Abstract
Objectives
Optimal therapy for patients with non-small cell lung carcinoma (NSCLC) presenting with synchronous brain-only oligometastases (SBO) is not well defined. We sought to analyze the effect of differing therapeutic paradigms in this subpopulation.
Materials and Methods
We retrospectively analyzed NSCLC patients with 1-4 SBO diagnosed between 1/2000 and 1/2011 at our institution. Patients with T0 tumors or documented Karnofsky Performance Status <70 were excluded. Aggressive thoracic therapy (ATT) was defined as resection of the primary disease or chemoradiotherapy whose total radiation dose exceeded 45 Gy. Cox proportional hazards and competing risks models were used to analyze factors affecting survival and first recurrence in the brain.
Results
Sixty-six patients were included. Median follow-up was 31.9 months. Intrathoracic disease extent included 9 stage I, 10 stage II and 47 stage III patients. Thirty-eight patients received ATT, 28 did not. Patients receiving ATT were younger (median age 55 vs. 60.5 years, p=0.027) but were otherwise similar to those who did not. Receipt of ATT was associated with prolonged median overall survival (OS) (26.4 vs. 10.5 months; p<0.001) with actuarial 2-year rates of 54% vs. 26%. ATT remained associated with OS after controlling for age, thoracic stage, performance status and initial brain therapy (HR 0.40, p=0.009). On multivariate analysis, the risk of first failure in the brain was associated with receipt of ATT (HR 3.62, p=0.032) and initial combined modality brain therapy (HR 0.34, p=0.046).
Conclusion
Aggressive management of thoracic disease in NSCLC patients with SBO is associated with improved survival. Careful management of brain disease remains important, especially for those treated aggressively.
Keywords: Non-small cell lung cancer, brain metastases, oligometastases, aggressive therapy, radiation therapy, whole brain radiotherapy, stereotactic radiosurgery
Introduction
Non-small cell lung cancer is the leading cause of cancer-related death across the world, in part because over 50% of patients have metastatic disease at diagnosis. Twenty percent of NSCLC patients will ultimately develop brain metastases, making the brain one of the most common sites of distant spread [1]. The prognosis for patients with brain metastases from NSCLC is poor, with an estimated median survival of 7 months, and therapy is generally given to extend life or palliate symptoms rather than for cure [2].
Brain metastases are usually accompanied by diffuse extracranial disease and mortality is driven by the competing risks of both intra- and extracranial progression. Patients with NSCLC presenting with synchronous brain-only oligometatases (SBO) may represent a population where the risk of death from widespread dissemination is lower, and such patients may benefit from aggressive therapy directed to known areas of disease. Previous studies have suggested that some in this subgroup have the potential for long-term survival if the primary tumor is managed aggressively [3-8]. These previous series largely selected patients for a specific therapy directed to either the thorax or brain, however, so the comparative value of different therapeutic approaches is unclear. We retrospectively analyzed patients presenting with NSCLC and SBO at our institution to determine how treatment paradigms were associated with survival and relapse.
Patients and Methods
We retrospectively identified patients presenting to our institution with NSCLC and SBO between January 1, 2000 and January 1, 2011 following approval by the institutional review board. Patients with identifiable primaries, 1-4 synchronous brain metastases by CT or MRI, and no other evidence of distant metastatic disease confirmed by torso CT +/− bone scan or PET were included. Patients with a previous diagnosis of cancer or a documented Karnofsky Performance Status (KPS) of <70 were excluded (N=3). All patients were seen and discussed in the setting of a multidisciplinary thoracic Oncology clinic. While patients may have been eligible to participate in appropriate NSCLC protocols, there were no existing protocols designed specifically for this subgroup of patients during the study period. Aggressive thoracic therapy (ATT) was defined as either surgical resection of the primary disease and/or the administration of thoracic radiation therapy (RT) with dose > 45 Gy. Combined CNS therapy was defined as whole brain radiotherapy (WBRT) plus surgery or stereotactic radiosurgery (SRS) or both.
Statistical considerations
Baseline patient characteristics were compared using the Wilcoxon rank-sum or Fisher’s exact test. Overall survival and time to first failure were calculated from the date of diagnosis. Overall survival was estimated by the Kaplan-Meier method and compared using the log-rank test. A multivariate Cox proportional hazards model was constructed to evaluate factors independently associated with survival. First failure in the central nervous system (CNS) was estimated with death and non-CNS failure as competing risks and compared using Gray’s test. Competing risks regression using the method of Fine and Gray was used for multivariate analysis to evaluate factors independently associated with the risk of first failure in the CNS [9]. All p-values are two-sided. Statistical analysis was performed using SAS 9.2 and the cmprsk package in R version 2.6.2.
Results
Patient characteristics
66 patients met all eligibility criteria, 38 of whom received ATT and 28 who did not. Median age was 57 years, and median follow-up among survivors was 31.9 months. Other characteristics are shown in Table 1. Patients receiving ATT were younger than those that did not (median 55 vs. 60.5 years, p=0.027) but there were no other significant differences between the groups including initial CNS therapy (Table 2).
Table 1.
Characteristics of 66 NSCLC patients presenting with 1-4 synchronous brain metastases as their only site of disease.
| Characteristic | N | % | |
|---|---|---|---|
| Age (years) | (Median 57, Range 35-90) | ||
| >60 years | 25 | 36% | |
| ≤60 years | 41 | 64% | |
| Sex | |||
| Male | 24 | 36% | |
| Female | 42 | 64% | |
| Histology | |||
| Adenocarcinoma | 38 | 58% | |
| Squamous cell carcinoma | 9 | 14% | |
| NSCLC NOS | 19 | 29% | |
| KPS* | |||
| 100 | 12 | 18% | |
| 80-90 | 31 | 47% | |
| 70 | 13 | 20% | |
| Thoracic stage | |||
| IA | 3 | 5% | |
| IB | 6 | 9% | |
| IIA | 7 | 11% | |
| IIB | 3 | 5% | |
| IIIA | 34 | 51% | |
| IIIB | 13 | 20% | |
| Number of brain metastases | |||
| 1 | 33 | 50% | |
| 2 | 22 | 33% | |
| 3 | 10 | 15% | |
| 4 | 1 | 2% | |
| Size of largest brain metastasis (cm) | (Median 1.8, Range 0.1-6.1) | ||
| ≤ 3 cm | 55 | 83% | |
| > 3 cm | 10 | 15% | |
| Unknown | 1 | 2% | |
| Initial CNS therapy | |||
| WBRT + SRS + Surgery | 3 | 5% | |
| WBRT + SRS | 11 | 17% | |
| WBRT + Surgery | 21 | 32% | |
| WBRT only | 7 | 11% | |
| SRS + Surgery | 4 | 6% | |
| SRS only | 18 | 27% | |
| None | 2 | 3% | |
| Initial thoracic therapy | |||
| Surgery + RT | 5 | 8% | |
| Surgery only | 14 | 21% | |
| RT > 45 Gy + chemotherapy | 19 | 29% | |
| RT ≤ 45 Gy +/− chemotherapy | 8 | 12% | |
| Chemotherapy alone | 17 | 26% | |
| Supportive care alone | 3 | 5% |
NSCLC: Non-small cell lung cancer; NOS: not otherwise specified; KPS: Karnofsky performance status; CNS: central nervous system; WBRT: whole brain radiotherapy; SRS: stereotactic radiosurgery; RT: radiation therapy.
Totals do not add up due to missing data.
Table 2.
Patient characteristics associated with the receipt of aggressive thoracic therapy.
| Characteristic | ATT | Non-ATT | p |
|---|---|---|---|
| N | 38 | 28 | |
| Age, median | 55 years | 60.5 years | 0.027 |
| Sex | 1.000 | ||
| Male | 14 (37%) | 10 (36%) | |
| Female | 24 (63%) | 18 (64%) | |
| KPS* | 0.200 | ||
| 80-100 | 27 (84%) | 16 (67%) | |
| 70 | 5 (16%) | 8 (33%) | |
| Histology | 0.104 | ||
| Adenocarcinoma | 25 (66%) | 13 (46%) | |
| Squamous cell | 6 (16%) | 3 (11%) | |
| NSCLC NOS | 7 (18%) | 12 (43%) | |
| Thoracic stage | 0.390 | ||
| I | 7 (18%) | 2 (7%) | |
| II | 6 (16%) | 4 (14%) | |
| III | 25 (66%) | 12 (79%) | |
| Number of brain metastases | 1.000 | ||
| 1 | 19 (50%) | 14 (50%) | |
| 2-4 | 19 (50%) | 14 (50%) | |
| Size of largest brain metastasis* | 0.495 | ||
| ≤ 3 cm | 30 (81%) | 25 (89%) | |
| > 3 cm | 7 (19%) | 3 (11%) | |
| Initial CNS therapy | 0.480 | ||
| WBRT + SRS and/or Surgery | 21 (55%) | 14 (50%) | |
| WBRT only or Surgery +/− SRS | 15 (39%) | 14 (50%) | |
| None | 2 (5%) | 0 (0%) |
Totals do not add up due to missing data.
NOS: not otherwise specified; KPS: Karnofsky performance status; NSCLC: non-small cell lung cancer; CNS: central nervous system; WBRT: whole brain radiotherapy; SRS: stereotactic radiosurgery; ATT: aggressive thoracic therapy
Overall survival
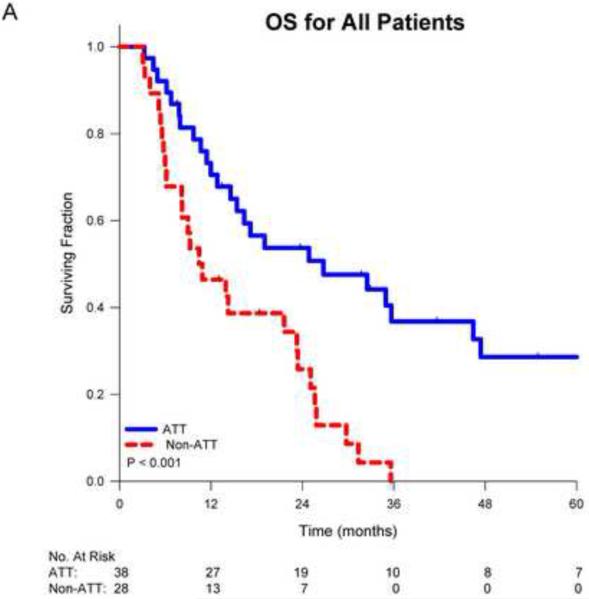
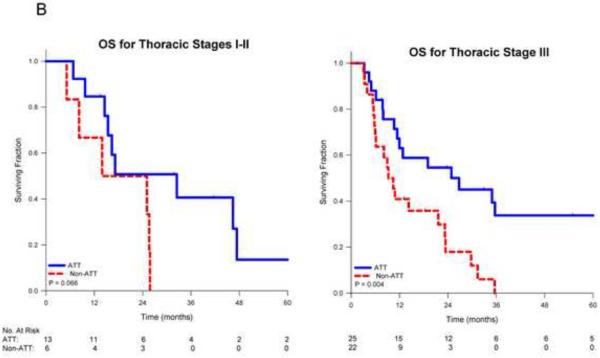
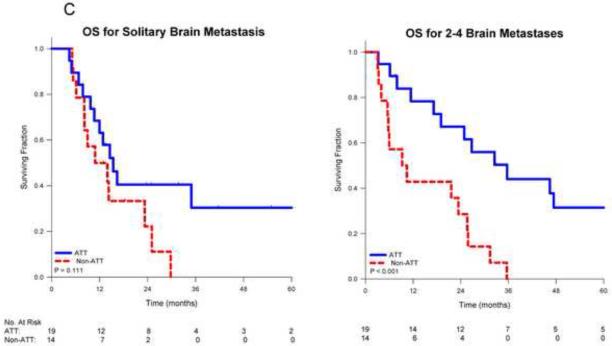
On univariate analysis, receipt of ATT was associated with improved overall survival (p<0.001). Median survival for patients receiving ATT was 26.4 months vs. 10.5 months for those receiving non-ATT. Actuarial 1-, 2- and 5-year survival for those receiving ATT was 71%, 54% and 29% respectively vs. 46%, 26% and 0% for those receiving non-ATT (Figure 1A). When stratified by stage, the benefit of ATT remained significant for patients with stage III disease (p=0.004), but was borderline significant for those with stage I-II disease (p=0.066; Figure 1B). When stratified by the number of SBO, patients with multiple metastases who received ATT had significantly improved survival (p<0.001) vs. non-ATT while those with a solitary metastasis who received ATT had a trend towards improved survival (p=0.111; Figure 1C).
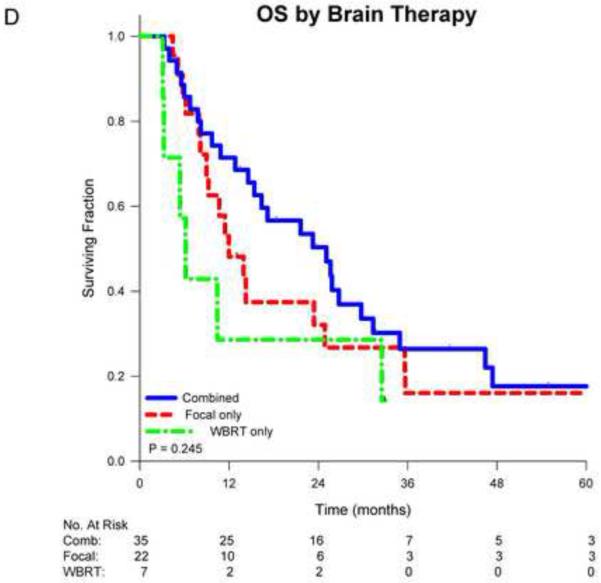
Figure 1.
Kaplan-Meier survival curves for non-small cell lung cancer patients comparing aggressive thoracic therapy (ATT) to non-aggressive thoracic therapy (non-ATT) for A) all patients, B) patients stratified by thoracic stage or C) patients stratified by number of brain metastases, and D) comparing overall survival by CNS therapy type.
There was a suggestion of an early survival benefit for patients initially treated with combined CNS therapy (especially compared to WBRT alone), however these differences did not reach statistical significance (p=0.245; Figure 1D). Multivariate analysis of overall survival is shown in Table 3. After adjusting for age, thoracic stage, performance status and initial CNS therapy, ATT remained the only factor independently associated with survival (HR 0.40, p=0.009).
Table 3.
Multivariate Cox regression analysis of factors associated with overall survival
| Covariate | HR | p |
|---|---|---|
| ATT | 0.40 | 0.009 |
| Combined brain therapy | 0.82 | 0.560 |
| Age (per year) | 1.02 | 0.262 |
| KPS 70 vs. 80-100 | 1.26 | 0.545 |
| Thoracic stage I-II vs. III | 0.96 | 0.909 |
ATT: aggressive thoracic therapy; KPS: Karnofsky performance status
CNS first failure
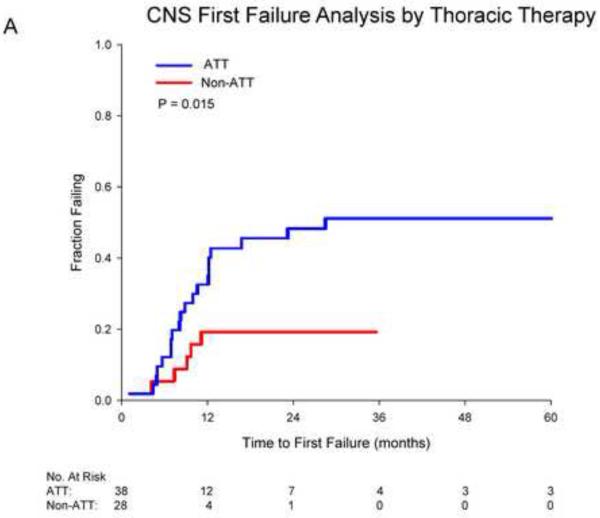
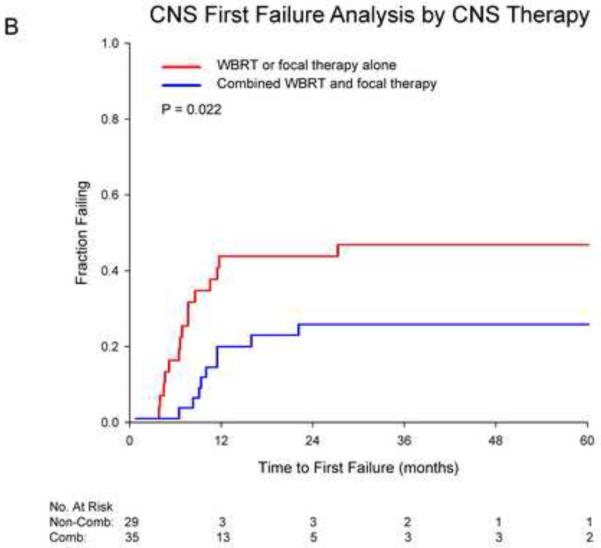
Cumulative incidence curves for first failure in the CNS are shown in figure 2. Receipt of ATT was associated with significantly higher rates of CNS first failure (48% vs. 18% at 2 years, p=0.015). Receipt of initial combined CNS therapy was associated with significantly decreased CNS first failure (26% vs. 44% at 2 years, p=0.022). For those who received ATT, combined CNS therapy was borderline significant for reducing CNS first failures (p=0.061). For those not receiving ATT, no significant benefit for combined therapy was seen (p=0.148). On multivariate competing risks analysis adjusting for age, performance status and thoracic stage, receipt of ATT was associated with a significant increase in CNS first failures (HR 3.62, p=0.032) while initial combined CNS therapy was associated with a significant reduction (HR 0.34, p=0.046; Table 4).
Figure 2.
Cumulative incidence curves depicting first failure in the CNS for patients stratified by A) receipt of aggressive thoracic therapy (ATT) and B) receipt of combined whole brain radiotherapy (WBRT) and focal therapy.
Table 4.
Multivariate competing risks analysis of factors associated with first failure in the brain
| Covariate | HR | p |
|---|---|---|
| ATT | 3.62 | 0.032 |
| Combined brain therapy | 0.34 | 0.046 |
| Age (per year) | 0.72 | 0.500 |
| KPS 70 vs. 80-100 | 0.52 | 0.280 |
| Thoracic stage I-II vs. III | 0.62 | 0.480 |
ATT: aggressive thoracic therapy; KPS: Karnofsky performance status
Discussion
Patients with NSCLC and brain metastases generally have a poor prognosis. This population is not homogeneous however; the Graded Prognostic Assessment index demonstrates median survivals ranging from 3 months to 14.8 months depending on the presence or absence of several prognostic factors [2]. Two of these prognostic factors relate to the competing risks for mortality: NSCLC patients with extracranial metastases and those with higher numbers of brain metastases have worse overall survival. While these variables are not modifiable, differences in management strategies can affect outcome, though no clear consensus on appropriate treatment exists for this subgroup [10]. Brain-directed therapy is guided by randomized data, but the relative benefits of WBRT or focal therapies in the context of limited extracranial disease are not well described. The potential benefit of ATT in this patient population has never been evaluated in a randomized fashion.
In our study, ATT was associated with improved overall survival for all thoracic stages. Only patients receiving ATT survived beyond 3 years. This impact on longer-term survival is important to note given the potential immortal time bias associated with retrospective analysis of lengthy treatment courses. As there is a clear tail to the survival curve for ATT, the perceived benefit appears to be not just definitional but representative of durable disease control. Indeed, the finding of potential long-term survivors in patients with oligometastatic lung cancer has been shown in multiple studies [11]. Significant variability is present, however, both in the definition of oligometastases and in the outcomes for such patients. As such, clearer evidence to help define an appropriate treatment path for these patients is needed, though mounting evidence suggests that aggressive treatment may be warranted given that oligometastatic NSCLC may indeed be a curable disease state.
Our actuarial 5-year survival estimate of 29% for patients receiving ATT compares favorably with other published results for patients with NSCLC and SBO. Several surgical series looking at combined surgery for the brain and chest disease have reported 5-year survival rates between 7% and 27% [3, 4, 12-16]. Patients included in these studies all had focal management of their brain metastases with variable use of WBRT. Other series have reported more variable management of the primary disease, allowing for comparisons. Hu et al. reported a 5-year survival of 7.6% in 84 cases, all of whom underwent focal therapy to their brain metastases followed by WBRT in 37% [7]. No patients in this cohort underwent surgery for their primary disease, however, and only 25% received any type of radiation therapy to the chest (median dose 45 Gy). Even so, any treatment (chemotherapy or radiation) to address extracranial disease was associated with an overall survival benefit. Despite this, the authors concluded that aggressive therapy was only likely to benefit those with stage I disease. This recommendation is generally in line with U.S. practice guidelines which do not recommend ATT for those with thoracic stage III disease and SBO [17]. This conclusion conflicts with the findings of our study, however, which suggest that patients of all thoracic stages may benefit from ATT. Indeed there are emerging prospective data that many patients with limited metastatic disease have the potential for long-term survival if treated aggressively [18].
Flannery et al. reported a 21% 5-year survival for patients presenting with NSCLC and a solitary synchronous brain metastasis. Similar to our study, for those who underwent ATT the 5-year survival rate was 35% vs. 0% for those who did not [6]. Recent European guidelines, however, do not recommend ATT for patients with more than a solitary brain metastasis [19]. By comparison, Arrieta et al. reported a remarkable median survival of 31.8 months and 2-year survival of 61% for 30 patients with one or multiple SBO who were treated with WBRT and induction chemotherapy followed by chemoradiotherapy for their primary disease [20]. This study would support our finding that ATT confers a survival benefit for patients with multiple SBO.
For patients with a single brain metastasis, the addition of local brain therapy (SRS or surgery) to WBRT is associated with improved overall survival compared to WBRT alone [21, 22]. The converse, however, is not true as several randomized trials have failed to demonstrate improved overall survival when WBRT is added to focal therapies despite better intracranial disease control [23-25]. This decoupling of intracranial disease progression from survival may be related to robust salvage of recurrences, to extracranial disease progression dominating as a competing risk, or a combination of factors. Patients with SBO, especially after ATT, may have a reduced competing risk of extracranial disease progression, potentially making intracranial control more important. This is supported by our data showing better control of the thoracic disease shifts the pattern of first failure to the brain. By comparison, for patients with NSCLC and SBO, Ampil et al. reported that surgical resection of the brain metastases was the only factor associated with improved overall survival [26] and Chidel et al. found that both initial WBRT and ATT were correlated with better overall survival [5]. Our analyses suggest that WBRT combined with focal therapy may be associated with a trend for improved survival in those receiving ATT.
Limitations of this study are related to its retrospective nature including the potential for selection and immortal time bias as previously noted. Though we attempted to control for known prognostic variables by multivariate analysis, the potential for patient selection based on non-identified factors remains. Of note, our study also contains a significantly higher proportion of female patients than is seen in other reports. This may represent the unique makeup of patients at our institution, but also may limit generalizability. Additionally, given the time period covered in our study, EGFR mutation status was not available for the bulk of our patients. Given these limitations, our results should be interpreted with caution and used only for hypothesis generation. The clear benefits seen for aggressive management in our cohort, however, should prompt the design of prospective studies to verify the effects of such therapy on survival and also identify effects of such therapy on patient quality of life.
Conclusions
Patients presenting with SBO from NSCLC may represent a population with the potential for long-term survival when managed aggressively. Until rigorous prospective data becomes available, these data and similar studies support ongoing review of current clinical practice guidelines for this subgroup. Future research should focus on identifying clinical and molecular markers which can be used to identify which patients are most likely to benefit from ATT.
Summary.
Optimal therapy for patients with non-small cell lung carcinoma presenting with synchronous brain-only oligometastases is not well defined. We retrospectively analyzed 66 non-small cell lung cancer patients with 1-4 synchronous brain-only oligometastases diagnosed over an 11 year period at our institution. We compared outcomes for patients who received aggressive thoracic therapy (surgical resection of the primary disease or definitive radiotherapy) to those who received less aggressive local therapy. We also analyzed the effect of differing treatment paradigms for the brain disease on outcomes. At a median follow-up of 31.9 months, receipt of aggressive thoracic therapy was associated with prolonged median overall survival (26.4 vs. 10.5 months). This association remained significant after controlling for age, thoracic stage, performance status and initial brain therapy. The risk of first failure in the brain was higher in those patients who received aggressive thoracic therapy and lower in those who received up-front combined whole brain radiotherapy and local therapy (surgery or stereotactic radiosurgery).
Highlights.
We analyzed outcomes for 66 patients with non-small cell lung cancer and brain-only metastases.
Receipt of aggressive therapy for the thoracic primary was associated with improved survival.
Receipt of combined whole brain and stereotactic radiotherapy decreased first brain failures.
Acknowledgments
Funding: B.Y. Yeap was supported by the Biostatistics Core of the Dana-Farber/Harvard Cancer Center funded in part by NIH Cancer Center Support Grant P30 CA06516.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: Dr. Jackman previously served as a consultant for Genentech, Foundation Medicine and Chugai Pharmaceuticals and has received honorariums from CE Outcomes and the Cowen Group. The remaining authors have no conflicts of interest to disclose.
References
- 1.Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 2.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billing PS, Miller DL, Allen MS, et al. Surgical treatment of primary lung cancer with synchronous brain metastases. J Thorac Cardiovasc Surg. 2001;122:548–553. doi: 10.1067/mtc.2001.116201. [DOI] [PubMed] [Google Scholar]
- 4.Bonnette P, Puyo P, Gabriel C, et al. Surgical management of non-small cell lung cancer with synchronous brain metastases. Chest. 2001;119:1469–1475. doi: 10.1378/chest.119.5.1469. [DOI] [PubMed] [Google Scholar]
- 5.Chidel MA, Suh JH, Greskovich JF, et al. Treatment outcome for patients with primary nonsmall-cell lung cancer and synchronous brain metastasis. Radiat Oncol Investig. 1999;7:313–319. doi: 10.1002/(SICI)1520-6823(1999)7:5<313::AID-ROI7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Flannery TW, Suntharalingam M, Regine WF, et al. Long-term survival in patients with synchronous, solitary brain metastasis from non-small-cell lung cancer treated with radiosurgery. Int J Radiat Oncol Biol Phys. 2008;72:19–23. doi: 10.1016/j.ijrobp.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 7.Hu C, Chang EL, Hassenbusch SJ, et al. Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer. 2006;106:1998–2004. doi: 10.1002/cncr.21818. [DOI] [PubMed] [Google Scholar]
- 8.Lind JS, Lagerwaard FJ, Smit EF, et al. Time for reappraisal of extracranial treatment options? Synchronous brain metastases from nonsmall cell lung cancer. Cancer. 2011;117:597–605. doi: 10.1002/cncr.25416. [DOI] [PubMed] [Google Scholar]
- 9.Fine J, Gray R. A proportional hazard model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 10.Halasz LM, Weeks JC, Neville BA, et al. Use of stereotactic radiosurgery for brain metastases from non-small cell lung cancer in the United States. Int J Radiat Oncol Biol Phys. 2013;85:e109–116. doi: 10.1016/j.ijrobp.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashworth A, Rodrigues G, Boldt G, Palma D. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung cancer. 2013;82:197–203. doi: 10.1016/j.lungcan.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 12.Getman V, Devyatko E, Dunkler D, et al. Prognosis of patients with non-small cell lung cancer with isolated brain metastases undergoing combined surgical treatment. Eur J Cardiothorac Surg. 2004;25:1107–1113. doi: 10.1016/j.ejcts.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Lo CK, Yu CH, Ma CC, et al. Surgical management of primary non-small-cell carcinoma of lung with synchronous solitary brain metastasis: local experience. Hong Kong Med J. 2010;16:186–191. [PubMed] [Google Scholar]
- 14.Magilligan DJ, Duvernoy C, Malik G, et al. Surgical approach to lung cancer with solitary cerebral metastasis: twenty-five years' experience. Ann Thorac Surg. 1986;42:360–364. doi: 10.1016/s0003-4975(10)60536-x. [DOI] [PubMed] [Google Scholar]
- 15.Mussi A, Pistolesi M, Lucchi M, et al. Resection of single brain metastasis in non-small-cell lung cancer: prognostic factors. J Thorasc Cardiovasc Surg. 1996;112:146–153. doi: 10.1016/s0022-5223(96)70190-3. [DOI] [PubMed] [Google Scholar]
- 16.Rossi NP, Zavala DC, VanGilder JC. A combined surgical approach to non-oat-cell pulmonary carcinoma with single cerebral metastasis. Respiration. 1987;51:170–178. doi: 10.1159/000195199. [DOI] [PubMed] [Google Scholar]
- 17.Ettinger DS, Akerley W, Borghaei H, et al. Non-Small Cell Lung Cancer, Version 2.2013. J Natl Compr Canc Netw. 2013;11:645–653. doi: 10.6004/jnccn.2013.0084. [DOI] [PubMed] [Google Scholar]
- 18.De Ruysscher D, Wanders R, van Baardwijk A, et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: long-term results of a prospective phase II trial (Nct01282450) J Thorac Oncol. 2012;7:1547–1555. doi: 10.1097/JTO.0b013e318262caf6. [DOI] [PubMed] [Google Scholar]
- 19.Peters S, Adjei AA, Gridelli C, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii56–64. doi: 10.1093/annonc/mds226. [DOI] [PubMed] [Google Scholar]
- 20.Arrieta O, Villarreal-Garza C, Zamora J, et al. Long-term survival in patients with non-small cell lung cancer and synchronous brain metastasis treated with whole-brain radiotherapy and thoracic chemoradiation. Radiat Oncol. 2011;6:166. doi: 10.1186/1748-717X-6-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 22.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 23.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 24.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 26.Ampil F, Caldito G, Milligan S, et al. The elderly with synchronous non-small cell lung cancer and solitary brain metastasis: does palliative thoracic radiotherapy have a useful role? Lung Cancer. 2007;57:60–65. doi: 10.1016/j.lungcan.2007.02.006. [DOI] [PubMed] [Google Scholar]