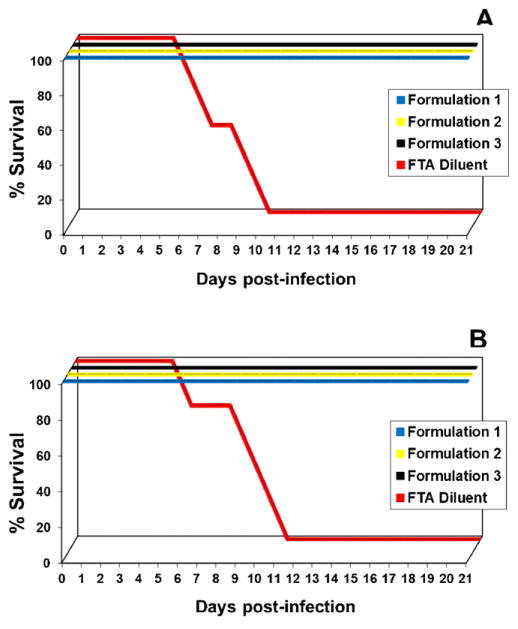
Fig. 4.
Protection afforded after vaccination with different DENVax formulations. Groups of eight AG129 mice were immunized subcutaneously with DENVax bearing different ratios of component vaccine viruses. Additional control groups were injected by the same route only with FTA. A booster SC injection of the same range of doses was given on day 42 post-priming. On day 70 all animals were split into two groups of four animals per group and were challenged intraperitoneally with 106 pfu of DENV-1 Mochizuki virus (A) or 106 pfu of mouse adapted DENV-2 New Guinea C virus (B). Animals were monitored daily for clinical signs of disease for three weeks and survival rates were recorded.