Abstract
Formulations of chimeric dengue vaccine (DENVax) viruses containing the pre-membrane (prM) and envelope (E) genes of serotypes 1–4 expressed in the context of the attenuated DENV-2 PDK-53 genome were tested for safety, immunogenicity and efficacy in interferon receptor knock-out mice (AG129). Monovalent formulations were safe and elicited robust neutralizing antibody responses to the homologous virus and only limited cross-reactivity to other serotypes. A single dose of monovalent DENVax-1, -2, or -3 vaccine provided eighty or greater percent protection against both wild-type (wt) DENV-1 (Mochizuki strain) and DENV-2 (New Guinea C strain) challenge viruses. A single dose of monovalent DENVax-4 also provided complete protection against wt DENV-1 challenge and significantly increased the survival times after challenge with wt DENV-2. In studies using tetravalent mixtures, DENVax ratios were identified that: (i) caused limited viremia, (ii) induced serotype-specific neutralizing antibodies to all four DENV serotypes with different hierarchies, and (iii) conferred full protection against clinical signs of disease following challenge with either wt DENV-1 or DENV-2 viruses. Overall, these data highlight the immunogenic profile of DENVax, a novel candidate tetravalent dengue vaccine and the advantage of sharing a common attenuated genomic backbone among the DENVax monovalent vaccines that confer protection against homologous or heterologous virus challenge.
Keywords: Dengue, Dengue vaccines, DENVax, AG129 mice, Dengue 2 PDK-53 chimeras
1. Introduction
Dengue viruses (DENV) are among the most common and important causative agents of emerging mosquito-borne viral disease in humans today [1,2]. These viruses belong to the family Flaviviridae and comprise four distinct antigenic serotypes (DENV-1 through DENV-4) that are transmitted to humans primarily by Aedes aegypti mosquitoes. Several factors such as travel, demographic and economic changes and the geographical expansion of the mosquito vector have contributed to the dramatic spread of the disease [3,4]. Infection with DENV leads to dengue fever (DF) of varying severity. The most severe consequences of infection – dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) – are life threatening. It is estimated that DENV cause 50–100 million cases of debilitating DF, 500,000 cases of DHF/DSS, and more than 20,000 deaths each year [4,5].
Host immune responses play a critical role in the resolution and protection against primary and secondary DENV infections. After primary infection IgG antibodies predominantly recognize the virus structural and non-structural proteins [6]. Following reinfection with the same DENV serotype, the humoral immune response has broader specificity and the antibodies are cross-reactive with other DENV serotypes since they are structurally related [6]. However, the immune response to DENV may also contribute to the severity of disease due to pre-existing immunity following a secondary infection with a different DENV serotype [7–9]. It is believed that at least in part this phenomenon is a result of the presence of sub neutralizing anti-DENV antibody levels that exacerbate disease by increasing infection of cells bearing Fc receptors, a phenomenon termed antibody-dependent enhancement of infection (ADE) [7]. Therefore, an ideal candidate DEN vaccine should simultaneously provide long lasting protective immunity against all four DENV serotypes [3,10]. Currently, there are several tetravalent vaccine candidates in development, including mixtures of four different inactivated viruses, recombinant live attenuated viruses, protein subunit and DNA vaccines [11]. In this study, we investigated a live-attenuated DENV tetravalent vaccine (DENVax) consisting of infectious cDNA clone-derived DENV-2 PDK-53, and three chimeric viruses containing the preM and E genes of DENV-1, -3, or -4 in the DENV-2 PDK-53 genome background [12–15]. The original cell culture derived DENV-2 PDK-53 vaccine virus has been tested as a monovalent vaccine in Phase 1 and as a component of a tetravalent vaccine in Phase 2 clinical trials. The DENV-2 PDK-53 vaccine was shown to be well-tolerated and to generate long-lasting neutralizing antibody and cell-mediated immune responses to DENV-2 [16–21]. The mutations necessary and sufficient for the attenuated phenotype of DENV-2 PDK-53 virus genetically identified were shown to reside outside of the structural gene regions of the viral genome [14,15].
The main objectives of this study were: (i) to test the safety and further characterize the immunogenic profile of monovalent DENVax vaccines in AG129 mice, (ii) examine the effect of immunization with different component ratios of tetravalent DENVax on the hierarchy of neutralizing antibody responses, and (iii) evaluate the protective efficacy of monovalent DENVax vaccines or various tetravalent DENVax formulations against intraperitoneal challenge with a lethal dose of DENV-1 Mochizuki or mouse brain adapted DENV-2 New Guinea C virus. These studies supported the initiation of Phase I clinical trials of DENVax to assess the safety of the vaccine as well as measure the immune responses after administration to healthy adults.
2. Materials and methods
2.1. Viruses and cell culture
Wild-type DENV-1 16007, DENV-2 16681, DENV-3 16562, and DENV-4 1036 viruses were obtained from the Division of Vector-Borne Diseases, Centers for Disease Control and Prevention (CDC), Fort Collins, CO. DENVs were grown in Vero cells in Dulbecco’s modified minimal essential medium (DMEM) containing penicillin-streptomycin.
2.2. DENVax viruses
The DENVax viruses consist of cDNA clone-derived DENV- 2 VV45R virus (based on genome of DENV-2 PDK-53), and the DENV-2VV45R-based chimeras expressing the prM and E genes of wild-type (wt) DENV-1 16007, DENV-3 16562 or DENV-4 1036. The construction and characterization of these viruses has been previously reported [12–15]. To complete preclinical and clinical development of DENVax, new viral stocks were generated by introducing RNAs transcribed from infectious cDNA clones (pD2-VV45R [15] and chimeric pD2/1V, /3V, and/4V plasmids [12]) into certified vaccine production Vero cells under Good Manufacturing Practice (GMP) conditions at Shantha Biotechnics, Ltd., Hyderabad, India. The resulting re-derived viruses were amplified, plaque purified, characterized and sequenced. Based on these analyses, a formal pre-master virus seed was chosen and then was amplified to generate the master virus seed for each DENVax serotype (Huang et al., manuscript in preparation). For the studies reported here we used either GMP quality master seed virus stocks or surrogate master seed prepared in our laboratory from individual pre-master cGMP seeds. These surrogate master seed viruses were grown in serum-free media under vaccine production conditions.
Three tetravalent vaccine formulations containing different ratios of the individual DENVax components were prepared. For each, surrogate vaccine preparations were mixed into a novel FTA diluent (final concentration 1% F127 polyoxyethylene–polyoxypropylene block copolymer), 15% tre-halose, and 0.1% human serum albumin) that has shown to improve the thermal stability of these DENVax vaccine viruses [22]. Formulation 1 (3333) contained 103 plaque forming units (pfu) of each of the four DENVax viruses per dose. Formulation 2 (3355) was prepared to contain 103 pfu each of DENVax-1 and DENVax-2, and 105 pfu each of DENVax-3 and DENVax-4 per dose. Formulation 3 (5555) contained 105 pfu of each of the four DENVax viruses per dose. To validate the virus concentrations, the titer of each DENVax virus was determined by an immunofocus assay described below.
2.3. Telemetry
For measurement of body temperature, animals were implanted subcutaneously with BMDS IPTT-300 transponder (chips), purchased from BioMedic Data Systems, Inc. (BMDS, Seaford, DE) two to three days prior to start of the study and were monitored for signs of infection or migration of the transponder. Chips were scanned using a DAS-6007 transponder reader (BMDS).
2.4. Animal studies
Treatment of animals was in accordance with the University of Wisconsin-Madison Standard Operating Procedures, which adhere to the regulations outlined in the USDA Animal Welfare Act (9 CFR Parts 1, 2 and 3) and the conditions specified in the Guide for the Care and Use of Laboratory Animals (1996). All animal study protocols were approved by the UW Institutional Animal Care and Use Committee. AG129 mice (supplied by B & K Universal Ltd., UK) lack the IFN-α/β and IFN-γ receptor genes and have been successfully used as a mouse model for DENV studies [12,13,23]. Although they possess IFN receptor deficiencies, AG129 mice otherwise exhibit immunological competence and are able to mount antibody and cellular immunity to variety of pathogens [24].
Immunogenicity studies with monovalent DENVax vaccines were conducted in groups of 5–8 week-old AG129 mice (n = 6) by subcutaneously (SC) injecting a dose of 105 pfu of each of the individual DENVax vaccines in 0.5 ml. Control mice were injected by the same route with PBS. All animals received primary and secondary immunizations on days 0 and 42, respectively. Mice were monitored daily for morbidity and mortality. Serum samples collected at days 0, 42, and 56 were tested for DENV serotype-specific neutralizing antibodies.
A subsequent study evaluated the protective capacity of each monovalent DENVax vaccine using non-GMP surrogate vaccine preparations. Groups of ten 8–9 week old AG129 mice received a SC injection of 0.1 ml containing 105 pfu of each surrogate master seed virus. Control mice were inoculated via the same route with FTA (the virus diluent). At five weeks after the single vaccine administration each group was split into two sub-groups of five mice and were challenged by intraperitoneal (IP) administration of 106 pfu of DENV-1 Mochizuki or DENV-2 New Guinea C viruses in 0.1 ml. Following challenge, all mice were monitored twice a day for five weeks for morbidity (weight loss, temperature, neurological signs), and survival rates were recorded.
The immunogenicity and protective efficacy of tetravalent DEN-Vax formulations (Formulation 1, 2 and 3) were tested in groups of eight 5–8 week old AG129 mice. Each of the three formulations and the FTA diluent was administered SC on day 0 and 42. Viremia levels were measured in samples collected on day 3 post-primary vaccination, the peak of dengue virus replication in the AG129 mouse model [25]. Serum neutralizing antibody responses against all four serotypes were measured in individual blood samples collected on days 30 and 56 post-priming. On day 56 each group of eight mice was sub-divided into two groups of four mice and challenged IP with 106 pfu of DENV-1 Mochizuki or DENV-2 New Guinea C virus. Animals were monitored daily for three weeks for clinical signs of disease and survival rates were recorded.
2.5. Virus plaque titration and immune focus assay
Virus plaque forming units were measured by plaque titration performed in double agarose overlays in six-well plates of conflu-ent Vero cells as previously described [12]. The plaque titration was performed for each monovalent DENVax virus. For tetravalent DENVax formulations and viremia measurements of animals vaccinated with tetravalent DENVax, we performed immune focus assay using serotype-specific monoclonal antibodies (MAbs), 1F1 (DENV-1), 3H5 (DENV-2), 8A-1 (DENV-3), and 1H10 (DENV-4) to differentiate titers for each DENVax serotype. The immune focus assay was conducted in confluent Vero cells in 6-well plates. Cells were adsorbed with serially diluted samples for 1.5 h at 37 °C and overlaid with 3 ml/well of YE-LAH medium [12] containing 0.7% high viscosity carboxymethylcellulose (CMC) (Sigma). After 7-day incubation at 37 °C with 5% CO2, overlay was aspirated, and cell sheets were washed 3 times with PBS before they were fixed by adding cold 85% acetone or 100% methanol for 30 min at −20 °C. After fixation, plates were air dried, washed once with PBS, and blocked with a buffer containing 2.5% (w/v) nonfat dry milk, 0.5% Triton X-100, 0.05% Tween-20 in PBS at 37 °C for 30 min. After blocking, cells were incubated with diluted DENV serotype-specific MAbs in the blocking buffer at 37 °C for 1 h or 4 °C for overnight, washed 3 times with washing buffer (0.05% Tween-20 in PBS), and incubated with diluted alkaline phosphatase-conjugated affinity pure goat anti-mouse IgG (Jack-son Immuno Research Laboratories) at 37 °C for 45 min. Plates were washed three times again with washing buffers and stained with 0.4 ml/well of 1-Step NBT/BCIP plus suppressor (Pierce). Immune foci were visible after 20 min at room temperature and color development was stopped by washing the plates with water within an hour to avoid dark cell background. Immune foci were counted to calculate the immune focus units (ifu)/ml of the input samples.
2.6. Neutralization assays
Serum samples collected for neutralization assay were heated at 56 °C for 30 min to inactivate complement and possible adventitious agents. Heat-inactivated sera were tested for neutralizing antibodies by the 50% plaque reduction neutralization test (PRNT50) as previously described [12]. The neutralizing antibody titer was identified as the highest serum dilution that reduced the input number of virus plaques by at least 50% (PRNT50). All samples were assayed in duplicate. The input virus numbers were calculated by back titration with 2-fold serial dilutions of the input viruses in each assay. Neutralizing antibody titers of less than 10 were considered negative and were assigned an inverse titer of 1 for calculation purposes. Duplicate PRNT50 values for each sample were geometrically averaged and geometric mean titers (GMT) were calculated for each group of animals.
3. Results
3.1. Safety, immunogenicity and antigenic specificity of monovalent chimeric DENVax vaccines
Groups of AG129 mice were immunized with monovalent chimeric DENVax vaccines and monitored for clinical signs of infection, weight loss and temperature changes. After vaccination there was no mortality and all animals showed no evidence of abnormal elevation of body temperatures or weight loss as compared to the control groups. The mean body weights and temperature of mice vaccinated with each monovalent DENVax vaccine are presented in Tables 1 and 2, respectively.
Table 1.
Mean body temperatures (°C) of AG129 mice immunized with 105 pfu of DENVax-1, DENVax-2, DENVax-3 or DENVax-4 compared to PBS treated mice.
| Group | Day 0 | Day 1 | Day 3 | Day 5 | Day 7 | Day 11 | |
|---|---|---|---|---|---|---|---|
| PBS | Mean | 36.58 | 36.53 | 36.92 | 35.60 | 35.83 | 36.12 |
| SD | 1.18 | 1.06 | 1.65 | 1.73 | 1.80 | 1.94 | |
| DENVax-1 | Mean | 37.33 | 37.28 | 37.47 | 37.22 | 36.77 | 37.28 |
| SD | 0.66 | 0.91 | 0.79 | 0.59 | 1.71 | 1.68 | |
| DENVax-2 | Mean | 35.97 | 34.38 | 35.98 | 35.25 | 35.67 | 35.18 |
| SD | 0.66 | 4.10 | 1.19 | 1.56 | 1.76 | 1.83 | |
| DENVax-3 | Mean | 36.37 | 36.57 | 36.92 | 36.05 | 36.35 | 36.40 |
| SD | 0.43 | 0.75 | 0.40 | 1.27 | 0.67 | 1.40 | |
| DENVax-4 | Mean | 36.56 | 36.42 | 36.76 | 36.06 | 37.43 | 36.58 |
| SD | 0.71 | 1.05 | 1.56 | 2.05 | 0.69 | 0.46 |
Table 2.
Mean body weights (grams) of AG129 mice immunized with 105 pfu of DENVax-1, DENVax-2, DENVax-3 or DENVax-4 compared to PBS treated mice.
| Group | Day 0 | Day 1 | Day 3 | Day 5 | Day 7 | Day 11 | |
|---|---|---|---|---|---|---|---|
| PBS | Mean | 19.28 | 19.95 | 20.45 | 19.82 | 20.08 | 19.95 |
| SD | 2.57 | 2.20 | 2.41 | 2.52 | 2.52 | 2.59 | |
| DENVax-1 | Mean | 18.60 | 19.07 | 19.42 | 17.87 | 18.78 | 18.90 |
| SD | 2.42 | 2.22 | 2.74 | 2.86 | 2.67 | 2.80 | |
| DENVax-2 | Mean | 18.55 | 18.82 | 20.20 | 19.63 | 19.78 | 20.00 |
| SD | 1.12 | 1.11 | 1.23 | 1.05 | 1.32 | 1.35 | |
| DENVax-3 | Mean | 18.53 | 18.60 | 19.57 | 19.23 | 18.63 | 19.60 |
| SD | 1.75 | 1.84 | 1.71 | 1.87 | 1.86 | 1.76 | |
| DENVax-4 | Mean | 19.98 | 20.15 | 19.92 | 19.95 | 19.98 | 20.97 |
| SD | 1.32 | 1.48 | 1.37 | 1.20 | 1.47 | 1.32 |
Following vaccination with each monovalent DENVax, serum samples collected after the primary and secondary immunization were tested for serotype-specific neutralizing antibody responses against the four DENV serotypes. As shown in Table 3, monovalent vaccines elicited strong primary and secondary neutralizing antibody responses against their wild-type homologous virus strains. In addition, all monovalent vaccines elicited low levels of cross-neutralizing antibodies to the three other serotypes with the highest cross-neutralizing reactivity observed against DENV-3 in the DENVax-1, DENVax-2, and DENVax-4 immunization groups. A weak anti-DENV-3 neutralizing activity in the serum of PBS immunized mice, suggesting possible non-specific serum factors that might neutralize this virus.
Table 3.
Serum neutralizing antibody titers of AG129 mice immunized with 105 pfu of DENVax-1, DENVax-2, DENVax-3 or DENVax-4 compared to PBS treated mice. Titers determined for pool sera obtained at days 40 and 56 after primary vaccination.
| Group | Neutralizing antibody titers (PRNT50)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| DEN-1
|
DEN-2
|
DEN-3
|
DEN-4
|
|||||
| D40 | D56 | D40 | D56 | D40 | D56 | D40 | D56 | |
| PBS | 1 | 1 | 1 | 1 | 11 | 30 | 1 | 1 |
| DENVax-1 | 1920 | 5120 | 21 | 20 | 120 | 480 | 11 | 30 |
| DENVax-2 | 30 | 40 | 1280 | 1280 | 320 | 320 | 20 | 20 |
| DENVax-3 | 21 | 80 | 1 | 20 | 1280 | 1920 | 30 | 40 |
| DENVax-4 | 1 | 1 | 50 | 20 | 120 | 480 | 200 | 480 |
3.2. Protective efficacy of monovalent chimeric DENVax vaccines against homologous or heterologous DENV challenge
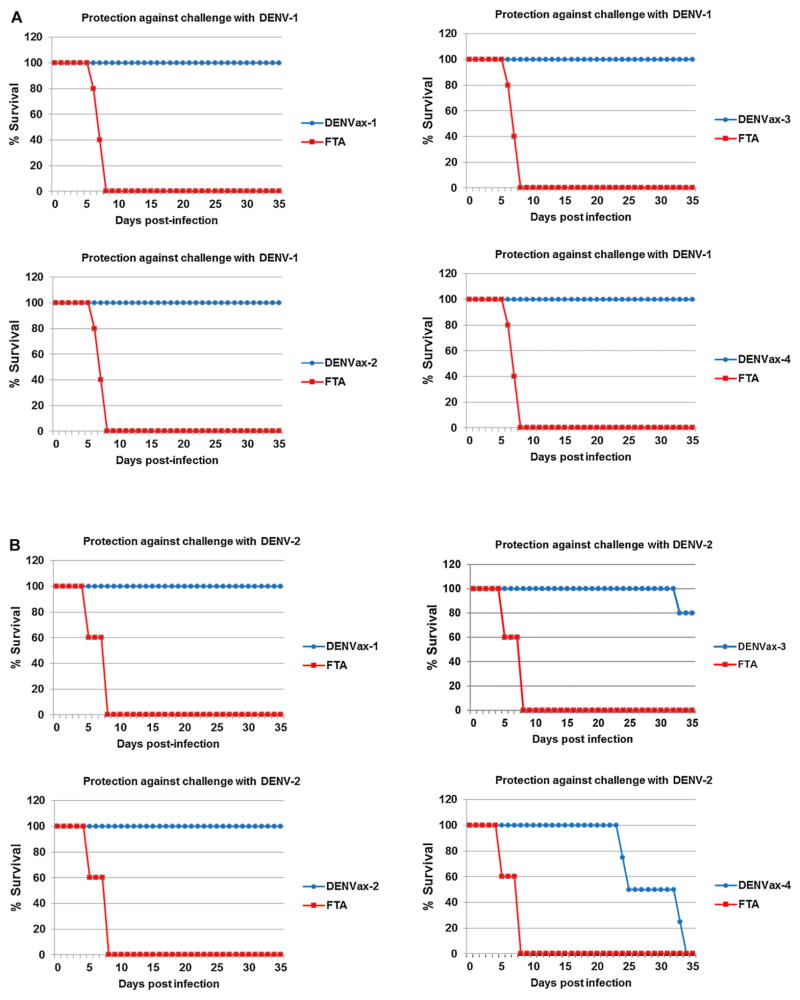
A single immunization of AG129 mice with each of the monovalent DENVax vaccine formulations provided complete protection against lethal challenge with wt DENV-1 Mochizuki virus, administered IP at five weeks post priming (Fig. 1A). Similarly, monovalent chimeric DENVax-1 or DENVax-2 conferred 100% protection against lethal IP challenge with DENV-2 New Guinea C virus. Immunization with DENVax-3 provided significant protection (P = 0.0031 based on a Log-rank test) from challenge with DENV-2 (Fig. 1B). Interestingly, while vaccination with monovalent DENVax-4 did not protect against DENV-2 challenge, the median survival time was significantly longer (P = 0.0067 based on a Log-rank test) than that of non-vaccinated control animals (32 and 7.5 days, respectively). None of the vaccinated animals displayed any sign of morbidity. In contrast, control infected animals succumbed to infection after early signs of morbidity (ruffled coat and/or hunched posture) followed by paralysis and sudden death or humane euthanasia.
Fig. 1.
Protection afforded after vaccination with a single dose of monovalent DENVax. Groups of 10 AG129 mice were immunized SC with each of the monovalent DENVax vaccines. Additional control groups were injected by the same route only with FTA. On day 35 all animals were split into two groups of five animals per group and were challenged IP with 106 pfu of DENV-1 Mochizuki virus (A) or 106 pfu of mouse adapted DENV-2 New Guinea C virus (B). Animals were monitored daily for clinical signs of disease for five weeks and survival rates were recorded.
3.3. Viremia following immunization with the tetravalent DENVax vaccine formulations
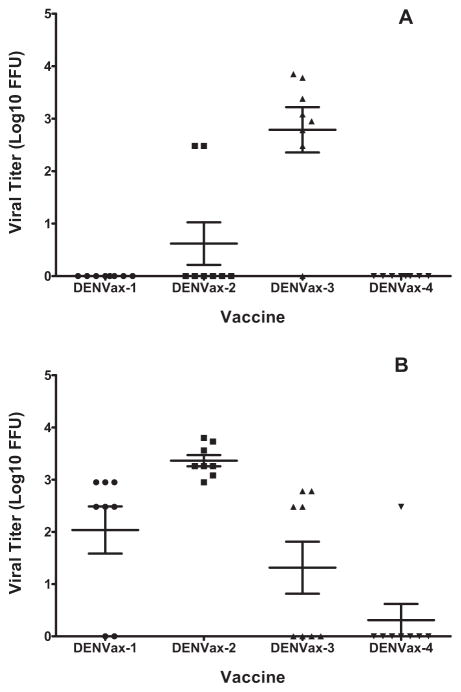
Mice were immunized with the three formulations of the four DENVax strains. In one study, the replication of DENVax viruses was assessed by measuring viremia in blood samples collected shortly after the primary immunization. The magnitude of viremia following SC immunization with DENVax Formulation 2 (103, 103, 105, 105 of DENVax-1, -2, -3 and -4, respectively) and DENVax Formulation 3 (105 of each strain) varied between the four vaccine strains and were dependent on the ratio of component chimeric viruses. After administration of Formulation 3, the DENVax-2 showed the highest viremia with detectable levels in all 8 mice of average value 3.36 log10 ifu/ml followed by the DENVax-1 (6 out of 8 mice), DENVax-3 (4 out of 8 mice) and DENVax-4 (1 out of 8 mice) with average values of 2.0, 1.3, and 0.3 log10 ifu/ml, respectively (Fig. 2B). After administration of Formulation 2 there were only two mice that had detectable viremia for DENVax-2 (average value 0.62 log10 ifu/ml), and 7 out of 8 mice for DENVax-3 (average value 2.78 log10 ifu/ml) (Fig. 2A). No mice demonstrated detectable DENVax-1 or DENVax-4 viremia. Comparison of viremia levels between formulation 2 and 3 for each monovalent DENVax vaccine using one-way ANOVA test reveal significant differences for DENVax-1 (P = 0.0005), DENVax- 2 (P < 0.0001) and DENVax-3 (P = 0.0424) but not for DENVax-4 (P = 0.3343). Viremia after administration of DENVax Formulation 1 was not assessed due to insufficient quantity of available serum samples.
Fig. 2.
Viremia following vaccination with monovalent DENVax viruses. Groups of eight AG129 mice were immunized SC with DENVax Formulation 2 (A) or 3 (B). On day three post vaccination blood samples were collected and serum from each individual mouse was analyzed for viremia by immune focus assay with DENV serotype-specific MAbs.
3.4. Neutralizing antibody responses elicited by different formulations of tetravalent DENVax vaccine
Immunization of AG129 mice with formulations containing different ratios of the four DENVax viruses (Formulations 1, 2 or 3) elicited neutralizing antibody responses against all four serotypes (Fig. 3). After the booster immunization the neutralizing antibody response induced by Formulation 3 was dominated by the anti-DENV-2 and DENV-1 response followed by the anti-DENV-3 and anti-DENV-4 response. In Formulation 2, the neutralizing antibody response was dominated by the anti-DENV-3 and followed by anti-DENV-1, anti-DENV-2, and anti-DENV-4 response. Finally, neutralizing antibody responses elicited by Formulation 1 were lower than those induced by Formulations 2 and 3. It is interesting to point out that following a booster injection with Formulation 1, there was no clear boosting effect on DENV-1 and DENV-3 neutralizing antibody titers on sampling day 56, which could be accounted to differences in the kinetics of antibody responses to these two serotypes. In all tested formulations, the DENVax-4 vaccine was the least immunogenic of the DENVax vaccine components (Fig. 3). Overall, Formulation 3 gave significantly higher neutralizing antibody responses to all four serotypes than formulations 1 and 2 (P < 0.05) for both sampling days using repeated measures by ANOVA test.
Fig. 3.
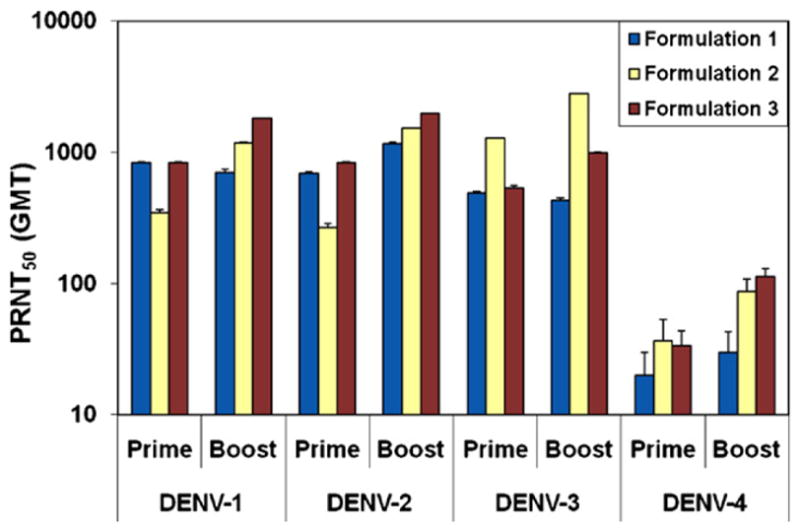
Neutralizing antibody responses against all four DEN virus serotypes following immunization with DENVax Formulations 1, 2 and 3. Groups of eight AG129 mice were immunized SC with DENVax bearing different ratios of component vaccine viruses. Additional control groups were injected by the same route only with FTA. A booster SC injection of the same range of doses was given on day 42 post-priming. Blood samples were collected at 30 and 56 days post-primary vaccination and used to measure the neutralizing antibody response to the four DEN viruses by PRNT50. Bars represent geometric mean titer (GMT) ± SE. Geometric mean titer of neutralizing antibody responses elicited in control mice immunized with FTA were lower than 40 against all four DEN virus serotypes.
3.5. Ability of the tetravalent DENVax formulations to protect against challenge of AG129 mice with wild-type DENV-1 and DENV-2
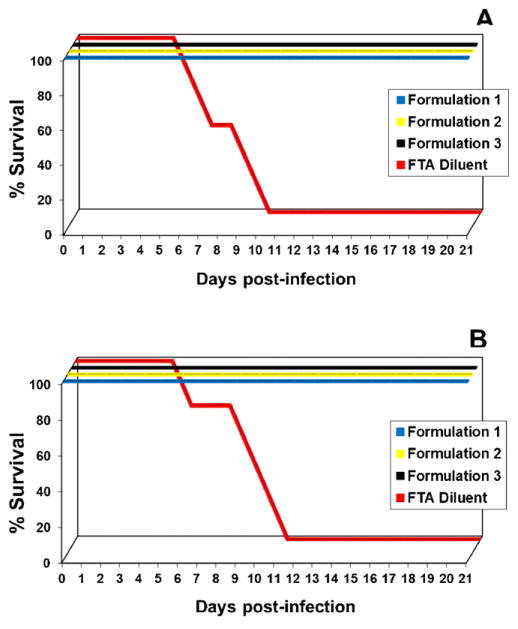
Two weeks after the second DENVax administration all tetravalent-vaccinated and control mice were subdivided into two groups and challenged with a lethal dose of DENV-1 Mochizuki (Fig. 4A) or DENV-2 New Guinea C viruses (Fig. 4B). All DENVax immunized mice survived infection while control animals succumbed to infection between 6 and 11 days after challenge. All vaccinated animals failed to show any clinical signs of disease following viral challenge independent of the DENVax formulation. In contrast, by day 5 after challenge all control animals showed clinical signs of illness (e.g. ruffled coat, hunched posture and hind limb paralysis). On day 6 post-challenge, one animal from each of the challenged groups died and, by day 11 post-challenge, all control animals had succumbed to infection or been euthanized (Fig. 4A and B).
Fig. 4.
Protection afforded after vaccination with different DENVax formulations. Groups of eight AG129 mice were immunized subcutaneously with DENVax bearing different ratios of component vaccine viruses. Additional control groups were injected by the same route only with FTA. A booster SC injection of the same range of doses was given on day 42 post-priming. On day 70 all animals were split into two groups of four animals per group and were challenged intraperitoneally with 106 pfu of DENV-1 Mochizuki virus (A) or 106 pfu of mouse adapted DENV-2 New Guinea C virus (B). Animals were monitored daily for clinical signs of disease for three weeks and survival rates were recorded.
4. Discussion
The development of an effective vaccine against DENVs represents an important step towards the prevention and control of this global emerging disease. Current efforts are focused on the development of tetravalent vaccines that simultaneously provide protection against all four DENV serotypes [3,10]. In this study we tested a novel tetravalent DENVax that consists of the live, attenuated viruses based on recombinant DENV-2 PDK-53.
The safety, immunogenicity and efficacy of monovalent DENVax were evaluated in the AG129 mouse model. All four vaccines were well tolerated with no signs of morbidity, weight loss or febrile response. After priming each monovalent vaccine elicited a robust neutralizing antibody response to the homologous virus and these responses were further elevated following a booster injection with the exception of DENVax-2. In general, DENVax-1, -2, and -3 were very immunogenic as compared to DENVax-4 which was the least immunogenic vaccine candidate. Neutralizing antibodies to each monovalent DENVax vaccine were weakly cross-reactive with non-homologous DENV-1, DENV-2, and DENV-4, while demonstrating high cross-reactivity against DENV-3. Such cross reactive epitopes are normally located at domains I and II and elicit low avidity cross-reactive neutralizing antibody responses although cross-reactive epitopes have been also identified in DIII [26].
Antibodies can provide long-term protection against the serotype responsible for the homologous challenge virus and cross-reactive neutralizing antibodies may contribute to short-term protection against heterologous virus challenge. As indicated by our data, AG129 mice immune to each monovalent DENVax vaccine were protected against homologous and heterologous DENV challenge with the exception of DENVax-4 which conferred partial protection against DENV-2 virus. It is still not clear what levels of cross-neutralizing antibodies are required for protection and to what degree this protection is similar to that elicited by the homologous virus. Our data are consistent with studies by Kyle et al. [27] highlighting the role of antibodies in heterologous protection against secondary DENV infection in the AG129 mouse model. The low-level cross-neutralizing antibodies might exert their effect in concert with cross-reactive anti-virus antibodies that induce conformational changes that increase the avidity to closely related antibodies. Alternatively, cross reactive antibodies might inhibit heterologous virus uptake by Fcγ receptors through the formation of viral aggregates hence preventing antibody-dependent enhancement of DENV infection [28]. Even weak levels of cross-neutralizing antibodies may be sufficient to confer protection alone or together with induced cross-reactive effector T cells. In particular, the non-structural NS3 protein could be a potential source of such cross-reactive CTL epitopes [29,30]. We are currently pursuing a systematic study to assess the functional role of passively transferred antibodies in protection against homologous and/or heterologous DENV challenge in conjunction with adoptive transfer studies. Nevertheless, irrespective of which arm of adaptive immunity is involved in protection afforded by each monovalent DENVax vaccine, our findings highlight the capability of candidate DENVax vaccine viruses to afford at least a short-term protection against heterologous virus challenge. In this mouse model we are unable to conduct long-term vaccine efficacy studies against homologous or heterologous DENV challenge since mice become resistant with age and do not succumb to infection. An alternative approach to evaluate the long-term cross protective efficacy of each monovalent DENVax vaccine could be based on measuring virus replication in tissues following challenge, as indicated by Kyle et al. [27] and currently we are pursuing this option.
Following priming with the tetravalent DENVax vaccine the profile of viremia was dependent on the ratio of component monovalent vaccine viruses. Viremia titers in DENVax Formulation 2 (3355) were generally low. Only two mice out of eight had detectable viremia to DENVax-2 and seven out of eight against DENVax-3. In contrast, detectable viremia against all DENVax vaccines was measured in mice immunized with the DENVax Formulation 3 (5555). In the current study direct evidence for DENVax-4 viremia in mice was absent (with the exception of one mouse in the Formulation 3); however, AG129 mice mounted an anti-DENV-4 neutralizing antibody response, irrespective of the DENVax vaccine ratio. A previous study has shown that the chimeric DENVax-4 has a relatively slower replication than the other three DENVax in cell culture [12]. Therefore, it is very likely that on day 3 post immunization measuring viremia may be too early for DENVax-4 infection. Due to the limited amount and frequency of serum samples allowed to be collected in animals, blood samples were collected after priming on day 3 based on previous study of dengue infection in AG129 mice [25]. In addition, previous studies using a chimeric yellow fever-DENV tetravalent vaccine in non-human primates [31] and our own studies conducted with DENVax in this species [32] have shown that there is not necessarily a direct correlation of viremia with neutralizing antibody responses in animals immunized with the live attenuated DEN vaccine.
All tested DENVax formulations were immunogenic and elicited primary and secondary neutralizing antibody responses to all four DEN viruses. However, responses to certain serotypes were more dominant. This finding is consistent with studies showing that antibodies are sometimes preferentially solicited against one or two dominant serotypes due to intrinsic differences in immunogenicity and/or virus interference [33]. In addition, DENVax-immune mice were protected against challenge with DENV-1 or DENV-2 consistent with previous studies demonstrating the efficacy of tetravalent DENV-2 PDK-53 based recombinant vaccine in the AG129 mouse model [12,13]. Immunogenicity studies conducted in previous reports [12,13] were through IP immunization. In this study, we showed that the SC route was also very effective for immunizing AG129 mice with DENVax. Due to lack of any strains of wt DENV-3 and DENV-4 that have been shown to be lethal for AG129 mice, we were unable to directly determine protective efficacies of the tetravalent DENVax formulations against lethal challenge with these viruses. However, given that all tetravalent mixtures elicited anti-DENV-3 and DENV-4 neutralizing titers, particularly after the boost, it is anticipated that they should be protective. In addition, a single monovalent DENVax-3 or DENVax-4 provided full or partial protection against heterogonous wt DENV-1 or DENV-2 lethal challenge (Fig. 1A and B), indicating these two vaccine components should be able to protect against homologous wt DENV-3 or DENV-4 challenge as well.
In conclusion, although immunogenicity studies in mice may not accurately reflect the human response to DENV infection, the findings of this study highlight the safety, immunogenicity and protective efficacy profile of the monovalent and various tetravalent DENVax formulations in a novel FTA formula by the subcutaneous route in one of the most accepted DENV mouse model, the AG129 mice. These findings support further clinical development and optimization of the tetravalent DENVax.
Acknowledgments
These studies were partially supported by NIH Grant 5-U01-AI070443. We are grateful to Cooper Rosin and Joanna Paykel for technical assistance and Steven Radecki for statistical analysis.
References
- 1.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10:S98. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 2.Beatty ME, Stone A, Fitzsimons DW, Hanna JN, Lam SK, Vong S, et al. Dengue: a continuing global threat. PLoS Negl Trop Dis. 2010;4:e890. doi: 10.1371/journal.pntd.0000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–96. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilder-Smith A, Gubler DJ. Geographic expansion of dengue: the impact of international travel. Med Clin North Am. 2008;92:1377–90. doi: 10.1016/j.mcna.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Guzman MG, Kouri G. Dengue and dengue hemorrhagic fever in the Americas: lessons and challenges. J Clin Virol. 2003;27:1–13. doi: 10.1016/s1386-6532(03)00010-6. [DOI] [PubMed] [Google Scholar]
- 6.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, et al. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8(12 suppl):S7–16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halstead SB, Mahalingam S, Marovich MA, Ubol S, Mosser DM. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: disease regulation by immune complexes. Lancet Infect Dis. 2010;10:712–22. doi: 10.1016/S1473-3099(10)70166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothman AL. Dengue: defining protective versus pathologic immunity. J Clin Invest. 2004;113:946–51. doi: 10.1172/JCI21512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothman AL. T lymphocyte responses to heterologous secondary dengue virus infections. Ann NY Acad Sci. 2009;1171:E36–41. doi: 10.1111/j.1749-6632.2009.05055.x. [DOI] [PubMed] [Google Scholar]
- 10.Halstead SB. Vaccines aplenty. Curr Opin Infect Dis. 2002;15:461–3. doi: 10.1097/00001432-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nat Rev Microbiol. 2007;5:518–28. doi: 10.1038/nrmicro1690. [DOI] [PubMed] [Google Scholar]
- 12.Huang CY-H, Butrapet S, Tsuchiya KR, Bhamarapravati N, Gubler DJ, Kinney RM. Dengue 2 PDK-53 virus as a chimeric carrier for tetravalent dengue vaccine development. J Virol. 2003;77:11436–47. doi: 10.1128/JVI.77.21.11436-11447.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang CY-H, Butrapet S, Pierro DJ, Chang G-JJ, Hunt AR, Bhamarapravati N, et al. Chimeric dengue type 2 (vaccine strain PDK-53)/dengue type 1 virus as a potential candidate dengue type 1 virus vaccine. J Virol. 2000;74:3020–8. doi: 10.1128/jvi.74.7.3020-3028.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinney RM, Butrapet S, Chang GJ, Tsuchiya KR, Roehrig JT, Bhamarapravati N, et al. Construction of infectious cDNA clones for dengue 2 virus: strain 16681 and its attenuated vaccine derivative, strain PDK-53. Virology. 1997;230:300–8. doi: 10.1006/viro.1997.8500. [DOI] [PubMed] [Google Scholar]
- 15.Butrapet S, Huang CY, Pierro DJ, Bhamarapravati N, Gubler DJ, Kinney RM. Attenuation markers of a candidate dengue type 2 vaccine virus, strain 16681 (PDK-53), are defined by mutations in the 5′ noncoding region and nonstructural proteins 1 and 3. J Virol. 2000;74:3011–9. doi: 10.1128/jvi.74.7.3011-3019.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaughn DW, Hoke CH, Jr, Yoksan S, LaChance R, Innis BL, Rice RM, et al. Testing of a dengue 2 live-attenuated vaccine (strain 16681 PDK 53) in ten American volunteers. Vaccine. 1996;14:329–36. doi: 10.1016/0264-410x(95)00167-y. [DOI] [PubMed] [Google Scholar]
- 17.Sabchareon A, Lang J, Chanthavanich P, Yoksan S, Forrat R, Attanath P, et al. Safety and immunogenicity of tetravalent live-attenuated dengue vaccines in Thai adult volunteers: role of serotype concentration, ratio, and multiple doses. Am J Trop Med Hyg. 2002;66:264–72. doi: 10.4269/ajtmh.2002.66.264. [DOI] [PubMed] [Google Scholar]
- 18.Kanesa-thasan N, Sun W, Kim-Ahn G, Van Albert S, Putnak JR, King A, et al. Safety and immunogenicity of attenuated dengue virus vaccines (Aventis Pasteur) in human volunteers. Vaccine. 2001;19:3179–88. doi: 10.1016/s0264-410x(01)00020-2. [DOI] [PubMed] [Google Scholar]
- 19.Bhamarapravati N, Yoksan S, Chayaniyayothin T, Angsubphakorn S, Bun-yaratvej A. Immunization with a live attenuated dengue-2-virus candidate vaccine (16681-PDK 53): clinical, immunological and biological responses in adult volunteers. Bull World Health Organ. 1987;65:189–95. [PMC free article] [PubMed] [Google Scholar]
- 20.Dharakul T, Kurane I, Bhamarapravati N, Yoksan S, Vaughn DW, Hoke CH, et al. Dengue virus-specific memory T cell responses in human volunteers receiving a live attenuated dengue virus type 2 candidate vaccine. J Infect Dis. 1994;170:27–33. doi: 10.1093/infdis/170.1.27. [DOI] [PubMed] [Google Scholar]
- 21.Rothman AL, Kanesa-thasan N, West K, Janus J, Saluzzo JF, Ennis FA. Induction of T lymphocyte responses to dengue virus by a candidate tetravalent live attenuated dengue virus vaccine. Vaccine. 2001;19:4694–9. doi: 10.1016/s0264-410x(01)00236-5. [DOI] [PubMed] [Google Scholar]
- 22.Wiggan O, Livengood JA, Silengo SJ, Kinney RM, Osorio JE, Huang CY, et al. Novel formulations enhance the thermal stability of live-attenuated flavivirus vaccines. Vaccine. 2011;43:7456–62. doi: 10.1016/j.vaccine.2011.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson AJ, Roehrig JT. New mouse model for dengue virus vaccine testing. J Virol. 1999;73:783–6. doi: 10.1128/jvi.73.1.783-786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Broek MF, Müller U, Huang S, Zinkernagel RM, Aguet M. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol Rev. 1995;148:5–18. doi: 10.1111/j.1600-065x.1995.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 25.Schul W, Liu W, Xu H-Y, Flamand M, Vasudevan SG. A dengue fever viremia model in mice shows reduction in viral replication and suppression of the inflammatory response after treatment with antiviral drugs. J Inf Dis. 2007;195:665–74. doi: 10.1086/511310. [DOI] [PubMed] [Google Scholar]
- 26.Wahala WM, Kraus AA, Haymore LB, Accavitti-Loper MA, de Silva AM. Dengue virus neutralization by human sera: role of envelope protein domain III-reactive antibody. Virology. 2009;392:103–13. doi: 10.1016/j.virol.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyle JL, Balsitis SJ, Zang L, Beatty PR, Harris E. Antibodies play a greater role than immune cells in heterologous protection against secondary dengue virus infection in a mouse model. Virology. 2008;380:296–303. doi: 10.1016/j.virol.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan KR, Zang SL-X, Tan HC, Chan YK, Chow A, Lim APC, et al. Ligation of Fc gamma receptor IIB inhibits antibody-dependent enhancement of dengue virus infection. Proc Natl Acad Sci USA. 2011;108:12479–84. doi: 10.1073/pnas.1106568108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurane I, Zeng L, Brinton MA, Ennis FA. Definition of an epitope on NS3 recognized by human CD4+ cytotoxic T lymphocyte clones cross-reactive for dengue virus types 2, 3 and 4. Virology. 1998;240:169–74. doi: 10.1006/viro.1997.8925. [DOI] [PubMed] [Google Scholar]
- 30.Spaulding AC, Kurane I, Ennis FA, Rothman AL. Analysis of murine CD8+ T cell clones specific for the dengue virus NS3 protein: fla-vivirus cross-reactivity and influence of infecting serotype. J Virol. 1999;73:398–403. doi: 10.1128/jvi.73.1.398-403.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guirakhoo F, Pugachev K, Zhang Z, Myers G, Levenbook I, Draper K, et al. Safety and efficacy of chimeric yellow fever-dengue virus tetravalent vaccine formulations in non human primates. J Virol. 2004;78:4761–75. doi: 10.1128/JVI.78.9.4761-4775.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osorio JE, Brewoo JN, Silengo SJ, Arguello J, Moldovan IR, Tary-Lehmann M, et al. Efficacy of a tetravalent chimeric dengue vaccine (DENVax) in cynomologus macaques. Am J Trop Med Hyg. 2011;84:978–87. doi: 10.4269/ajtmh.2011.10-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guy B, Barban V, Mantel N, Aguirre M, Gulia S, Pontvianne J, et al. Evaluation of interferences between dengue vaccine serotypes in a monkey model. Am J Trop Med Hyg. 2009;80:302–11. [PubMed] [Google Scholar]