Abstract
Background
The aims of the study were to examine the association between CKD and the metabolic syndrome (MetS) and its components in older adults. We also explored two possible pathways linking the metabolic syndrome with CKD: inflammation as measured by high sensitivity C-Reactive Protein (hsCRP) and insulin resistance as measured by HOMA-IR.
Methods
Community-dwelling non-diabetic 70+ adults from the Einstein Aging Study participated in the study. We defined CKD as eGFR below 60mL/min/1.73m2. MetS was defined according to recent guidelines from the National Cholesterol Education Program. Binary logistic regressions were used to assess the association between the metabolic syndrome, its components and CKD with adjustments for demographics, HOMA-IR and hsCRP.
Results
Of 616 participants (mean age = 79.3 years, 65.5% female), 25% had MetS and 26.5% had CKD. Participants with CKD had a significantly higher prevalence of the MetS than individuals without CKD (34.4% vs. 24.3%). Binary logistic regression models showed that CKD was associated with MetS (OR = 1.72, 95%CI = 1.13–2.61). The association was unaltered by adjustment for hsCRP but altered by adjustment for HOMA-IR. As the number of MetS components increased the relative odds of CKD also increased. None of the individual components was independently associated with CKD.
Conclusion
MetS is associated with CKD in non-diabetic older adults. Results showed that as the number of MetS components increased so did the odds for CKD. HOMA-IR seems to be in the casual pathway linking MetS to CKD.
Introduction
About 47 million people in the US have the metabolic syndrome (MetS), 42% of whom are over the age of 70 [1]. Up to 20 million individuals have chronic kidney disease (CKD), 47% of whom are over the age of 70 [2,3]. Due to their increasing prevalence rates, both the MetS and CKD have emerged as significant public health problems [4].
The MetS is characterized by a constellation of CVD risk-factors and morbidities that include the presence of at least three of the following components: elevated fasting glucose, elevated triglycerides, elevated blood pressure, elevated waist circumference, and low high-density lipoprotein (HDL) cholesterol [1]. There is increasing evidence that the MetS is associated with incident and prevalent CKD, often defined by a poor glomerular filtration rate (GFR) below 60mL/min/1.73m [2,5]. Indeed individuals with CKD have higher prevalence rates of MetS components than individuals without CKD. Persons with CKD have higher rates of traditional and nontraditional cardiovascular risk factors, including insulin resistance and elevation of inflammatory markers [6, 7]. Insulin resistance is associated with diabetes, cerebrovascular disease, central obesity and hypertension [8–11]; it is characterized by a complex network of nutritional and metabolic changes that also include inflammation, oxidative stress, vitamin D deficiency, anemia, and malnutrition [12]. High sensitivity C-reactive protein (hsCRP), which is generally considered a marker of low-grade chronic inflammation, has also been associated with central obesity, atherosclerosis, hypertension and other components of the MetS [11, 13, 14]. It is thought that insulin resistance and inflammation are the main culprits in the development of CKD [5, 12, 15, 16].
Inflammation and insulin resistance have rarely been examined simultaneously as potential mediators of the influence of MetS on CKD [11, 17–21]. The Atherosclerosis Risk in Community (ARIC) Study, which consists of 15,000 individuals between the ages of 45 and 64 [20] and the Third National Health and Nutrition Examination Survey (NHANES), which includes over 6,000 adults over the age of 20 [17] as well as international studies (e.g. Ming et al.[21] investigated the CKD-MetS association in 15,987 Chinese individuals over the age of 20) have investigated the association between the MetS and CKD. However, these studies overlooked major risk factors, such as inflammation and insulin resistance that are also highly associated with CKD [17, 19] and that are potential pathways which may attenuate the MetS and CKD association. Exploring the interrelationships of the MetS, its components as well as insulin resistance and inflammation with CKD will help identify potential casual pathways. Research in community-dwelling elderly is also still lacking, mainly because most studies make use of lifespan datasets [11, 17, 19, 20]. Determining the associations of these metabolic risk factors with CKD in persons over age 70 is crucial to determining whether prevention strategies and treatment should be tailored to this older segment of the population.
In this study we aimed to explore the association between MetS and CKD. We also examined two potential casual pathways, inflammation and insulin resistance, to determine if they attenuate the associations between MetS and CKD. Our hypotheses were that the MetS is associated with CKD; insulin resistance and inflammation moderate this association but do not fully attenuate it.
We planned a set of secondary aims that included exploring the association between CKD and number of MetS components present (i.e. from 0 up to 4+), and exploring the association between CKD and individual components of the MetS to determine the number of components and the specific components that are associated with the MetS.
Methods
This analysis was cross-sectional and was conducted within a subset of the Einstein Aging Study (EAS) cohort. EAS enrolls community-dwelling, English-speaking residents of Bronx county in New York who are 70 years or older. Participants were systematically recruited from the Health Care Financing Administration/Centers for Medicaid and Medicare Services rosters for Medicare-eligible persons who were 70 years or older between 1993 and 2004, and from New York City Board of Elections from 2004 onwards. Individuals are first mailed introductory letters about the study and research assistants then followed up by phoning to obtain oral consent and administer a brief screening interview. Participants were excluded if they had visual and/or auditory impairments that interfere with neuropsychological testing, psychiatric symptomatology that interferes with test completion, or a nonambulatory status. The study protocol was approved by the Albert Einstein College of Medicine Institutional Review Board. Written informed consent is obtained on their first clinical visit [22]. Individuals with dementia and diabetes at baseline status for eGFR were excluded from these analyses. A diagnosis of dementia was assigned at case conferences attended by a study neurologist, neuropsychologist, and a geriatric nurse clinician, using standardized criteria from the Diagnostic and Statistical Manual, Fourth Edition (DSM-IV) [23], which required impairment in memory plus at least 1 additional cognitive domain, accompanied by evidence of functional decline. History of diabetes was defined if the participant replied yes during the clinical interview to the question: “Did a doctor ever tell you that you have diabetes?” Baseline status here refers to the first wave of data for which participants have eGFR data.
Demographic characteristics
We used age, gender, race, and years of education, current smoking status and alcohol intake in the past month as covariates in our models. These were collected from the clinical interview. Smoking and alcohol were included in the demographic covariates due to their known association with CKD.
Definitions
We defined the metabolic syndrome as three of more of the following criteria, according to the National Cholesterol Education Program Adult Treatment Panel III [24]:
Elevated waist circumference (≥102cm in men, and ≥88cm in women),
Elevated triglycerides (≥150mg/dl),
Reduced HDL cholesterol (<40mg/dL in men and <50mg/dL in women),
High blood pressure (≥103/≥85mmHg or the use of antihypertensive medications)
Elevated fasting glucose (≥100mg/dL).
We used high sensitivity C—reactive protein (hsCRP, mg/L) to assess inflammation. We used this as a continuous variable. We ran models using both raw units of hsCRP and its log transformation; since results remained similar after log transformation, we used the raw scores for our analyses.
Insulin resistance (IR) was defined using the homeostasis model assessment insulin resistance (HOMA-IR) equation [25]: [insulin (ulu/mL) x glucose (mmol/L) / 22.5]. We also used this as a continuous variable.
Chronic Kidney Disease (CKD) was defined as eGFR below 60 mL/min/1.73m2. We estimated eGFR in mL/min/1.73m2 using the Modification of Diet in Renal Disease (MDRD [26]) formula:
eGFR = 186 × Serum Creatinine-1.154 × Age-0.203 × [1.210 if Black] × [0.742 if Female]
The MDRD formula has been recommended for use in older people [27].
Statistical analysis
Participants were divided into two subgroups; with and without metabolic syndrome. Differences in demographic and clinical characteristics were analyzed for the two subgroups. The independent t-test was used for continuous variables and the chi-square for categorical variables.
Participants were also divided according to with and without CKD to analyze means and prevalence rates of the metabolic syndrome and its individual components.
First, binary logistic regressions were used to assess the association of each of the metabolic syndrome components and CKD. The first model was adjusted for demographics, the second and third models were adjusted for hsCRP and HOMA-IR to find out if these mediated any associations. The fourth model was adjusted for demographics, hsCRP and HOMA-IR. Binary logistic regression models with the same adjustments were also used to assess the association between the number of components of the metabolic syndrome and CKD and lastly, to assess the association between individual components of the MetS and CKD. In these models we also included hsCRP and HOMA-IR to examine whether the association between number of, and individual metabolic syndrome components and CKD is modified by the presence of inflammation and/or insulin resistance. A Bonferroni correction [28] factor (with an adjusted p value of 0.01 for the five metabolic components (a = 0.05, five metabolic components) and for the number of metabolic components present (0, 1, 2, 3, or 4+; a = 0.05, five possible scenarios) was used to correct for Type I error in models that included multiple testing.
Results
Table 1 lists the demographic and clinical characteristics of the population according to participants with and without the metabolic syndrome. Of the 616 participants included in the study, the mean age was 79.3 (SD = 5.5), 34.7% were male and 25.8% were black. 25% of the cohort met criteria for the metabolic syndrome and 26.5% were classified as having CKD. In contrast to participants without the metabolic syndrome, those with the metabolic syndrome had fewer years of education, significantly lower levels of eGFR and significantly higher prevalence rates of CKD. They also had higher mean level of hsCRP, and significantly higher levels of insulin resistance (Table 1). Fig 1 shows the most prevalent components of the metabolic syndrome, which were high blood pressure (n = 500, 81.2%) and elevated waist circumference (n = 276, 44.8%); and Fig 2 shows that most of these older individuals had at least 2 metabolic syndrome component risk factors present (n = 226, 32.7%). In total there were 151 (26.5%) participants defined as having CKD on the basis of eGFR. Participants classified as CKD had a significantly higher proportion of the metabolic syndrome than those without CKD (34.4% vs. 24.3%, p = .017). They were also on average significantly older (80.5 vs. 78.9, p = .004) and showed significantly higher HOMR-IR (8.29 vs. 5.42, p = .012) and higher mean hsCRP, although not significantly different from the no CKD group (4.04 vs. 3.39, p = 2.00). Table 2 shows the means and prevalence of individual metabolic syndrome components according to presence of CKD. Participants with CKD had significantly higher prevalence of elevated fasting glucose, elevated triglycerides and low HDL cholesterol than individuals without CKD.
Table 1. Demographic and clinical characteristics of the study participants with and without the metabolic syndrome.
| Metabolic syndrome | |||
|---|---|---|---|
| No | Yes | P | |
| (n = 416, 73%) | (n = 154, 27%) | ||
| Demographics | |||
| Age (y) | 79.5 (5.6) | 78.3 (5.3) | .017 |
| Male (%) | 164 (39.4) | 49 (31.8) | .096 |
| Non-Hispanic white (%) | 286 (68.8) | 108 (70.1) | .904 |
| Non-Hispanic black (%) | 107 (25.7) | 40 (26.0) | |
| Other (%) | 23 (5.5) | 6 (3.9) | |
| Education (y) | 14.4 (3.4) | 14.1 (3.2) | .209 |
| Current smokers (%) | 17 (4.1) | 7 (4.6) | .818 |
| Alcohol consumption (%) | 59 (14.2) | 25 (16.2) | .547 |
| Clinical characteristics | |||
| CKD (%) | 99 (23.8) | 52 (33.8) | .017 |
| eGFR (1.73/m2) | 72.6 (18.7) | 66.0 (17.8) | .000 |
| Waist circumference (cm) | 90.3 (11.3) | 100.0 (10.8) | .000 |
| Triglycerides (mg/dL) | 93.0 (93.4) | 164.1 (87.6) | .000 |
| HDL cholesterol (mg/dL) | 62.2 (15.4) | 49.1 (12.4) | .000 |
| Systolic blood pressure (mmHg) | 136.0 (18.0) | 137.6 (16.2) | .304 |
| Diastolic blood pressure (mmHg) | 77.3 (9.2) | 77.5 (9.9) | .754 |
| Fasting glucose (mg/dL) | 89.7 (11.2) | 105.7 (23.1) | .000 |
| High sensitivity C-Reactive Protein (mg/L) | 3.46 (5.7) | 4.10 (4.3) | .243 |
| HOMA insulin resistance | 4.49 (5.9) | 10.8 (14.5) | .000 |
Note. CKD = chronic kidney disease. eGFR = estimated glomerular filtration rate. HDL cholesterol = high density lipoprotein cholesterol. HOMA-IR = Homeostasis Model Assessment Insulin resistance.
Fig 1. Proportion of participants with each component of the MetS.
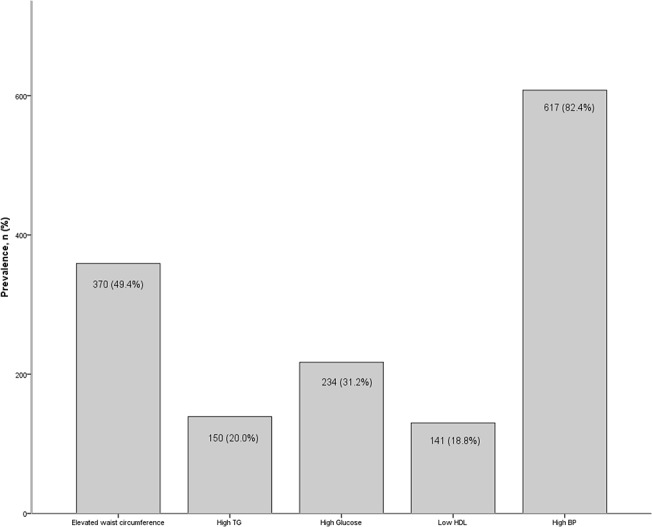
(A) TG = triglycerides. HDL = high-density lipoprotein cholesterol. BP = blood pressure.
Fig 2. Proportion of study participants with specific number of components of the metabolic syndrome.
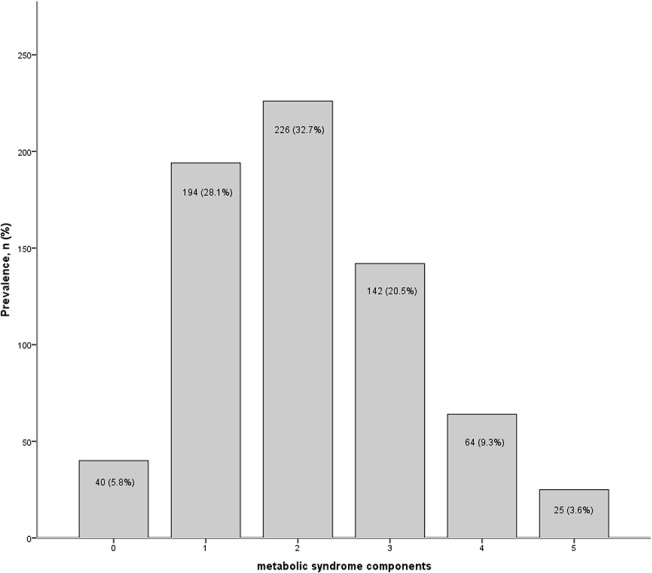
Table 2. Prevalence of individual components of the metabolic syndrome in participants with and without chronic kidney disease.
| Component | Chronic kidney disease | ||||
|---|---|---|---|---|---|
| Missing, n (%) | No, n (%) | Yes, n (%) | p value | ||
| 419 (73.5) | 151 (26.5) | ||||
| Fasting glucose | Mean (mg/dL) | 92.3 (15.2) | 98.6 (20.1) | .000 | |
| Elevated fasting glucose | Proportion ≥ 100 mg/dL | 1 (0.1%) | 91 (20.0) | 48 (29.8) | .010 |
| Waist circumference | Mean (cm) | 92.3 (12.2) | 94.8 (11.1) | .028 | |
| Elevated waist circumference | Proportion ≥102cm in men or ≥ 88cm in women | 41 (5.3%) | 194 (45.2) | 82 (5.6) | .116 |
| Triglycerides | Mean (mg/dL) | 108.4 (60.5) | 121.6 (64.5) | .020 | |
| Elevated triglycerides | Proportion ≥150mg/dL | 1 (0.1%) | 75 (16.5) | 38 (23.6) | .046 |
| HDL cholesterol | Mean (mmHg) | 59.3 (16.0) | 55.8 (14.9) | .018 | |
| Reduced HDL cholesterol | Proportion <40mg/dL in men or <50mg/dL in women | 1 (0.1%) | 69 (15.2) | 37 (23.0) | .025 |
| Systolic blood pressure | Mean (mmHg) | 136.7 (17.4) | 135.9 (18.1) | .636 | |
| Diastolic blood pressure | Mean (mmHg) | 78.0 (8.8) | 75.6 (10.8) | .015 | |
| Elevated blood pressure | Proportion ≥130/85 mmHg | 50 (6.5%) | 362 (85.2) | 138 (91.4) | .053 |
| Metabolic syndrome | Proportion with MetS | 46 (7.5%) | 102 (24.3) | 52 (34.4) | .017 |
| HOMA-IR | Mean | 5.42 (7.29) | 8.29 (13.3) | .012 | |
| High sensitivity C-Reactive protein | Mean (mg/L) | 3.39 (5.1) | 4.04 (5.4) | .200 | |
Note. HOME-IR = Homeostasis Model Assessment Insulin Resistance. HDL = high-density lipoprotein.
Table 3 shows the odds ratio of CKD for metabolic syndrome defined as a dichotomous variable (presence of >3 components vs. < 3). Results showed that the MetS was associated with CKD independent of demographics and inflammation (OR = 1.83, CI = 1.16–2.88, p = .009); however, when HOMA-IR was entered in the model, the association between the MetS and CKD was attenuated, and HOMA-IR was significantly and independently associated with CKD (OR = 1.02, CI = 1.00–1.05, p = .038).
Table 3. Odds ratios for chronic kidney disease predicted by the metabolic syndrome with adjustments as described below.
| Odds Ratios (95% Confidence Intervals) | ||||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Adjusted for demographics | Adjusted for demographics + hsCRP | Adjusted for demographics + HOMA-IR | Adjusted for demographics + hsCRP + HOMA-IR | |
| Metabolic syndrome | 1.72** (1.13–2.61) | 1.83** (1.16–2.88) | 1.45 (0.92–2.78) | 1.48 (0.93–2.41) |
| hsCRP | - | 1.03 (0.99–1.07) | - | 1.04 (0.99–1.08) |
| HOMA-IR | - | - | 1.02* (1.00–1.05) | 1.02* (1.00–1.05) |
Note. Demographics = age, gender, race, education, smoking and alcohol intake. hsCRP = high sensitivity C-reactive protein. IR = insulin resistance. HDL = high-density lipoprotein cholesterol.
*p>.05
**p < .01
In Table 4 we show the odds ratios of CKD associated with one, two, three, or four + components of the MetS compared to no components present. In the fully adjusted model, for individuals presenting with 4+ components, the OR for CKD was 7.65 (CI = 1.49–36.26, p = .015), independent of inflammation and HOMR-IR.
Table 4. Odds ratios for chronic kidney disease predicted by number of components of the metabolic syndrome with adjustments as described below.
| Odds Ratio (95% Confidence Interval) | ||||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Adjusted for demographics | Adjusted for demographics + hsCRP | Adjusted for demographics + HOMA-IR | Adjusted for demographics + hsCRP + HOMA-IR | |
| 0 components | Ref. | Ref. | Ref. | Ref. |
| 1 component | 2.13 (0.77–5.89) | 2.90 (0.82–10.31) | 2.33 (0.76–7.12) | 3.62 (0.80–16.35) |
| 2 components | 2.77 (1.01–7.57) | 3.78 (1.07–13.39) | 2.82 (0.93–8.56) | 4.31 (0.96–19.48) |
| 3 components | 3.05 (1.07–8.74) | 4.54 (1.23–16.78) | 2.98 (0.94–9.48) | 4.90 (1.04–22.99) |
| 4+ components | 6.18*** (2.03–18.82) | 9.21** (2.03–36.81) | 4.98* (1.45–17.10) | 7.65* (1.49–39.26) |
| hsCRP | - | 1.03 (0.99–1.07) | - | 1.03 (0.99–1.08) |
| HOMA-IR | - | - | 1.02 (1.00–1.04) | 1.02 (1.00–1.04) |
Note. Demographics = age, gender, race, education, smoking and alcohol intake. hsCRP = high sensitivity C-reactive protein. HOMA-IR = Homeostasis Model Assessment insulin resistance. HDL cholesterol = high-density lipoprotein cholesterol. A p value < .01 was considered statistically significant.
*p>.05
**p < .01
***p < .001
Table 5 shows the relative odds ratio of CKD associated with individual components of the metabolic syndrome. None of the MetS components were significantly associated with CKD. Elevated waist circumference was associated with CKD in models that included only demographics (OR = 1.44, CI = 1.01–2.03, p = .01, results not shown); however, once all other components were entered into the model this association was attenuated.
Table 5. Odds ratios for chronic kidney disease predicted by individual components of the metabolic syndrome with adjustments as described below.
| Odds ratio (95% confidence interval) | ||||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Adjusted for demographics | Adjusted for demographics and hsCRP | Adjusted for demographics and HOMA-IR | Adjusted for demographics and hsCRP and HOMA-IR | |
| Reduced HDL cholesterol | 1.23 (0.73–2.08) | 1.29 (0.73–2.29) | 1.21 (0.70–2.10) | 1.15 (0.63–2.07) |
| Elevated fasting glucose | 1.71 (1.08–2.71) | 1.70 (1.03–2.79) | 1.42 (0.86–2.33) | 1.38 (0.81–2.35) |
| Elevated waist circumference | 1.33 (0.88–2.01) | 1.45 (0.93–2.26) | 1.31 (0.86–2.01) | 1.38 (0.88–2.17) |
| Elevated triglycerides | 1.15 (0.68–1.94) | 1.13 (0.64–1.98) | 1.03 (0.59–1.79) | 1.03 (0.57–1.85) |
| Elevated blood pressure | 1.70 (0.89–3.26) | 2.01 (0.92–4.37) | 1.58 (0.80–3.12) | 2.07 (0.91–4.70) |
| hsCRP | - | 1.03 (0.99–1.07) | - | 1.03 (0.99–1.08) |
| HOMA-IR | - | - | 1.02 (1.00–1.04) | 1.02 (0.99–1.04) |
Note. Demographics = age, gender, race, education, smoking and alcohol intake. hsCRP = high sensitivity C-reactive protein. IR = insulin resistance. HDL = high-density lipoprotein cholesterol. A p value of < .01 was considered statistically significant.
Discussion
In this cross-sectional study, our aim was to characterize the associations of metabolic components with CKD in community-residing older adults using i) the MetS as a dichotomous variable (3+ vs <3 components) ii) the total number of components present (0 to 4+), and iii) individual components of the MetS. We also evaluated two potential mediators that may explain the association between the MetS and CKD: inflammation as measured by hsCRP, and insulin resistance as measured by HOMA-IR.
Individuals with MetS had higher odds for CKD; HOMA-IR attenuated the association between the MetS and CKD, though hsCRP did not. This suggests that the explanatory variance of MetS for CKD is better accounted for by HOMA-IR. hsCRP does not attenuate the association of MetS with CKD. Examining the total number of components present, we showed that as the numbers of MetS components increased so did the odds of CKD [F = (1, 1) = 13.82, p < .001]. Thus, as we added components starting from 0 and moving on to 4+ components of MetS (aim ii), odds for CKD also increased (OR = 7.98, CI = 1.57–40.60, p = .015 for 4+ components). Although not significant as a predictor of CKD, adding inflammation to the model (Table 4) increased the odds of CKD given components of the MetS (Models 2 and 4 in Table 4). Because hsCRP was not associated with CKD and did not attenuate the association between MetS and CKD (Tables 3 and 4), we suggest that inflammation as measured here may not be in the casual pathway linking the MetS to CKD. On the other hand, once HOMA-IR was entered in the models, MetS risk odds became weaker for CKD (Models 3 and 4 in Table 4). Thus, in line with previous research, our results also suggested that HOMA- IR may be in the casual pathway to CKD7 and that inflammation may not be associated with CKD-related events as some previous studies have suggested [29].
Our results are supported by previous studies. Results from the ARIC and the NHANES showed that the MetS was associated with CKD independent of diabetes and hypertension; individuals with up to five components present, as opposed to those without any components present, had an odds of 2.45 (CI = 1.32–4.54) of developing CKD in the ARIC [20] and a 5.85 odds ratio [confidence interval (CI) = 3.11–5.19] in the NHANES [17] Other studies also showed similar results. Ming et al.’s [21] study, showed that the odds ratio of CKD in individuals with MetS was 1.46 (CI = 1.15–1.86) as opposed to those without MetS; the odds still remained high (OR = 1.32, CI– 1.08–1.62) after excluding individuals with diabetes and controlling for demographics, physical activity, smoking and alcohol drinking. Similarly, Chen et al. [30] also explored this association in 15,160 Chinese adults between the ages of 35 and 74. Results also showed an independent increasing risk of CKD with higher prevalence of 5 MetS components (an OR of up to 2.72, CI = 1.50–4.39) compared to those without any components.
In our study, HOMA-IR was a strong predictor of CKD even though we excluded individuals with diabetes. HOMA-IR has played an important role in previous studies that excluded participants with diabetes too, such as in Chen et al. [17] and Kurella et al.[20] who found associations between HOMA-IR and CKD, and between CKD and MetS. Results from the NHANES data [17] showed that individuals with the highest insulin resistance had higher odds for CKD (OR = 2.65, CI = 1.25–5.62). However, this study did not explore other MetS components and limited the CKD risk-factors to just insulin-related variables, such as serum-insulin, HbA1C and insulin resistance. In a study on 2,380 Native Americans between the ages of 45 and 74 [31], the association between the MetS and CKD was stronger in individuals who developed diabetes during follow-up. HOMA-IR is increasingly becoming recognized as a nontraditional risk factor for CKD [12]. This measure is associated with worsening renal hemodynamics, sodium retention, overproduction of low-density lipoprotein cholesterol, and hypertriglyceridemia [12, 16, 32]. Insulin resistance is also associated with endothelial dysfunction attributed to structural arteriolar changes that lead to limited vasodilation and consequent reduction in endothelial nitric oxide synthase (eNOS) [33]. Metabolic syndrome and insulin resistance are risk factors for stroke and cardiovascular events [34, 35] and all-cause mortality [35, 36].
We therefore suggest future research to identify if the insulin resistance-MetS-kidney function association also extends to the brain. Since previous research has also found kidney-cognitive associations [20, 37, 38] and MetS-cognitive associations [39, 40], we encourage work that attempts to identify if these associations extend to brain and cognitive function using imaging techniques and neuropsychological testing. We also suggest investigating further the association between HOMA-IR and specific components of the MetS in stratified analysis analyzing individuals with CKD separately from those without. Lastly, we suggest that future research also investigate other non-traditional risk factors that may be associated with CKD with the aim of developing clinical trials specifically targeted at treating these risk factors. Although there is not much evidence available that shows that by preventing or treating the symptoms of MetS protects against, or reverses CKD, there are some insulin resistance treatments that aim at targeting metabolic acidosis, anemia, uremic toxins, vitamin D deficiency, malnutrition and even physical fitness [12, 16]. Although traditional risk factors, such as hypertension and diabetes, are high associates of CKD, they do not fully explain this condition. It seems that biomarkers of pathophisologies contributing to CKD may help in predicting and identify CKD in more precise terms.
A particular strength of this study was the defined age-group and the systemic community-based sample. The vast majority of studies use large age-ranges; limiting to one age-group may show specific associations to that particular group. Older adults are at higher risk of complications and comorbidity; learning what puts them at risk and what prevents it may lead to better prevention and treatment plans, longer independence, and better health and health-care management. Our study also had some limitations. Our sample size was relatively small when compared to other large databases. Furthermore, our participants were relatively healthy compared to the rest of their age group–this is because we excluded individuals with diabetes and dementia, and also because our study only includes independent community-dwelling individuals, thus excluding anyone who may be hospitalized or living in a nursing home and therefore more likely to have disease. This may be evident in the proportion that met the criteria for MetS in our sample (25%), (in the introduction we state that 42% of older adults have MetS1). Although we excluded participants with diabetes, this was only based on self-report, so it may be possible that we included some participants with undiagnosed diabetes. We only had available a single marker of inflammation (hsCRP). Lastly, this study was cross-sectional, aiming to identify associations and risk factors; however, it is encouraged that longitudinal work is followed up on these variables to find out if these associations become stronger over time, and if MetS and insulin resistance predict incident CKD.
Conclusion
In conclusion, results from this study showed that i) the single definition of the MetS does not survive adjustment for HOMA-IR; ii) MetS studied by number of components present is a better measure of the MetS than by it single definition; iii) single components of the MetS on their own are not significant independent risk factors for CKD; and that iv) hsCRP per se may not be of key importance in the prevalence of CKD, but rather HOMA-IR seems to have a more prominent role. The association between insulin resistance and CKD should be further investigated.
Acknowledgments
We thank the EAS research participants. We thank Charlotte Magnotta, Diane Sparracio and April Russo for assistance in participant recruitment; Betty Forro, Alicia Gomez, Wendy Ramratan, and Mary Joan Sebastian for assistance in clinical and neuropsychological assessments; Michael Potenza for assistance in data management.
Data Availability
Due to restrictions set by the Albert Einstein College of Medicine Institutional Review Board regarding potentially identifying information, data are available upon request. Requests for data may be sent to Mindy Katz (Mindy.Katz@einstein.yu.edu).
Funding Statement
This research was supported by the Einstein Aging Study (PO1 AG03949) from the National Institutes on Aging program; the National Institutes of Health CTSA (1UL1TR001073) from the National Center for Advancing Translational Sciences (NCATS); the Sylvia and Lenard Marx Foundation; and the Czap Foundation. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NI.
References
- 1. Ford ES, Giles WH, Dietz WH: Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA 2002, 287: 356–359. [DOI] [PubMed] [Google Scholar]
- 2. Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003, 41:1–12. [DOI] [PubMed] [Google Scholar]
- 3. Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, et al. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol 2005, 16:180–188. [DOI] [PubMed] [Google Scholar]
- 4. Locatelli F, Pozzoni P, Del Vecchio L. Renal manifestations in the metabolic syndrome. J Am Soc Nephrol 2006, 17:S81–5. [DOI] [PubMed] [Google Scholar]
- 5. Cheng H-T, Huang J-W, Chiang C-K, Yen C-J, Hung K–Y, Wu K–D. Metabolic syndrome and insulin resistance as risk factors for development of chronic kidney disease and rapid decline in renal function in elderly, JECM 2012, 97: 1268–1276. [DOI] [PubMed] [Google Scholar]
- 6. Muntner P, Hamm LL, Kusek JW, Chen J, Whelton PK, He J: The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med 2004, 140: 9–17. [DOI] [PubMed] [Google Scholar]
- 7. Chen J, Muntner P, Hamm L, Fonseca V, Batuman V, Whelton PK, et al. : Insulin resistance and chronic kidney disease in nondiabetic US adults. J Am Soc Nephrol 2003, 14: 469–477, 2003. [DOI] [PubMed] [Google Scholar]
- 8. Boden G. Pathogenesis of type 2 diabetes. Insulin resistance. Endocrinol Metab Clinics North Amer 2001, 30: 801–815. [DOI] [PubMed] [Google Scholar]
- 9. Ginsberg HN: Insulin resistance and cardiovascular disease. J Clin Inves 2000, 106: 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lebovitz HE: Insulin resistance: Definition and consequences. Exper Clin Endocrinol Diabetes 2001, 109: S135–148. [DOI] [PubMed] [Google Scholar]
- 11. Chen S, Liu H, Liu X, Li Y, Li M, Liang Y, et al. : Central obesity, C-reactive protein and chronic kidney disease: a community-based cross-sectional study in southern China. Kidney Blood Press R 2013, 37: 392–401. 10.1159/000355718 [DOI] [PubMed] [Google Scholar]
- 12. Liao M-T, Sung C-C, Hung K-C, Wu C-C, Lo L, Lu K-C. Insulin resistance in patients with chronic kidney disease. J Biomed Biotechnol 2012, 691369: 1–12. 10.1155/2012/691369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beddhu S, Kimmel PL, Ramkumar N, Cheung AK: Associations of metabolic syndrome with inflammation in CKD: results From the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 2005, 46:577–586. [DOI] [PubMed] [Google Scholar]
- 14. Ridker PM, Hennekens CH, Buring JE, Rifai N: C- Reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women N Engl J Med 2000, 342:836–843. [DOI] [PubMed] [Google Scholar]
- 15. Nashar K, Egan BM. Relationship between chronic kidney disease and metabolic syndrome: current perspectives. Diabetes Metab Syndr Obes 2014, 7, 421–35. 10.2147/DMSO.S45183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gluba A, Mikhailidis D, Lip GYH, Hannam S, Rysz J, Banach M. Metabolic syndrome and renal disease. Inter J Cardiol 2013, 164: 141–50. [DOI] [PubMed] [Google Scholar]
- 17. Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Int Med 2004, 140: 167–74. [DOI] [PubMed] [Google Scholar]
- 18. Chen S, Chen Y, Liu X, Li M, Wu B, Li Y, et al. : Association of Insulin Resistance with Chronic Kidney Disease in Non-diabetic Subjects with Normal Weight. PLoS One 2003, 8:e74058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee JE, Choi SY, Huh W, Kim YG, Kim DJ, Oh HY: Metabolic syndrome, C-reactive protein, and chronic kidney disease in nondiabetic, nonhypertensive adults. Am J Hypertens 2007, 20: 1189–94. [DOI] [PubMed] [Google Scholar]
- 20. Kurella M, Chertow GM, Fried LF, Cummings SR, Harris T, Simonsick E, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol: JASN 2005, 16: 2127–33. 10.1681/ASN.2005010005 [DOI] [PubMed] [Google Scholar]
- 21. Ming J, Xu S, Yang C, Gao B, Wan Y, Xing Y, et al. Metabolic syndrome and chronic kidney disease in general Chinese adults: results from the 2007–08 China National Diabetes and Metabolic Disorders Study. Clin Chim Acta 2014, 430, 115–20. 10.1016/j.cca.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 22. Katz MJ, Lipton RB, Hall CB, Zimmerman ME, Sanders AE, Verghese J, et al. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord 2012, 26:335–43. 10.1097/WAD.0b013e31823dbcfc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV. Washington DC: American Psychiatric Association. [Google Scholar]
- 24. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection. Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 25. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resis- tance and cell function from fasting plasma glucose and insulin concentration in man. Diabetologia 1985, 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 26. Levey AS, Bosch JP, Breyer LJ, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 1999, 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 27. Cirillo M, Anastasio P, De Santo NG. Relationship of gender, age and body mass index to errors in predicting kidney function. Nephrol Dial Transplant 2005, 20: 1791–8. [DOI] [PubMed] [Google Scholar]
- 28.Bonferroni CE. Teoria statistica delle classi e calcolo delle probabilità, Pubblicazioni del R Istituto Superiore di Scienze Economiche e Commerciali di Firenze 1936.
- 29. Yang T, Chou YC, Chu CH, Lin SH, Hsieh PC, Hsu CH, et al. (2014). Metabolic syndrome and C-reactive protein concentration as independent correlates of chronic kidney disease. Endoc Res 2014, 39(3), 94–8. [DOI] [PubMed] [Google Scholar]
- 30. Chen J, Gu D, Chen CS, Wu X, Hamm LL, Muntner P, et al. ,. Association between the metabolic syndrome and chronic kidney disease in Chinese adults. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association—European Renal Association 2007, 22(4), 1100–6. [DOI] [PubMed] [Google Scholar]
- 31. Lucove J, Vupputuri S, Heiss G, North K, Russell M. Metabolic syndrome and the development of CKD in American Indians: the Strong Heart Study. Am J Kid Dis 2008. [DOI] [PubMed] [Google Scholar]
- 32. Tsimihodimos V, Dounousi E, and Siamopoulos KC. Dyslipidemia in chronic kidney disease: an approach to pathogenesis and treatment. Am J Nephrol 2008, 28: 958–973. 10.1159/000144024 [DOI] [PubMed] [Google Scholar]
- 33. Deedwania P. Hypertension, dyslipidemia, and insulin resistance in patients with diabetes mellitus or the cardiometabolic syndrome: benefits of vasodilating β-blockers. J Clin Hypertens 2011, 13:52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol 2010, 56:1113–1132. 10.1016/j.jacc.2010.05.034 [DOI] [PubMed] [Google Scholar]
- 35. Ford ES. The metabolic syndrome and mortality from cardiovascular disease and all-causes: findings from the National Health and Nutrition Examination Survey II Mortality Study. Atherosclerosis 2004, 173:309–14. [DOI] [PubMed] [Google Scholar]
- 36. Wu SH, Hui WS, Liu Z, Ho SC. Metabolic syndrome and all-cause mortality: a meta-analysis of prospective cohort studies. Eur J Epidemiol 2010, 25: 375–384. 10.1007/s10654-010-9459-z [DOI] [PubMed] [Google Scholar]
- 37. Yaffe K, Ackerson L, Tamura MK, Blanc P. Le, John W, Sehgal AR, et al. Chronic kidney disease and cognitive function in older adults: Findings from the Chronic Renal Insufficiency Cohort (CRIC) cognitive study. J Am Geriatr Soc 2011, 58: 338–345. 10.1111/j.1532-5415.2009.02670.x.Chronic [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zammit AR, Katz MJ, Lai JY, Zimmerman ME, Bitzer M, Lipton RB. Renal function and cognitive composites of function in the Einstein Aging Study: A cross-sectional analysis, J Gerontol Med Sci 2014, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yaffe K, Kanaya A, Lindquist K, Simonsick E. M, Harris T, Shorr RI, et al. (2004). The metabolic syndrome, inflammation, and risk of cognitive decline. J Amer Med Assoc 2004, 292: 2237–42. [DOI] [PubMed] [Google Scholar]
- 40. Segura B, Jurado MA, Freixenet N, Albuin C, Muniesa J, Junqué C. Mental slowness and executive dysfunctions in patients with metabolic syndrome. Neurosci Lett 2009, 462, 49–53. 10.1016/j.neulet.2009.06.071 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to restrictions set by the Albert Einstein College of Medicine Institutional Review Board regarding potentially identifying information, data are available upon request. Requests for data may be sent to Mindy Katz (Mindy.Katz@einstein.yu.edu).


