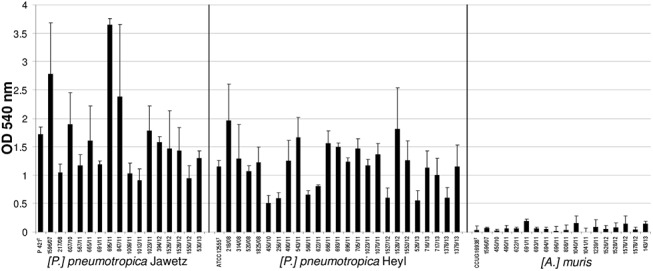
The biofilm formation was recorded after 24 h of growth by a standard crystal violet microtiter plate assay and measuring the absorbance at 540 nm (y-axis). Strains tested are shown on the x-axis and grouped by species. Bars represent the average absorbance + standard deviation from three independent experiments.
Fig 2. Biofilm formation by rodent Pasteurellaceae in glass tubes.

Representative pictures of biofilm formation in glass tubes by [P.] pneumotropica biotype Jawetz (A.), [P.] pneumotropica biotype Heyl (B.) and [A.] muris (C.) after 24 h incubation at 37°C and staining with crystal violet. The strain number is pasted on the corresponding tube. The picture of a tube containing sterile BHI was included in panel A for comparison purposes.
Fig 3. Confocal laser scanning microscopy analysis of rodent Pasteurellaceae biofilms.
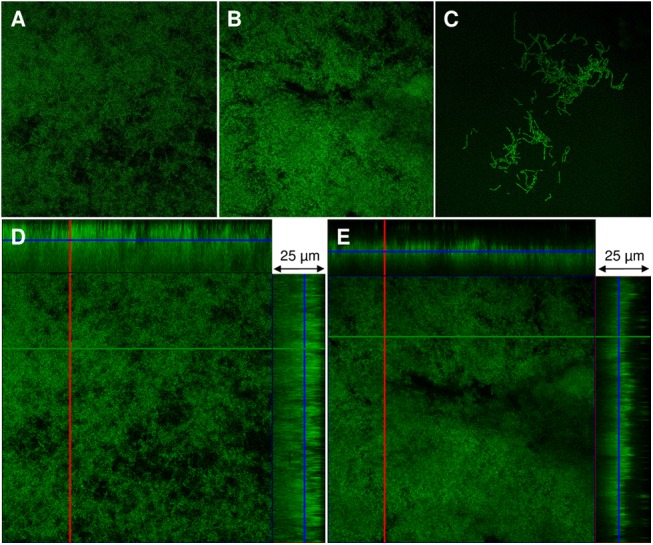
The type reference strains of the three rodent Pasteurellaceae species studied were allowed to produce biofilms for 24 h on glass coverslips and then examined by CLSM as described in Materials and Methods. The upper panel images are two-dimensional images of the biofilms formed by [P.] pneumotropica biotype Jawetz (A), [P.] pneumotropica biotype Heyl (B) and [A.] muris (C). The lower panels are orthogonal views of z-stacks of [P.] pneumotropica biotype Jawetz (D) and [P.] pneumotropica biotype Heyl biofilms (E) where the larger panel is a “bird´s eye” view of the biofilms whereas the right and the upper panels are side views of x- and y-axis sections respectively.
Inhibition of biofilm formation by proteinase K, DNase I and sodium periodate
Proteins, DNA and polysaccharides may play an important role in the extracellular matrix of bacterial biofilms. To address the functional role of these components in the [P.] pneumotropica biofilm formation, we studied the ability of proteinase K, DNase I and sodium periodate to inhibit biofilm development. For this, the components listed above were added into the growth media at the time of bacterial inoculation. Subsequently the cultures were allowed to develop biofilms for 24 h at 37°C.
Cultures of the type reference strains of [P.] pneumotropica biotypes Jawetz und Heyl in BHI containing a range of proteinase K concentrations from 100 to 6.25 μg/ml did not affect the growth of bacteria (S1 Fig). Interestingly, a proteinase K dose dependent inhibition in the formation of biofilms was observed for both type strains of [P.] pneumotropica used (S1 Fig). To test whether the proteinase K inhibition in the biofilm formation is spread among [P.] pneumotropica field isolates or was a specific characteristic of the reference strains, we tested the effect of 100 μg/ml proteinase K on all [P.] pneumotropica isolates included in this study. All but one of the 17 [P.] pneumotropica biotype Jawetz isolates were significantly inhibited in their ability to form biofilm by proteinase K (Fig 4A). Twenty-two of the 25 [P.] pneumotropica biotype Heyl isolates displayed as well a statistically significant reduction in the biofilm formation in the presence of proteinase K (Fig 4B). The p-values for the isolates 1528/12, 450/10, 256/11 and 566/11 which did not show statistically significant differences were 0.06, 0.08, 0.14 and 0.09.
Fig 4. Inhibition of biofilm formation by proteinase K and DNase I in [P.] pneumotropica biotypes Jawetz (A) and Heyl (B).
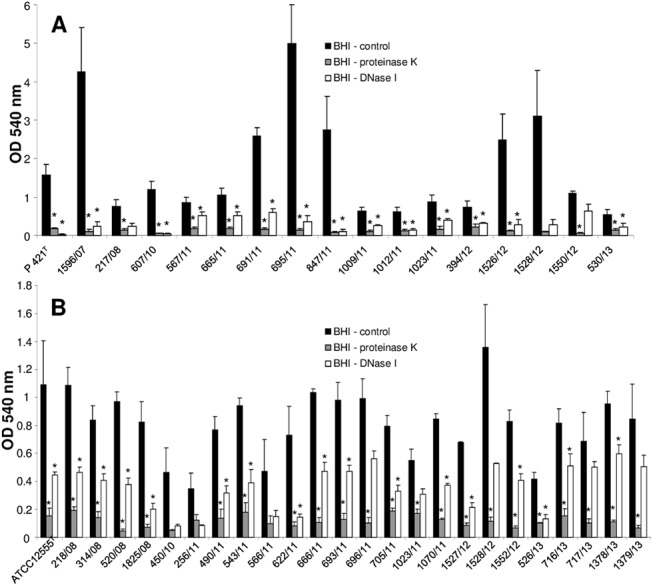
The bacterial strains indicated along the x-axis were grown statically for 24 h in BHI broth alone (black bars) or in BHI broth supplemented with 100 μg/ml proteinase K (grey bars) or 50 μg/ml DNase I (white bars). The biofilm formation was then quantified photometrical at 540 nm after staining with crystal violet. Bars represent mean values + standard deviation of at least three independent experiments. Asterisks (*) designate a p-value less than 0.05 between the treated group and the corresponding control.
Introduction of serial two-fold dilutions of DNase I (from 100 to 6.25 μg/ml) into the growing medium did not affect the growth of [P.] pneumotropica isolates. Similar to the proteinase K, a DNase I dose dependent inhibition in the biofilm formation was observed for the type reference strains (S2 Fig). In addition, the biofilm formation was significantly impaired by the presence of 50 μg/ml DNase I in the growth medium, by all but three of the [P.] pneumotropica biotype Jawetz strains (Fig 4A) and in all but eight [P.] pneumotropica biotype Heyl strains (Fig 4B) tested. Although no statistical significant differences were observed for some strains, probably due to the high standard deviation of the control group, a clear inhibition tendency in biofilm formation was observed (Fig 4) with p-values between 0.05 and 0.1 for all but one of the eleven strains.
To evaluate the role of β-1,6-linked polysaccharides in the biofilm formation, the two [P.] pneumotropica type reference strains were allowed to form biofilms in BHI containing two fold serial dilutions of 10 to 0.625 mM sodium periodate. Even the lowest concentration of sodium periodate (0.625 mM) inhibited the growth of [P.] pneumotropica biotype Jawetz, thus the reduction in the biofilm formation by 2.5 mM sodium periodate could be attributed to the impaired growth of bacteria (S3 Fig). A similar reduction of the biofilm formation at reduced bacterial growth displayed the type strain of [P.] pneumotropica biotype Heyl (S3 Fig). Due to the growth inhibition of the type strains by sodium periodate, no further tests using additional isolates were performed in these experimental settings.
Dispersal of biofilms by proteinase K, DNase I and sodium periodate
To characterize in more detail the role of proteins, DNA and polysaccharides in the structure of mature biofilms, we tested the capability of proteinase K, DNase I and sodium periodate to disperse pre-formed mature biofilms. For this, the same components used for the inhibition of biofilm formation were applied for 2 h on 24 h old biofilms and their ability to disperse biofilm in comparison to non treated controls was recorded.
Concentrations of 100 μg/ml of proteinase K were necessary to achieve a statistically significant disruption of the biofilm produced by both [P.] pneumotropica type strains in comparison with the non-treated controls (S1 Fig). Incubation of the mature biofilms from the field isolates of [P.] pneumotropica with 100 μg/ml proteinase K caused significant detachment in nearly all strains tested (Fig 5). By four strains of [P.] pneumotropica biotype Jawetz and eight strains of [P.] pneumotropica biotype Heyl no statistically significant (p—values between 0.06 and 0.14 and one value of 0.22) proteinase K dispersal of the biofilms was achieved, although visual differences could be recognized.
Fig 5. Dispersal of mature biofilms of [P.] pneumotropica biotypes Jawetz (A) and Heyl (B) by proteinase K.
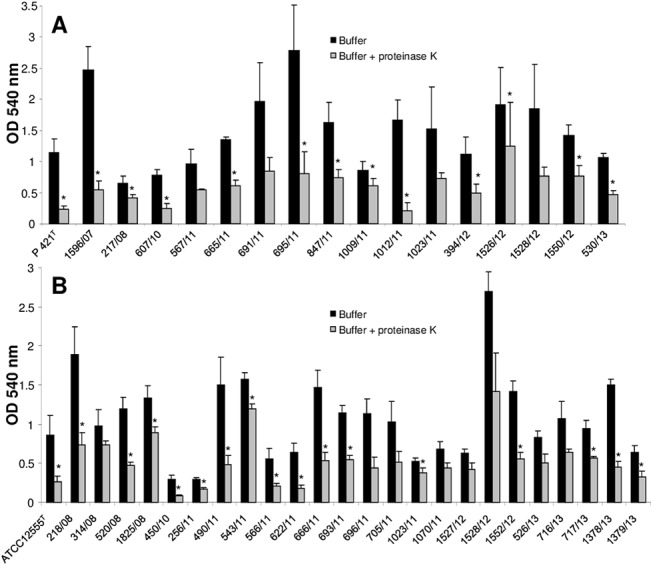
The supernatants of 24 h old biofilms of the strains indicated along the x-axis were replaced for 2 h by buffer alone (black bars) or buffer containing 100 μg/ml proteinase K (grey bars). Biofilm quantity was then recorded using a standard crystal violet assay by measuring the absorbance at 540 nm. Bars represent mean values + standard deviation of at least three independent experiments. Asterisks (*) designate a p-value less than 0.05 between the treated group and the corresponding control.
Treatment of the pre-formed biofilms with DNase I had a similar dispersal effect compared with proteinase K. S2 Fig shows that even 12.5 μg/ml DNase I was enough to cause a significant dispersal of the biofilm in the two type strains of [P.] pneumotropica biotypes Jawetz and Heyl. Notably, all but eight [P.] pneumotropica isolates tested were statistically sensitive to dispersal by 50 μg/ml of DNase I (Fig 6). For the remaining eight strains p-values between 0.06 and 0.09 were recorded.
Fig 6. Dispersal of mature biofilms of [P.] pneumotropica biotypes Jawetz (A) and Heyl (B) by DNase I.
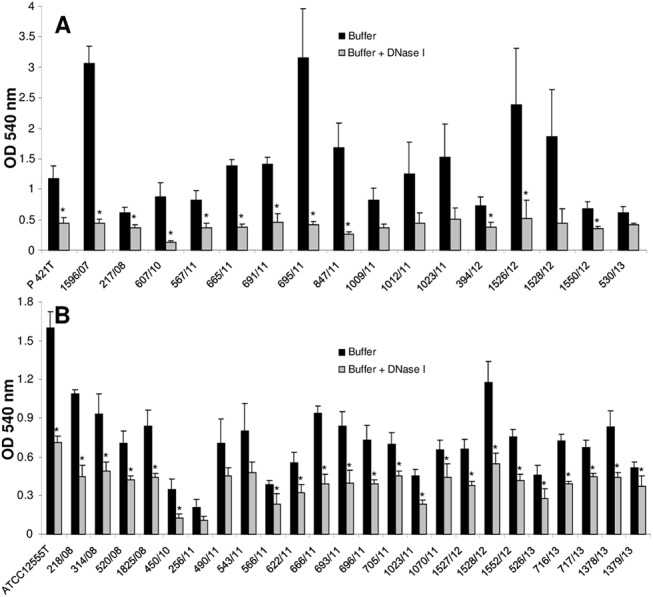
The supernatants of 24 h old biofilms of the strains indicated along the x-axis were replaced for 2 h by buffer alone (black bars) or buffer containing 50 μg/ml DNase I (grey bars). Biofilm quantity was then recorded using a standard crystal violet assay by measuring the absorbance at 540 nm. Bars represent mean values + standard deviation of at least three independent experiments. Asterisks (*) designate a p-value less than 0.05 between the treated group and the corresponding control.
Treatment of the pre-formed biofilms of the reference strains with sodium periodate resulted surprisingly in an increase in the biofilm mass at already 5 mM in the case of [P.] pneumotropica biotype Jawetz and at 10 mM in the case of [P.] pneumotropica biotype Heyl (S3 Fig). However, treatment of the [P.] pneumotropica biotype Jawetz isolates with 10 mM sodium periodate had a varying effect on biofilm dispersal. Six of the 17 strains tested did not show any modification in the biofilm amount after treatment with sodium periodate, whereas a significantly reduced amount of biofilm could be measured in strain 695/11. The remaining ten isolates displayed an increase in the biofilm quantity after treatments with sodium periodate (Fig 7A). The phenomenon of increase in the biofilm amount, after treatment with 10 mM sodium periodate was also observed for all but two of the [P.] pneumotropica biotype Heyl strains tested (Fig 7B).
Fig 7. Dispersal of mature biofilms of [P.] pneumotropica biotypes Jawetz (A) and Heyl (B) by sodium periodate.
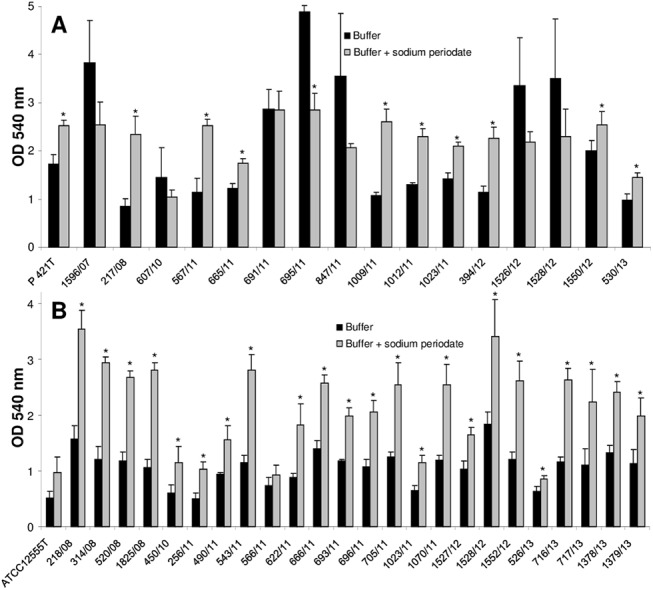
The supernatants of 24 h old biofilms of the strains indicated along the x-axis were replaced for 2 h by buffer alone (black bars) or buffer containing 10 mM sodium periodate (grey bars). Biofilm quantity was then measured by a standard crystal violet assay and measuring the absorbance at 540 nm. Bars represent mean values + standard deviation of at least three independent experiments. Asterisks (*) designate a p-value <0.05 between the treated group and the corresponding control.
Antibiotic susceptibility of planktonic and biofilm bacteria
The MICs of amoxicillin to all [P.] pneumotropica strains used were higher for the bacteria grown in biofilms than for planktonic cells (Table 2). The MICs of enrofloxacin was similar for both biofilm and planktonic cells in three of the strains used (ATCC12555T, CCUG12398T, 394/12), whereas for the strain 1070/11 it was higher for the bacteria grown in biofilm (Table 2). Fig 8 presents the reduction in viability of selected [P.] pneumotropica strains after exposure of planktonic cells or biofilms to amoxicillin and enrofloxacin in comparison to non treated controls. The antibiotics treatment reduced significantly the number of viable cells in both planktonic and biofilm cells in comparison to the non treated controls. However, the viable cells from the antibiotics treated samples were outnumbered by the control cells by a factor of about 10:1 in the case of biofilms and by a factor of about 105:1 in the case of planktonic cells, showing thus a more severe reduction in the cellular viability for the planktonic in comparison to biofilm bacteria.
Table 2. Minimal inhibitory concentrations (MICs) of amoxicillin and enrofloxacin for [P.] pneumotropica in planktonic and biofilm status.
| Strain | Growth | Antibiotic | |
|---|---|---|---|
| Amoxicillin (μg/ml) | Enrofloxacin (μg/ml) | ||
| ATCC12555T | Biofilm | 12.5 | 0.78 |
| Planktonic | <0.19 | 0.78 | |
| 1070/11 | Biofilm | 100 | 12.5 |
| Planktonic | <0.19 | <0.19 | |
| CCUG12398T | Biofilm | 100 | <0.19 |
| Planktonic | 12.5 | <0.19 | |
| 394/12 | Biofilm | 25 | <0.19 |
| Planktonic | <0.19 | <0.19 | |
Fig 8. Reduction of cellular viability of planktonic and biofilm cells of [P.] pneumotropica after 3 hours exposure to antibiotics.
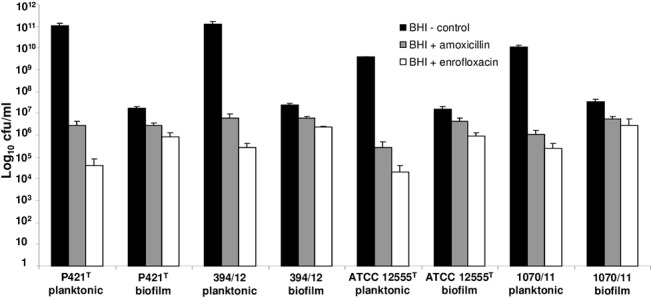
The y-axis indicates the log10 cfu/ml recovered from the controls without antibiotics (black bars) in comparison to bacteria treated with amoxicillin (grey bars) or enrofloxacin (white bars) in planktonic or biofilm status. Bars represent mean values + standard deviation of three independent experiments.
Discussion
Biofilm formation is spread among many bacterial pathogens and plays an important role in the pathogenesis of disease and protection from therapy [4]. To date it is known that many facultative pathogenic members of the Pasteurellaceae family such as P. multocida [39], A. pleuropneumoniae [33], A. actinomycetemcomitans [40], H. influenzae [41], H. somni [1] and H. parasuis [42] are able to form biofilms. In the present investigation we analyzed the capacity of [P.] pneumotropica biotypes Jawetz and Heyl, which can be regarded as two different species [9] and of [A.] muris to form biofilms in vitro. One can depict at first glance from the Figs 1 and 2 that the two [P.] pneumotropica biotypes species strains are able to form robust biofilms, in contrast to [A.] muris isolates which are no biofilm producers under the same experimental conditions. In correlation with the photometrical results, the CLSM imagines definitively show that only [P.] pneumotropica strains but not [A.] muris are able to produce biofilms with a thickness of about 20 μm under the conditions used in this study (Fig 3). Although all three species used in this study belong to the normal murine respiratory tract microbiota as shown recently [43], only [P.] pneumotropica biotypes Jawetz and Heyl possess a known pathogenic potential [13, 16, 44], whereas [A.] muris is considered to be non-pathogenic [21], suggesting that biofilm production might represent a virulence attribute for these bacteria.
To further characterize the mechanisms involved in the biofilm formation and their chemical composition, we tested whether substances targeting major components of the biofilm matrix like proteins, eDNA or polysaccharides could inhibit the biofilm formation or affect established biofilms. Enzymes like proteinase K, DNase I or compounds targeting β-1,6-linked polysaccharides such as sodium periodate have different kinds of actions on particular types of biofilms [35, 45].
Our study demonstrates that proteins are essential in biofilm formation in both [P.] pneumotropica biotypes, since the proteinase K at a concentration that does not affect cell growth, clearly inhibited the biofilm formation (Fig 4). Dispersal of the pre-formed biofilms by the proteinase K treatment demonstrates, using a different approach, that proteins are essential components of the [P.] pneumotropica biofilms (Fig 5). Due to their localization on the surface of bacteria, it is plausible that proteins play an important role in colonization and biofilm formation. Families of surface associated proteins involved in adherence and biofilm formation such as the biofilm-associated proteins (Bap), containing a core domain of tandem repeats that confer bacteria the ability to form a biofilm, seem to be present in many species of Gram-positive and Gram-negative bacteria [46]. In correlation with our findings, proteins have been shown to play a central role in the biofilms of other species belonging to the family of Pasteurellaceae such as Haemophilus parasuis [47] or in important human and animal pathogens such as MRSA [48].
DNase I at concentrations that do not affect bacterial growth, inhibited biofilm formation and dispersed preformed biofilms of [P.] pneumotropica (Figs 4 and 6), suggesting that eDNA similarly to proteins plays a decisive role in the biofilm construction. This finding is in agreement with other studies which also showed the importance of eDNA in biofilm formation for other related or not related bacteria [45, 47–49]. Autolysis is the common mechanism by which eDNA is released. eDNA has a crucial role in stabilizing biofilm structures and represents an important mechanism for horizontal gene transfer in bacteria. Furthermore, it is recognized by the innate immune system via the TLR family receptors [50].
It seemed that sodium periodate did not disturb the biofilm formation of the type reference strains of [P.] pneumotropica biotypes when added to the growth medium. However, the growth of the type strains of [P.] pneumotropica was inhibited by this substance (S3 Fig), thus making an objective evaluation of the role of β-1,6-linked polysaccharides in the biofilm formation in this experimental setting difficult. Consequently, no further inhibition experiments with additional bacterial isolates were performed. Interestingly, treatments of preformed biofilms of [P.] pneumotropica with sodium periodate produced varying effects. On one hand, this resulted in an increased biofilm production in all [P.] pneumotropica biotype Heyl and 10 out of 17 [P.] pneumotropica biotype Jawetz isolates (Fig 7). On the other hand, sodium periodate had no effect or even dispersed the biofilm in some strong biofilm producer isolates of [P.] pneumotropica biotype Jawetz. Capsular polysaccharides seem generally to interfere with the contact of bacteria to surfaces, by masking adhesive structures of other biochemical structure. For example, the capsule impairs the adherence of Streptococcus suis to epithelial cells [51]. Similarly, capsule reduction correlated with increased biofilm formation by some Streptococcus agalactiae strains [49]. Moreover, the capsule of A. pleuropneumoniae possess antibiofilm activity against both Gram-positive and Gram-negative bacteria [52]. [P.] pneumotropica possess a slight extracellular fibrous material associated with the cell wall when stained with ruthenium red [53]. One can speculate that the treatment of [P.] pneumotropica biofilms with sodium periodate and thus removal of the capsular polysaccharides lead to a better surface exposure of proteins important in biofilm formation and thus to an increased biofilm stability, more resistant to the washing steps which preceded the quantification of the biofilm amount. In contrast, it seems that for the strong biofilm producers of the biotype Jawetz, the polymers of β-1,6-linked polysaccharides are integrated in the biofilms, reminding on structures similar to poly-β-1,6-N-acetyl-D-glucosamine (PGA) of E. coli [54], which are also present in [P.] pneumotropica related species such as A. pleuropneumoniae [55], A. actinomycetemcomitans [56] and Haemophilus parasuis [47] or in Gram-positive bacteria such as Staphylococci [57]. The variable behaviour of [P.] pneumotropica biotype Jawetz to sodium periodate suggests that different serotypes might be present within this species as known for other related Pasteurellaceae such as A. pleuropneumoniae. However, further investigations are necessary to confirm these suppositions.
Bacterial biofilms confer an increased resistance of bacteria to antibiotics and represent thus one of the reasons why treatment with antibiotics fails [3]. To study whether [P.] pneumotropica biofilms are able to interfere with the sensitivity to antibiotics we compared the MICs and the viability of planktonic and biofilm cells in the presence of a cell wall synthesis inhibitor (amoxicillin) and of a DNA gyrase inhibitor (enrofloxacin) antibiotic. Several mechanisms have been assumed to contribute to the biofilm resistance phenotype to antibiotics [37]. Our results demonstrate that the MICs of amoxicillin were higher for bacteria in biofilm than for planktonic cells. Similarly, the MIC of enrofloxacin was higher for the biofilm as for the planktonic cells in strain 1070/11. However, for the three other strains tested the MICs to enrofloxacin did not differ in biofilm and planktonic status (Table 2). Attempts to eliminate [P.] pneumotropica from mice colonies by treatment with enrofoxacin proved to be successful as demonstrated recently [58], showing the capacity of this antibiotic to neutralize [P.] pneumotropica cells. Unfortunately, the growth inhibition methods do not give information on the viability of the bacteria. In order to clarify whether the biofilm status confer as well a survival advantage in comparison to planktonic state, we used a cfu viability test. Using this assay, we demonstrate that [P.] pneumotropica cells enclosed in the biofilms were less sensitive than the planktonic cells to both antibiotics tested (Fig 8), which is in agreement with other studies [37, 38, 59]. Whether some of the known biofilm antibiotics resistance mechanisms contributed as well to this phenotype in the case of [P.] pneumotropica was not addressed here.
In conclusion, this is the first report showing that [P.] pneumotropica biotypes Jawetz and Heyl are strong biofilm producers and that this phenotype is uniform distributed among the field isolates. Moreover, we demonstrate that these biofilms, which are composed mainly of proteins and eDNA, displayed an increased resistance to antibiotics in vitro in comparison to planktonic bacteria. It is additionally hypothesized that surface polysaccharides might play a biofilm inhibitory role in [P.] pneumotropica biotype Heyl and in a majority of [P.] pneumotropica biotype Jawetz strains, whereas they might be integrated in the biofilm structure by some other Jawetz isolates. Notably, this study opens several questions to be addressed in the future. For example, further efforts are necessary to identify the proteins and other molecules that are decisive in the biofilm formation. It would be of interest as well to study whether the biofilm formation in vitro implies a similar phenotype on host cells as shown with A. pleuropneumoniae [60] and display an in vivo correspondent into the upper respiratory tract and the genital mucosa of mice. These would represent excellent tools to understand the biofilm mechanisms in vivo for a species specific pathogen such as [P.] pneumotropica, which could represent even a prototype for related microorganisms whose host specificities do not allow their study in vivo as easy as in the mouse model.
Supporting Information
For A and B, the strains P421T and ATCC12555T were allowed to grow and form biofilms in the presence of different concentrations (x-axis) of proteinase K. After 24 h the bacterial growth (grey bars) and subsequently the biofilm amount (black bars) of the same wells were quantified photometrical. To determine the biofilm dispersal capacity of proteinase K (C, D), bacterial growth (grey bars) was recorded on 24 h old biofilms. Subsequently, the wells were treated for 2 h with different concentrations of proteinase K (x-axis), washed and the biofilm amount (black bars) was recorded photometrical (540 nm) by a standard crystal violet assay. Average plus standard deviation of at least three independent experiments are shown. Asterisks (*) assign a p-value <0.05 between the treated groups and the non-treated control.
(TIFF)
The strains P421T and ATCC12555T were allowed to grow and form biofilms in the presence of different concentrations (x-axis) of DNase I (A, B). Bacterial growth (grey bars) and the biofilm amount (black bars) were recorded photometrical by 540 nm after 24 h. For C and D, bacterial growth (grey bars) was recorded on 24 h old biofilms. Subsequently, the wells were treated for 2 h with different concentrations of DNase I (x-axis), washed and the biofilm amount (black bars) was recorded photometrical (540 nm) by a standard crystal violet assay. Average plus standard deviation of at least three independent experiments are shown. Asterisks (*) designate a p-value <0.05 between the treated groups and the non-treated control.
(TIFF)
The strains P421T and ATCC12555T were allowed to grow and form biofilms in the presence of different concentrations (x-axis) of sodium periodate (A, B). Bacterial growth (grey bars) and the biofilm amount (black bars) were assessed 24 h later by measuring the absorbance at 540 nm. To evaluate the dispersal effect on pre-formed biofilms (C, D), the growth of 24 h old biofilms was recorded photometrical (grey bars). Subsequently, the biofilms were treated for 2 h with different concentrations of sodium periodate (x-axis), washed and the biofilm amount (black bars) was recorded photometrical (540 nm) by a standard crystal violet assay. Average plus standard deviation of at least three independent experiments are shown. Asterisks (*) assign a p-value <0.05 between the treated groups and the non-treated control.
(TIFF)
Acknowledgments
We gratefully acknowledge Sonja Green, Andrea Grunwald, Theresa Ohly and Manuela Stockhausen for the excellent technical assistance. Magne Bisgaard and Henrik Christensen (University of Copenhagen, Denmark) are kindly acknowledged for providing the reference strains. We thank Stefanie Weidtkamp-Peters at the Center for Advanced Imaging of the Heinrich-Heine-University Düsseldorf for her support with the confocal microscopy.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Sandal I, Hong W, Swords WE, Inzana TJ. Characterization and comparison of biofilm development by pathogenic and commensal isolates of Histophilus somni. J Bacteriol. 2007;189(22):8179–85. Epub 2007/07/24. doi: JB.00479-07 [pii] 10.1128/JB.00479-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communities. Annu Rev Microbiol. 2002;56:187–209. Epub 2002/07/27. 10.1146/annurev.micro.56.012302.160705 012302.160705 [pii]. . [DOI] [PubMed] [Google Scholar]
- 3. Coenye T, Nelis HJ. In vitro and in vivo model systems to study microbial biofilm formation. J Microbiol Methods. 2010;83(2):89–105. Epub 2010/09/08. doi: S0167-7012(10)00284-8 [pii] 10.1016/j.mimet.2010.08.018 . [DOI] [PubMed] [Google Scholar]
- 4. Clutterbuck AL, Woods EJ, Knottenbelt DC, Clegg PD, Cochrane CA, Percival SL. Biofilms and their relevance to veterinary medicine. Vet Microbiol. 2007;121(1–2):1–17. Epub 2007/02/06. doi: S0378-1135(06)00527-X [pii] 10.1016/j.vetmic.2006.12.029 . [DOI] [PubMed] [Google Scholar]
- 5. Benga L, Benten WP, Engelhardt E, Christensen H, Sager M. Analysis of 16S-23S rRNA internal transcribed spacer regions in Pasteurellaceae isolated from laboratory rodents. J Microbiol Methods. 2012;90(3):342–9. Epub 2012/07/10. doi: S0167-7012(12)00226-6 [pii] 10.1016/j.mimet.2012.06.013 . [DOI] [PubMed] [Google Scholar]
- 6. Mutters R CH, Bisgaard M, editor. Genus I Pasteurella Trevisan 1887. 2nd ed. New York: Springer; 2005. [Google Scholar]
- 7. Jawetz E. A pneumotropic pasteurella of laboratory animals; bacteriological and serological characteristics of the organism. J Infect Dis. 1950;86(2):172–83. Epub 1950/03/01. . [DOI] [PubMed] [Google Scholar]
- 8. Heyl JG. A study of Pasteurella strains from animal sources. Antonie Van Leeuwenhoek. 1963;29:79–83. Epub 1963/01/01. . [DOI] [PubMed] [Google Scholar]
- 9. Christensen H, Foster G, Christensen JP, Pennycott T, Olsen JE, Bisgaard M. Phylogenetic analysis by 16S rDNA gene sequence comparison of avian taxa of Bisgaard and characterization and description of two new taxa of Pasteurellaceae. J Appl Microbiol. 2003;95(2):354–63. Epub 2003/07/16. doi: 1986 [pii]. . [DOI] [PubMed] [Google Scholar]
- 10. Dole VS, Banu LA, Fister RD, Nicklas W, Henderson KS. Assessment of rpoB and 16S rRNA genes as targets for PCR-based identification of Pasteurella pneumotropica. Comp Med. 2010;60(6):427–35. Epub 2011/01/26. [PMC free article] [PubMed] [Google Scholar]
- 11. Hayashimoto N, Takakura A, Itoh T. Genetic diversity on 16S rDNA sequence and phylogenic tree analysis in Pasteurella pneumotropica strains isolated from laboratory animals. Curr Microbiol. 2005;51(4):239–43. Epub 2005/09/28. 10.1007/s00284-005-4541-6 . [DOI] [PubMed] [Google Scholar]
- 12. Hayashimoto N, Ueno M, Takakura A, Itoh T. Phylogenetic analysis of isolates of Pasteurella pneumotropica from laboratory animals based on the gyrB gene sequence. Exp Anim. 2006;55(5):487–90. Epub 2006/11/09. doi: JST.JSTAGE/expanim/55.487 [pii]. . [DOI] [PubMed] [Google Scholar]
- 13. Artwohl JE, Flynn JC, Bunte RM, Angen O, Herold KC. Outbreak of Pasteurella pneumotropica in a closed colony of STOCK-Cd28(tm1Mak) mice. Contemp Top Lab Anim Sci. 2000;39(1):39–41. Epub 2001/02/15. . [PubMed] [Google Scholar]
- 14. Macy JD Jr, Weir EC, Compton SR, Shlomchik MJ, Brownstein DG. Dual infection with Pneumocystis carinii and Pasteurella pneumotropica in B cell-deficient mice: diagnosis and therapy. Comp Med. 2000;50(1):49–55. Epub 2000/09/15. . [PubMed] [Google Scholar]
- 15. Ueno Y, Shimizu R, Nozu R, Takahashi S, Yamamoto M, Sugiyama F, et al. Elimination of Pasteurella pneumotropica from a contaminated mouse colony by oral administration of Enrofloxacin. Exp Anim. 2002;51(4):401–5. Epub 2002/09/12. . [DOI] [PubMed] [Google Scholar]
- 16. Benga L, Benten WP, Engelhardt E, Gougoula C, Sager M. Spontaneous bacterial and fungal infections in genetically engineered mice: Is Escherichia coli an emerging pathogen in laboratory mouse? Berl Munch Tierarztl Wochenschr. 2015;128(7–8):278–84. . [PubMed] [Google Scholar]
- 17. Cuadrado-Gomez LM, Arranz-Caso JA, Cuadros-Gonzalez J, Albarran-Hernandez F. Pasteurella pneumotropica pneumonia in a patient with AIDS. Clin Infect Dis. 1995;21(2):445–6. Epub 1995/08/01. . [DOI] [PubMed] [Google Scholar]
- 18. Frebourg NB, Berthelot G, Hocq R, Chibani A, Lemeland JF. Septicemia due to Pasteurella pneumotropica: 16S rRNA sequencing for diagnosis confirmation. J Clin Microbiol. 2002;40(2):687–9. Epub 2002/02/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patten CC Jr, Myles MH, Franklin CL, Livingston RS. Perturbations in cytokine gene expression after inoculation of C57BL/6 mice with Pasteurella pneumotropica. Comp Med. 2010;60(1):18–24. Epub 2010/02/18. [PMC free article] [PubMed] [Google Scholar]
- 20. Bisgaard M. Actinobacillus muris sp. nov. isolated from mice. Acta Pathol Microbiol Immunol Scand B. 1986;94(1):1–8. Epub 1986/02/01. . [DOI] [PubMed] [Google Scholar]
- 21. Benga L, Benten WP, Engelhardt E, Gougoula C, Sager M. Specific detection and identification of [Actinobacillus] muris by PCR using primers targeting the 16S-23S rRNA internal transcribed spacer regions. J Microbiol Methods. 2013;94(2):88–93. Epub 2013/05/15. doi: S0167-7012(13)00153-X [pii] 10.1016/j.mimet.2013.05.002 . [DOI] [PubMed] [Google Scholar]
- 22. Sasaki H, Kawamoto E, Tanaka Y, Sawada T, Kunita S, Yagami K. Identification and characterization of hemolysin-like proteins similar to RTX toxin in Pasteurella pneumotropica. J Bacteriol. 2009;191(11):3698–705. Epub 2009/04/14. doi: JB.01527-08 [pii] 10.1128/JB.01527-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sasaki H, Ishikawa H, Kojima K, Itoh M, Matsumoto T, Itoh T, et al. Intranasal immunization with a non-adjuvanted adhesive protein descended from Pasteurella pneumotropica and its preventive efficacy against opportunistic infection in mice. Vaccine. 2013;31(48):5729–35. Epub 2013/10/05. doi: S0264-410X(13)01274-7 [pii] 10.1016/j.vaccine.2013.09.033 . [DOI] [PubMed] [Google Scholar]
- 24. See SB, Thomas WR. Protective anti-outer membrane protein immunity against Pasteurella pneumotropica infection of mice. Microbes Infect. 2013;15(6–7):470–9. Epub 2013/04/30. doi: S1286-4579(13)00073-7 [pii] 10.1016/j.micinf.2013.04.003 . [DOI] [PubMed] [Google Scholar]
- 25. Sasaki H, Ishikawa H, Asano R, Ueshiba H, Matsumoto T, Boot R, et al. Draft Genome Sequence of the Rodent Opportunistic Pathogen Pasteurella pneumotropica ATCC 35149T. Genome Announc. 2014;2(4). Epub 2014/08/12. doi: 2/4/e00771-14 [pii] 10.1128/genomeA.00771-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kawai T, Paster BJ, Komatsuzawa H, Ernst CW, Goncalves RB, Sasaki H, et al. Cross-reactive adaptive immune response to oral commensal bacteria results in an induction of receptor activator of nuclear factor-kappaB ligand (RANKL)-dependent periodontal bone resorption in a mouse model. Oral Microbiol Immunol. 2007;22(3):208–15. Epub 2007/05/10. doi: OMI348 [pii] 10.1111/j.1399-302X.2007.00348.x . [DOI] [PubMed] [Google Scholar]
- 27. Silva MJ, Kajiya M, AlShwaimi E, Sasaki H, Hong J, Ok P, et al. Bacteria-reactive immune response may induce RANKL-expressing T cells in the mouse periapical bone loss lesion. J Endod. 2012;38(3):346–50. Epub 2012/02/22. doi: S0099-2399(11)01462-2 [pii] 10.1016/j.joen.2011.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hart ML, Mosier DA, Chapes SK. Toll-like receptor 4-positive macrophages protect mice from Pasteurella pneumotropica-induced pneumonia. Infect Immun. 2003;71(2):663–70. Epub 2003/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sahagun-Ruiz A, Granados Martinez AP, Breda LC, Fraga TR, Castiblanco Valencia MM, Barbosa AS, et al. Pasteurella pneumotropica evades the human complement system by acquisition of the complement regulators factor H and C4BP. PLoS One. 2014;9(10):e111194 Epub 2014/10/28. 10.1371/journal.pone.0111194 PONE-D-14-16412 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mahler Convenor M, Berard M, Feinstein R, Gallagher A, Illgen-Wilcke B, Pritchett-Corning K, et al. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab Anim. 2014. Epub 2014/02/06. doi: 0023677213516312 [pii] 10.1177/0023677213516312 . [DOI] [PubMed] [Google Scholar]
- 31. Benga L, Benten WP, Engelhardt E, Bleich A, Gougoula C, Sager M. Development of a multiplex PCR assay based on the 16S-23S rRNA internal transcribed spacer for the detection and identification of rodent Pasteurellaceae. J Microbiol Methods. 2013;95(2):256–61. 10.1016/j.mimet.2013.09.005 . [DOI] [PubMed] [Google Scholar]
- 32. Labrie J, Pelletier-Jacques G, Deslandes V, Ramjeet M, Auger E, Nash JH, et al. Effects of growth conditions on biofilm formation by Actinobacillus pleuropneumoniae. Vet Res. 2010;41(1):3 Epub 2009/09/10. 10.1051/vetres/2009051v09366 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaplan JB, Mulks MH. Biofilm formation is prevalent among field isolates of Actinobacillus pleuropneumoniae. Vet Microbiol. 2005;108(1–2):89–94. Epub 2005/05/27. doi: S0378-1135(05)00080-5 [pii] 10.1016/j.vetmic.2005.02.011 . [DOI] [PubMed] [Google Scholar]
- 34. Kumar Shukla S, Rao TS. Dispersal of Bap-mediated Staphylococcus aureus biofilm by proteinase K. J Antibiot (Tokyo). 2013;66(2):55–60. Epub 2012/11/15. doi: ja201298 [pii] 10.1038/ja.2012.98 . [DOI] [PubMed] [Google Scholar]
- 35. Kaplan JB, Velliyagounder K, Ragunath C, Rohde H, Mack D, Knobloch JK, et al. Genes involved in the synthesis and degradation of matrix polysaccharide in Actinobacillus actinomycetemcomitans and Actinobacillus pleuropneumoniae biofilms. J Bacteriol. 2004;186(24):8213–20. Epub 2004/12/04. doi: 186/24/8213 [pii] 10.1128/JB.186.24.8213-8220.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Izano EA, Amarante MA, Kher WB, Kaplan JB. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl Environ Microbiol. 2008;74(2):470–6. Epub 2007/11/28. doi: AEM.02073-07 [pii] 10.1128/AEM.02073-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cerca N, Martins S, Cerca F, Jefferson KK, Pier GB, Oliveira R, et al. Comparative assessment of antibiotic susceptibility of coagulase-negative staphylococci in biofilm versus planktonic culture as assessed by bacterial enumeration or rapid XTT colorimetry. J Antimicrob Chemother. 2005;56(2):331–6. Epub 2005/06/28. doi: dki217 [pii] 10.1093/jac/dki217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grenier D, Grignon L, Gottschalk M. Characterisation of biofilm formation by a Streptococcus suis meningitis isolate. Vet J. 2009;179(2):292–5. Epub 2007/10/30. doi: S1090-0233(07)00322-X [pii] 10.1016/j.tvjl.2007.09.005 . [DOI] [PubMed] [Google Scholar]
- 39. Olson ME, Ceri H, Morck DW, Buret AG, Read RR. Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can J Vet Res. 2002;66(2):86–92. Epub 2002/05/07. [PMC free article] [PubMed] [Google Scholar]
- 40. Kaplan JB, Meyenhofer MF, Fine DH. Biofilm growth and detachment of Actinobacillus actinomycetemcomitans. J Bacteriol. 2003;185(4):1399–404. Epub 2003/02/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murphy TF, Kirkham C. Biofilm formation by nontypeable Haemophilus influenzae: strain variability, outer membrane antigen expression and role of pili. BMC Microbiol. 2002;2:7 Epub 2002/04/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jin H, Zhou R, Kang M, Luo R, Cai X, Chen H. Biofilm formation by field isolates and reference strains of Haemophilus parasuis. Vet Microbiol. 2006;118(1–2):117–23. Epub 2006/09/09. doi: S0378-1135(06)00258-6 [pii] 10.1016/j.vetmic.2006.07.009 . [DOI] [PubMed] [Google Scholar]
- 43. Yun Y, Srinivas G, Kuenzel S, Linnenbrink M, Alnahas S, Bruce KD, et al. Environmentally determined differences in the murine lung microbiota and their relation to alveolar architecture. PLoS One. 2014;9(12):e113466 Epub 2014/12/04. 10.1371/journal.pone.0113466 PONE-D-14-20659 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Foreman O, Kavirayani AM, Griffey SM, Reader R, Shultz LD. Opportunistic bacterial infections in breeding colonies of the NSG mouse strain. Vet Pathol. 2011;48(2):495–9. Epub 2010/09/08. doi: 0300985810378282 [pii] 10.1177/0300985810378282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fredheim EG, Klingenberg C, Rohde H, Frankenberger S, Gaustad P, Flaegstad T, et al. Biofilm formation by Staphylococcus haemolyticus. J Clin Microbiol. 2009;47(4):1172–80. Epub 2009/01/16. doi: JCM.01891-08 [pii] 10.1128/JCM.01891-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lasa I, Penades JR. Bap: a family of surface proteins involved in biofilm formation. Res Microbiol. 2006;157(2):99–107. Epub 2006/01/24. doi: S0923-2508(05)00272-X [pii] 10.1016/j.resmic.2005.11.003 . [DOI] [PubMed] [Google Scholar]
- 47. Bello-Orti B, Deslandes V, Tremblay YD, Labrie J, Howell KJ, Tucker AW, et al. Biofilm formation by virulent and non-virulent strains of Haemophilus parasuis. Vet Res. 2014;45:104. Epub 2014/11/28. doi: s13567-014-0104-9 [pii] 10.1186/s13567-014-0104-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nicholson TL, Shore SM, Smith TC, Frana TS. Livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) isolates of swine origin form robust biofilms. PLoS One. 2013;8(8):e73376 Epub 2013/08/21. 10.1371/journal.pone.0073376 PONE-D-12-28929 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. D'Urzo N, Martinelli M, Pezzicoli A, De Cesare V, Pinto V, Margarit I, et al. Acidic pH strongly enhances in vitro biofilm formation by a subset of hypervirulent ST-17 Streptococcus agalactiae strains. Appl Environ Microbiol. 2014;80(7):2176–85. Epub 2014/02/04. doi: AEM.03627-13 [pii] 10.1128/AEM.03627-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Montanaro L, Poggi A, Visai L, Ravaioli S, Campoccia D, Speziale P, et al. Extracellular DNA in biofilms. Int J Artif Organs. 2011;34(9):824–31. Epub 2011/11/19. doi: 30C13724-F540-40CF-B73C-2C653C6C414A [pii] 10.5301/ijao.5000051 . [DOI] [PubMed] [Google Scholar]
- 51. Benga L, Goethe R, Rohde M, Valentin-Weigand P. Non-encapsulated strains reveal novel insights in invasion and survival of Streptococcus suis in epithelial cells. Cell Microbiol. 2004;6(9):867–81. Epub 2004/07/27. 10.1111/j.1462-5822.2004.00409.x CMI409 [pii]. . [DOI] [PubMed] [Google Scholar]
- 52. Karwacki MT, Kadouri DE, Bendaoud M, Izano EA, Sampathkumar V, Inzana TJ, et al. Antibiofilm activity of Actinobacillus pleuropneumoniae serotype 5 capsular polysaccharide. PLoS One. 2013;8(5):e63844 Epub 2013/05/22. 10.1371/journal.pone.0063844 PONE-D-12-24878 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kawamoto E, Okiyama E, Sasaki H, Sawada T, Mikazuki K, Ueshiba H. Ultrastructural characteristics of the external surfaces of Pasteurella pneumotropica from mice and Pasteurella multocida from rabbits. Lab Anim. 2007;41(2):285–91. Epub 2007/04/14. 10.1258/002367707780378087 . [DOI] [PubMed] [Google Scholar]
- 54. Wang X, Preston JF, 3rd, Romeo T. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J Bacteriol. 2004;186(9):2724–34. Epub 2004/04/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Izano EA, Sadovskaya I, Vinogradov E, Mulks MH, Velliyagounder K, Ragunath C, et al. Poly-N-acetylglucosamine mediates biofilm formation and antibiotic resistance in Actinobacillus pleuropneumoniae. Microb Pathog. 2007;43(1):1–9. Epub 2007/04/07. doi: S0882-4010(07)00022-8 [pii] 10.1016/j.micpath.2007.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Izano EA, Sadovskaya I, Wang H, Vinogradov E, Ragunath C, Ramasubbu N, et al. Poly-N-acetylglucosamine mediates biofilm formation and detergent resistance in Aggregatibacter actinomycetemcomitans. Microb Pathog. 2008;44(1):52–60. Epub 2007/09/14. doi: S0882-4010(07)00101-5 [pii] 10.1016/j.micpath.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gotz F. Staphylococcus and biofilms. Mol Microbiol. 2002;43(6):1367–78. Epub 2002/04/16. doi: 2827 [pii]. . [DOI] [PubMed] [Google Scholar]
- 58. Towne JW, Wagner AM, Griffin KJ, Buntzman AS, Frelinger JA, Besselsen DG. Elimination of Pasteurella pneumotropica from a mouse barrier facility by using a modified enrofloxacin treatment regimen. J Am Assoc Lab Anim Sci. 2014;53(5):517–22. Epub 2014/09/26. [PMC free article] [PubMed] [Google Scholar]
- 59. Hu Q, Han X, Zhou X, Ding S, Ding C, Yu S. Characterization of biofilm formation by Riemerella anatipestifer. Vet Microbiol. 2010;144(3–4):429–36. Epub 2010/03/17. doi: S0378-1135(10)00113-6 [pii] 10.1016/j.vetmic.2010.02.023 . [DOI] [PubMed] [Google Scholar]
- 60. Auger E, Deslandes V, Ramjeet M, Contreras I, Nash JH, Harel J, et al. Host-pathogen interactions of Actinobacillus pleuropneumoniae with porcine lung and tracheal epithelial cells. Infect Immun. 2009;77(4):1426–41. Epub 2009/01/14. doi: IAI.00297-08 [pii] 10.1128/IAI.00297-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For A and B, the strains P421T and ATCC12555T were allowed to grow and form biofilms in the presence of different concentrations (x-axis) of proteinase K. After 24 h the bacterial growth (grey bars) and subsequently the biofilm amount (black bars) of the same wells were quantified photometrical. To determine the biofilm dispersal capacity of proteinase K (C, D), bacterial growth (grey bars) was recorded on 24 h old biofilms. Subsequently, the wells were treated for 2 h with different concentrations of proteinase K (x-axis), washed and the biofilm amount (black bars) was recorded photometrical (540 nm) by a standard crystal violet assay. Average plus standard deviation of at least three independent experiments are shown. Asterisks (*) assign a p-value <0.05 between the treated groups and the non-treated control.
(TIFF)
The strains P421T and ATCC12555T were allowed to grow and form biofilms in the presence of different concentrations (x-axis) of DNase I (A, B). Bacterial growth (grey bars) and the biofilm amount (black bars) were recorded photometrical by 540 nm after 24 h. For C and D, bacterial growth (grey bars) was recorded on 24 h old biofilms. Subsequently, the wells were treated for 2 h with different concentrations of DNase I (x-axis), washed and the biofilm amount (black bars) was recorded photometrical (540 nm) by a standard crystal violet assay. Average plus standard deviation of at least three independent experiments are shown. Asterisks (*) designate a p-value <0.05 between the treated groups and the non-treated control.
(TIFF)
The strains P421T and ATCC12555T were allowed to grow and form biofilms in the presence of different concentrations (x-axis) of sodium periodate (A, B). Bacterial growth (grey bars) and the biofilm amount (black bars) were assessed 24 h later by measuring the absorbance at 540 nm. To evaluate the dispersal effect on pre-formed biofilms (C, D), the growth of 24 h old biofilms was recorded photometrical (grey bars). Subsequently, the biofilms were treated for 2 h with different concentrations of sodium periodate (x-axis), washed and the biofilm amount (black bars) was recorded photometrical (540 nm) by a standard crystal violet assay. Average plus standard deviation of at least three independent experiments are shown. Asterisks (*) assign a p-value <0.05 between the treated groups and the non-treated control.
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


