Abstract
Purpose of review
The purpose of this study is to provide an overview of the role of endoplasmic reticulum (ER) stress and the unfolded protein response (UPR) in inflammatory bowel disease (IBD).
Recent findings
Human genetic studies have identified several UPR-related genes and autophagy-related genes as IBD risk loci. Impairment of each branch of the UPR causes spontaneous enteritis or creates higher susceptibility for intestinal inflammation in model systems. Deficiency of either UPR or autophagy in small intestinal epithelial cells promotes each other’s compensatory engagement, which is especially prominent in Paneth cells such that, in the absence of both, severe spontaneous enteritis emerges.
Summary
Interactions between the UPR and autophagy exhibit critical synergistic interactions within the intestinal epithelium and especially Paneth cells that are of considerable importance to the maintenance of homeostasis. When dysfunctional in the Paneth cell, spontaneous inflammation can emerge that may extend beyond the epithelium providing direct experimental evidence that subsets of Crohn’s disease may emanate from primary Paneth cell disturbances.
Keywords: autophagy, Crohn’s disease, endoplasmic reticulum stress, inflammatory bowel disease, Paneth cells
INTRODUCTION
The intestinal epithelium consists of six major cell types that are derived from the common stem cell: Paneth cells, goblet cells, tuft cells, microfold villus (M) cells, absorptive cells and enteroendocrine cells. These together form a single layer of cells that line the gastrointestinal tract and represent a critical interface between the host and enteric microorganisms. Paneth cells, which are primarily located at the base of small intestinal crypts under homeostatic conditions, secrete multiple antimicrobial peptides (AMPs) including defensins and lysozyme and thereby regulate the composition of the host commensal microbiota as well as play an important role in innate immunity and the function of the stem cell niche [1,2]. Goblet cells secrete abundant amounts of mucins, which constitute the mucus barrier and enteroendocrine cells produce significant levels of neuropeptides [3,4]. Further, the vast majority of absorptive epithelial cells also broadly regulate the composition of the commensal microbiota and subjacent mucosal immune system via secretion of a large array of cytokines and chemokines, which makes them an important component of the mucosal immune system [5]. These activities of intestinal epithelial cells (IECs) create a large secretory burden for this cell type and it demands appropriate cell biologic systems to manage this.
The unfolded protein response (UPR), in particular, is important for the development and survival of highly secretory cells such as IECs that synthesize high volumes of proteins, which requires careful management of the endoplasmic reticulum (ER) to avoid stress in this organelle [6]. As Paneth and goblet cells are among the most secretory of the IEC subtypes, these cells are particularly prone to abnormalities when the UPR is dysfunctional [7,8]. Consistent with this, the small intestine, which contains high numbers of Paneth cells, has higher baseline levels of UPR-related gene expression than the colon [9]. The UPR is not only of importance to the function of differentiated IEC subtypes but also gut stem cells in general as ER stress and UPR induction are critical to such cells in the intestine [10▪] and oesophagus [11]. Given these broad biologic effects and evidence that UPR-related genetic risk loci are associated with inflammatory bowel disease (IBD), it is important to understand how these pathways operate in the maintenance of intestinal homeostasis and disease.
THE UNFOLDED PROTEIN RESPONSE
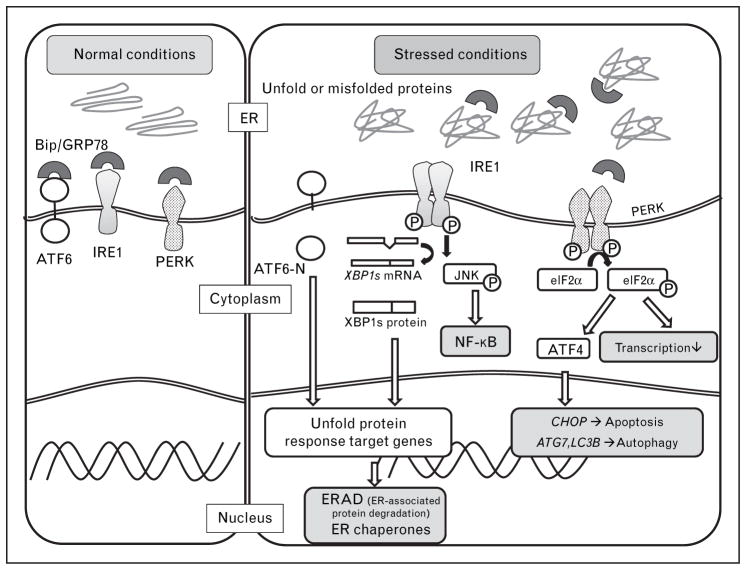
ER stress is caused by the accumulation of unfolded and misfolded proteins in the ER arising from either primary (genetic) or secondary (environmental) factors [12,13]. Highly secretory cells, such as plasma cells, insulin-secreting β-cells in the pancreas, IECs, (especially Paneth cells and goblet cells) are highly susceptible to ER stress [6]. The UPR is a highly conserved mechanism that enables the cell to respond to and resolve the ER stress. The UPR is characterized by three major response arms. Specifically, ER stress triggers the activity of three ER membrane resident proteins that sense the presence of unfolded proteins in the ER lumen: inositol-requiring transmembrane kinase endonuclease 1 (IRE1), pancreatic ER kinase (PERK) and activated transcription factor 6 (ATF6). IRE1, PERK and ATF6 exist as inactive complexes in association with immunoglobulin heavy chain binding protein (BIP), which is also known as glucose-regulated protein 78 (GRP78), in the absence of misfolded proteins in the ER lumen. BIP senses the presence of misfolded proteins and releases the three proteins to allow them to enter their active states. IRE1 is the most evolutionarily conserved pathway and is shared by yeast [14,15]. Mammalian cells have two highly conserved isoforms, IRE1α that is expressed ubiquitously [16] and IRE1β that is restricted to gut and lung epithelial cells [17]. IRE1 is dimerized and phosphorylated under ER stress. The activated IRE1 has both kinase activity that promotes activation of JUN N-terminal kinase (JNK) [18] and Nuclear factor-κB (NF-κB) [19] and endoribonuclease activity that excises a 26-nucleotide sequence of X-box binding protein 1 (XBP1) mRNA resulting in a frame shift and generation of a transcriptionally active isoform that functions as a transactivator of UPR target genes [20]. Such target genes play a role in expanding the ER, for example, to allow the cell an ability to deal with its increased secretory requirements and diminish the ER stress. In addition, IRE1 mediates the rapid degradation of a certain subset of mRNAs, a process that is termed RIDD (regulated IRE1-dependent decay) [21]. PERK possesses protein kinase activity and inactivates elongation initiation factor 2α (eIF2α) by phosphorylation that halts general protein synthesis but allows the translation of specific species of mRNA such as activating factor 4 (ATF4), which is translocated into the nucleus and activates genes necessary for the UPR. Finally, upon dissociation from GRP78, ATF6 translocates to the Golgi whereupon it is cleaved by site 1 and site 2 proteases (S1P and S2P) releasing the cytoplasmic domain of ATF6 (ATF6-N), which is transcriptionally active in inducing UPR-associated genes. ATF4 and the ATF6-N are critical to the activation of the apoptosis-related transcription factors, such as CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP) that leads to cell cycle arrest and/or apoptosis [22,23▪]. Thus, if ER stress is unresolved, cell death is the likely outcome (Fig. 1).
FIGURE 1.
Endoplasmic reticulum stress inducing autophagy. BIP (GRP78) senses the presence of misfolded proteins and releases ATF6, IRE1 and PERK to allow them to enter their active states, resulting in transcriptional programs that relieve the ER stress and activate autophagy. ATF4, downstream of the PERK-eIFα pathway, transactivates LC3b and Atg7, resulting in activation of the compensatory autophagic mechanism.
INOSITOL-REQUIRING TRANSMEMBRANE KINASE ENDONUCLEASE 1 – XBP1 BRANCH AND INTESTINAL INFLAMMATION
Several studies have shown that genetic deletion of molecules involved in the UPR are associated with either spontaneous intestinal inflammation and/or increased sensitivity to the experimental induction of colitis. IRE1β knockout mice are highly sensitive to dextran sodium sulphate (DSS)-induced colitis [24]. IRE1β-deficient mice exhibit increased basal levels of GRP78 in the intestinal epithelium prior to colitis induction suggesting ongoing ER stress and increased sensitivity to environmental agents that disrupt the IEC barrier and/or induce further ER stress [24]. IRE1β is particularly active in goblet cells such that IRE1β knockout (Ern2−/−) mice exhibit aberrant mucin 2 (MUC2) accumulation in the ER of goblet cells in contrast to IRE1α knockout (Ern1-ΔIEC) mice that have normal goblet cells [25▪]. This suggests that the sensitivity to DSS-induced colitis in Ern2−/− mice might be due to associated abnormalities in goblet cells in this model.
Mice with conditional deletion of XBP1 expression in IECs (Xbp1ΔIEC mice) have been observed to develop spontaneous inflammation in the small intestine with microscopic features that include crypt abscesses, mononuclear and polymorphonuclear cell infiltration and ulcerations that mimic certain features of human IBD [7]. Xbp1ΔIEC mice exhibit increased ER stress in the small intestine as detected by increased GRP78. As a consequence, IECs of Xbp1ΔIEC mice exhibit increased apoptosis and a regenerative response, resulting in a reduction of goblet cells and Paneth cells with a condensed ER and absence of their characteristic secretory granules, in association with decreased AMP production. Consistent with a defect in Paneth cells, Xbp1ΔIEC mice, such as Nod2−/− mice [26], are highly susceptible to infection with Listeria monocytogenes [7]. Although Xbp1ΔIEC mice do not exhibit spontaneous colitis, they are highly susceptible to DSS-induced colitis [7]. The mechanistic basis for this disparity between susceptibility to inflammation in the colon and small intestine in the presence of XBP1-deficiency and ER stress is not known. It has been pointed out that increased basal ER stress may exist in the small intestine [9]. It is also possible that the major effect of ER stress on Paneth cells may result in bacterial dysbiosis [7]. Both mechanisms are possible, as the spontaneous enteritis observed in Xbp1ΔIEC mice can be significantly reversed by rederivation under germ-free conditions [27▪▪]. Together, these studies show that when IECs are unable to properly manage ER stress, not only can spontaneous intestinal inflammation emerge in the small intestine, but also the intestine at large is exquisitely sensitive to environmental factors capable of triggering intestinal inflammation.
The kinase activity of IRE1 has been linked to the activation of NF-κB and JNK, both of which are critical molecules in intestinal inflammation [28]. Consistent with this, mechanistic studies have shown that IRE1-dependent NF-κB and JNK activation is increased in XBP1-deficient IECs in vivo. Further, the NF-κB hyperactivation observed in Xbp1ΔIEC mice is derived from increased IREα activation and responsible for the intestinal inflammation that is observed. Thus, small molecule blockade of NF-κB activation or genetic deletion Ern1 in IECs protects Xbp1ΔIEC mice from spontaneous enteritis. Further, consistent with the important role played by tumour necrosis factor-alpha (TNFα) in intestinal inflammation, Xbp1ΔIEC mice are protected from enteritis upon deletion of TNF-receptor 1 [27▪▪]. These studies indicate that the development of enteritis on Xbp1ΔIEC mice depends upon IRE1-NF-κB signalling in a pathway that is driven by TNFα, the cytokine targeted by the most potent therapeutics of human Crohn’s disease [29].
The IRE1 and XBP1 pathway also has an important role in tumourigenesis. Xbp1ΔIEC mice exhibit increased numbers of Lgr5+ and Olfm4+ intestinal stem cells (ISCs), which is dependent upon Ire1α [30], and a Stat3-mediated increase in the proliferative output of transit-amplifying cells [31▪], the latter being dependent upon inflammatory cytokines produced by the epithelium that is undergoing ER stress. It was specifically observed that colitis-associated cancer induced by azoxymethane (AOM) followed by DSS resulted in 10-fold more and larger colonic tumours in Xbp1ΔIEC mice than that observed in control mice. In addition, when Xbp1ΔIEC mice were crossed onto an Apcmin background (Xbp1ΔIEC; Apcmin), they developed twofold more tumours than their Xbp1Wt Apcmin littermates. Further evidence that ER stress may promote intestinal neoplasia is evidence that stem cells undergoing ER stress rapidly enter the transit-amplifying compartment and undergo significant proliferation [31▪].
OTHER ENDOPLASMIC RETICULUM STRESS RELATED MOLECULES AND INTESTINAL INFLAMMATION
Mice with deficiency in ATF6α (Atf6a−/− mice) are sensitive to DSS-induced colitis with evidence of increased ER stress, such as increased expression of GRP78, ATF4, CHOP and spliced Xbp1 [32▪]. P58IPK−/− mice, which are deficient in this important ER chaperone, have also been demonstrated to be sensitive to DSS-induced colitis with increased GRP78 and CHOP expression and phosphorylation of IRE1. Mice with conditional ablation of eIF2α phosphorylation exhibit defective UPR signalling and ER-associated mRNA translation, leading to protein secretion dysfunction in Paneth cell and increased sensitivity to Salmonella infection and DSS-induced colitis [33▪].
Anterior gradient 2 (AGR2), which is a member of the ER protein disulfide isomerase (PDI) gene family, is expressed in goblet, Paneth and enter-oendocrine cells in the small intestine [34,35]. Zhao et al. [36] have reported that Agr2−/− mice are observed to develop spontaneous ileocolitis with increased levels of ER stress markers; XBP1s, CHOP, PERK and GRP78 (BiP). These mice have decreased Mucin 2 expression in goblet cells and abnormal localization and expansion of Paneth cells in the small intestine. On the contrary, Chop−/− MEFs are resistant to ER stress induced apoptosis [37] and Chop−/− mice are protected from DSS colitis and colitis induced by 2,4,6-trinitrobenzenesulfonic acid (TNBS) [38]. CHOP may therefore be an interesting therapeutic target in the treatment of IBD.
Forward genetic approaches using N-ethyl-N-nitrosourea mutagenesis have resulted in several new mouse models that exhibit defects in the UPR. For example, mice with two distinct single missense mutations in Muc2 (Winnie and Eeyore) have been reported to develop spontaneous colitis resembling human ulcerative colitis [39]. These mutations cause mucin misfolding leading to accumulation of MUC2 precursor proteins in the ER, ER vacuolization and ER stress confirmed by increased levels of Xbp1 splicing and GRP78 [39]. The inflammatory response in this model involves both innate and adaptive immunity including the interleukin (IL)-23/Th17 inflammatory responses, as observed in human IBD [40]. Another mouse model, so-called woodrat mouse, possesses a loss-of-function mutation in site-specific protease 1 (S1P). S1P and S2P are responsible for the generation of the transcriptionally active form of ATF6-N through release of the cytoplasmic tail of ATF6 when it is translocated to the Golgi apparatus during ER stress [41]. The woodrat mouse exhibits increased sensitivity to DSS-induced colitis with the defect largely derived from the radio-resistant compartment in bone marrow chimera experiments pointing towards the intestinal epithelium as the likely source of the defect. One caveat of this model is due to the broad substrate specificity of S1P that includes ATF6, CREB-H and other related transcription factors [42]; hence, it cannot be concluded that the phenotype observed is directly due to ATF6.
OASIS, which is a basic leucine zipper transmembrane transcription factor activated in response to ER stress, is expressed in goblet cells of the large intestine. Oasis−/− mice have abnormal differentiation and maturation of goblet cell [43], resulting in increased susceptibility to DSS-induced colitis [44].
In summary, a large variety of defects in the UPR that result in ER stress render the intestinal epithelium susceptible to the development of spontaneous or induced enterocolitis. The determination of whether spontaneous or induced disease develops likely reflects the depth of the effect that the mutation has on the UPR and the susceptibility thereby to environmental stresses. In defects associated with Xbp1 or Agr2, for example, the pressure derived from the commensal microbiota per se is enough to tip the host over to spontaneous intestinal inflammation. Another aspect of these studies is greater apparent sensitivity of the small relative to the large intestine to the effects of ER stress. This is further buttressed by the exquisite sensitivity of the small intestine to the effects of ischemia/reperfusion (I/R) injury and implies that loss of Paneth cells due to ER stress is crucial for I/R-related disease [45]. I/R of the human small intestine has been shown to activate the UPR by evidence of increased splicing of XBP1 and increased BiP, CHOP and GADD34.
A ROLE OF AUTOPHAGY ON ENDOPLASMIC RETICULUM STRESS RELATED INFLAMMATION
Autophagy is an evolutionarily conserved and fundamental cellular mechanism that orchestrates the degradation of proteins, lipids and organelles in order to maintain homeostatic function. Autophagy is activated in response to multiple stresses that include processes such as hypoxia, infection, nutrient starvation and ER stress [46,47]. Autophagy is either considered as macroautophagy or microautophagy with the latter being directed at specific organelles [48]. Macroautophagy in response to pathogens is considered xenophagy.
Human genetic studies have revealed that autophagy is a major mechanism in the pathogenesis of Crohn’s disease through the discovery of autophagy-related 16-like 1 (ATG16L1) as a major genetic risk factor [49]. This has been significantly buttressed by the identification of further genetic defects that also support defects in autophagy as being critical to Crohn’s disease pathogenesis. These include genes such as nucleotide-binding oligomerization domain-containing protein 2 (NOD2), leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2) and immunity-related GTPase M (IRGM), which have been identified as genetic risk factors for IBD [50].
Most attention in this area has been on ATG16L1, which is one of the strongest risk factors in Crohn’s disease. The majority of genetic risk related to ATG16L1 is derived from a single non-synonymous single nucleotide polymorphism (SNP) that encodes for a single nonsynonymous amino acid substitution (Thr300Ala) [49]. This has been modelled in mice that express hypomorphic ATG16L1 function, so-called Atg16l1 hypomorphic mice (Atg16l1HM). Such mice do not develop spontaneous intestinal inflammation but exhibit morphologic and functional evidence of Paneth cell dysfunction as do humans who are homozygous for the Thr300Ala allele [51]. The morphologic defect observed is notable for Paneth cells with decreased quantities of intracellular granules and of reduced size together with evidence of increased pro-inflammatory mediator production such serum amyloid A [52]. More recently, a functional mechanism of Thr300Ala variant has been unveiled using Thr300Ala knock-in mice (Thr316Ala in mice), which have defective clearance of Yersinia enterocolitica and an elevated inflammatory cytokine response. The variant increases ATG16L1 sensitization to caspase-3-mediated degradation, resulting in diminished autophagy and pathogen clearance [53]. Given that autophagy is highly active in Paneth cells [27▪▪], and recent evidence that Paneth cell autophagy plays an important compensatory role in the context of ER stress, it is likely that environmental factors that induce ER stress are particularly problematic for the host that is unable to mount an effective autophagic response. Specifically, it is well known that the UPR in response to ER stress is a major inducer of autophagy [46,54–56]. In the presence of unresolved ER stress in the intestinal epithelium as caused by XBP1-deficiency or potentially yet-to-be defined environmental factors, the inability to effectively engage autophagy due to genetic deficiency in autophagy as modelled by ATG16L1 or ATG7-deficiency is associated with severe spontaneous enteritis that is able to extend transmurally when both pathways are dysfunctional [27▪▪]. It is notable that the superficial spontaneous enteritis associated with intestinal epithelial deficiency of XBP1 is converted into an inflammatory response that extends into the submucosa when autophagy is disabled. Interestingly, the mechanistic link between the UPR and autophagy induction in the intestinal epithelium is mainly derived from the PERK-eIF2α-ATF4 pathway that may be amenable to pharmacologic manipulation. Perhaps most importantly, these pathways are most active in Paneth cells such that specific deletion of genes in this cell type such as Xbp1 and Atg7 partially phenocopies what is observed when these genetic targets are extinguished throughout the intestinal epithelium [57▪▪]. Thus, mouse model systems suggest that intestinal inflammation associated Crohn’s disease can emerge primarily from the intestinal epithelium and the Paneth cell in particular, suggesting that a subset of small intestinal Crohn’s disease might be a specific disorder of the Paneth cell and potentially other IEC subtypes. As increased GRP78 and pEIF2α has been detected in Paneth cells in patients with Crohn’s disease who possess the ATG16L1T300A risk allele and in the setting of small intestinal disease, it is possible that these observations are extensible to humans [57▪▪].
ENDOPLASMIC RETICULUM STRESS, UNFOLDED PROTEIN RESPONSE AND HUMAN GASTROINTESTINAL DISEASE
Human genetic research of IBD has identified several UPR-related genes that are associated with Crohn’s disease and ulcerative colitis. Candidate gene approaches for IBD have identified AGR2 and XBP1 as risk loci associated with Crohn’s disease and ulcerative colitis. Regarding the former, Agr2−/− mice develop spontaneous ileocolitis with ER stress as described above. Genetic linkage studies from three independent microsatellite-based genome scans revealed a locus on chromosome 22 close to the XBP1 gene as a risk locus for Crohn’s disease and ulcerative colitis. Deep sequencing for all exons, splice sites and promoter regions of XBP1 gene revealed that several rare variants including two hypomorphic variants are more frequently present in the Crohn’s disease and ulcerative colitis than in the control cohort [7]. Genome-wide association studies found Orosomucoid-like 3 (ORMDL3), which is involved in ER calcium homeostasis, as the risk for Crohn’s disease [58] and ulcerative colitis [59].
Several recent studies have shown evidence for increased ER stress in the intestines of humans with Crohn’s disease and ulcerative colitis. For example, GRP78 expression and XBP1 splicing have been observed to be increased in the small intestine and colon of Crohn’s disease patients relative to that observed in healthy controls [7,60,61]. In ulcerative colitis, GRP78 expression is increased in the colon, especially in inflamed mucosa [7,39,60]. Treton et al. [62] have specifically reported higher expression levels of unspliced and spliced XBP1, GRP78, GRP94 and ER degradation enhancer mannosidase alpha-like 1 (EDEM1) in colonic mucosa in association with increased levels of the active form of ATF6 (ATF6α-p50) together with an impairment of the eIF2α-ATF4-CHOP pathway in ulcerative colitis. In contrast, Hu et al. [63] reported increased p-eIF2α in the colonic mucosa of Crohn’s disease and ulcerative colitis patients. Although these studies need to be reconciled, they suggest that in ulcerative colitis, and potentially Crohn’s disease, an active UPR may be uncoupled from autophagy induction that would be predicted to promote intestinal inflammation.
CONCLUSION
The IEC is highly susceptible to ER stress, which is likely due to the unique environmental conditions within which the IEC must function. As such, the IEC is highly dependent upon the UPR for the maintenance of homeostasis that assists in expanding the capabilities of the ER and proteostasis. In addition, the UPR critically engages autophagy to compensate for ER stress in the intestinal epithelium. When either the UPR and/or autophagy are disabled due to genetic and/or environmental factors, intestinal inflammation can emerge. Genetic factors that affect the UPR include those associated with XBP1, AGR2 and potentially ORMDL3, whereas those that affect autophagy include ATG16L1, IRGM and potentially LRRK2. Interestingly, IRGM defects have recently been shown to be associated with defects in Paneth cells [64]. Environmental factors that affect the UPR include factors such as hypoxia, microbial toxins (e.g. Shiga toxigenic factors that degrade GRP78) and dietary factors (e.g. iron) [65,12]. Similarly, environmental factors that potentially affect autophagy include infectious pathogens that activate intracellular caspases such as caspase 3 to which the Thr300Ala allele of ATG16L1 is highly susceptible and cleaved into an inactive state [53]. Not only do such insights shed light on our understanding of the pathogenesis of IBD but also may lead to new therapeutic strategies such as the use of chemical chaperones to enhance the UPR, mammalian target of rapamycin (mTOR) blockers to increase autophagy or inhibitors of activated IRE1a or CHOP to diminish a pathogenic UPR.
KEY POINTS.
Several UPR-related genes and autophagy-related genes have been identified as inflammatory bowel disease risk loci.
Genetic and environmental factors induce ER stress in the intestinal epithelium and especially Paneth cells.
Autophagy is induced by ER stress and plays an important compensatory role.
Uncompensated ER stress due to autophagy defects promotes small intestinal and likely large intestinal inflammation.
Intestinal inflammation can emerge directly from the intestinal epithelium and the Paneth cell in particular.
Acknowledgments
We thank Timon Erik Adolph, Magdalena B. Flak, Joep Grootjans and Lukas Niederreiter for helpful discussions.
Financial support and sponsorship
This work was supported by NIH grants DK044319, DK051362, DK053056, DK088199, the Harvard Digestive Diseases Center (HDDC) DK0034854 and High Point Foundation (to R.S.B.); and by the European Research Council (ERC) under the European Community’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant agreement no. 260961, the National Institute for Health Research Cambridge Biomedical Research Centre, and the Addenbrooke’s Charitable Trust (to A.K.).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest to disclose.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Ouellette AJ. Paneth cells and innate mucosal immunity. Curr Opin Gastroenterol. 2010;26:547–553. doi: 10.1097/MOG.0b013e32833dccde. [DOI] [PubMed] [Google Scholar]
- 2.Salzman NH, Hung K, Haribhai D, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGuckin MA, Linden SK, Sutton P, et al. Mucin dynamics and enteric pathogens. Nat Rev Microbiol. 2011;9:265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 4.Harrison E, Lal S, McLaughlin JT. Enteroendocrine cells in gastrointestinal pathophysiology. Curr Opin Pharmacol. 2013;13:941–945. doi: 10.1016/j.coph.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Olszak T, Neves JF, Dowds CM, et al. Protective mucosal immunity mediated by epithelial CD1d and IL-10. Nature. 2014;509:497–502. doi: 10.1038/nature13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Todd DJ, Lee A-H, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol. 2008;8:663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- 7.Kaser A, Lee A-h, Franke A, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGuckin MA, Eri RD, Das I, et al. ER stress and the unfolded protein response in intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;298:G820–G832. doi: 10.1152/ajpgi.00063.2010. [DOI] [PubMed] [Google Scholar]
- 9.Bogaert S, De Vos M, Olievier K, et al. Involvement of endoplasmic reticulum stress in inflammatory bowel disease: a different implication for colonic and ileal disease? PLoS One. 2011;6:e25589. doi: 10.1371/journal.pone.0025589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10▪.Heijmans J, van Lidth de Jeude Jooske F, Koo B-K, et al. ER stress causes rapid loss of intestinal epithelial stemness through activation of the unfolded protein response. Cell Rep. 2013;3:1128–1139. doi: 10.1016/j.celrep.2013.02.031. This work describes an important role of the UPR in regulating intestinal epithelial stem cell differentiation. [DOI] [PubMed] [Google Scholar]
- 11.Rosekrans SL, Heijmans J, Buller NV, et al. ER stress induces epithelial differentiation in the mouse oesophagus. Gut. 2014 Apr 30; doi: 10.1136/gutjnl-2013-306347. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Kaser A, Martínez-Naves E, Blumberg RS. Endoplasmic reticulum stress: implications for inflammatory bowel disease pathogenesis. Curr Opin Gastroenterol. 2010;26:318–326. doi: 10.1097/MOG.0b013e32833a9ff1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaser A, Adolph TE, Blumberg RS. The unfolded protein response and gastrointestinal disease. Semin Immunopathol. 2013;35:307–319. doi: 10.1007/s00281-013-0377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- 15.Mori K, Ma W, Gething MJ, et al. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993;74:743–756. doi: 10.1016/0092-8674(93)90521-q. [DOI] [PubMed] [Google Scholar]
- 16.Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 1998;12:1812–1824. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang XZ, Harding HP, Zhang Y, et al. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. Embo J. 1998;17:5708–5717. doi: 10.1093/emboj/17.19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urano F. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 19.Kaneko M, Niinuma Y, Nomura Y. Activation signal of nuclear factor-kappa B in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol Pharm Bull. 2003;26:931–935. doi: 10.1248/bpb.26.931. [DOI] [PubMed] [Google Scholar]
- 20.Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol. 2008;8:663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- 21.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 22.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 23▪.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. An excellent review focusing on the ER stress induced cell death. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertolotti A, Wang X, Novoa I, et al. Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J Clin Invest. 2001;107:585–593. doi: 10.1172/JCI11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25▪.Tsuru A, Fujimoto N, Takahashi S, et al. Negative feedback by IRE1beta optimizes mucin production in goblet cells. Proc Natl Acad Sci U S A. 2013;110:2864–2869. doi: 10.1073/pnas.1212484110. This study describes that IRE1β, but not IRE1α, promotes efficient protein folding and secretion in goblet cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi KS, Chamaillard M, Ogura Y, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 27▪▪.Adolph TE, Tomczak MF, Niederreiter L, et al. Paneth cells as a site of origin for intestinal inflammation. Nature. 2013;503:272–276. doi: 10.1038/nature12599. This work revealed that a compensatory mechanism of UPR and autophagy on Paneth cell is related to intestinal inflammation, and a mechanism of development of enteritis on Xbp1ΔIEC mice depends on the IRE1-NF-κB signalling pathway driven by TNF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogler G, Brand K, Vogl D, et al. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- 29.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Flier LG, Haegebarth A, Stange DE, et al. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137:15–17. doi: 10.1053/j.gastro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 31▪.Niederreiter L, Fritz TMJ, Adolph TE, et al. ER stress transcription factor Xbp1 suppresses intestinal tumorigenesis and directs intestinal stem cells. J Exp Med. 2013;210:2041–2056. doi: 10.1084/jem.20122341. This study describes an important role of IRE1 and XBP1 pathway in tumour genesis of colitis-associated cancer, depending on Ire1-mediated increase in Lgr5+ and Olfm4+ ISC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32▪.Cao SS, Zimmermann EM, Chuang BM, et al. The unfolded protein response and chemical chaperones reduce protein misfolding and colitis in mice. Gastroenterology. 2013;144:989–1000. doi: 10.1053/j.gastro.2013.01.023. This work shows that Atf6a−/− mice are sensitive to DSS-induced colitis with increased ER stress, and chemical chaperones, tauroursodeoxycholate (TUDCA) and 4-phenylbutyrate (PBA), reverse DSS-induced colitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33▪.Cao SS, Wang M, Harrington JC, et al. Phosphorylation of eIF2alpha is dispensable for differentiation but required at a posttranscriptional level for paneth cell function and intestinal homeostasis in mice. Inflamm Bowel Dis. 2014;20:712–722. doi: 10.1097/MIB.0000000000000010. This study describes an important role of eIF2α phoshorylation in Paneth cell function and mucosal homeostasis by activating UPRs. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z, Hao Y, Lowe AW. The adenocarcinoma-associated antigen, AGR2, promotes tumor growth, cell migration, and cellular transformation. Cancer Res. 2008;68:492–497. doi: 10.1158/0008-5472.CAN-07-2930. [DOI] [PubMed] [Google Scholar]
- 35.Komiya T, Tanigawa Y, Hirohashi S. Cloning of the gene gob-4, which is expressed in intestinal goblet cells in mice. Biochim Biophys Acta. 1999;1444:434–438. doi: 10.1016/s0167-4781(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 36.Zhao F, Edwards R, Dizon D, et al. Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2−/− mice. Dev Biol. 2010;338:270–279. doi: 10.1016/j.ydbio.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zinszner H, Kuroda M, Wang X, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Namba T, Tanaka K, Ito Y, et al. Positive role of CCAAT/enhancer-binding protein homologous protein, a transcription factor involved in the endoplasmic reticulum stress response in the development of colitis. Am J Pathol. 2009;174:1786–1798. doi: 10.2353/ajpath.2009.080864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heazlewood CK, Cook MC, Eri R, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eri RD, Adams RJ, Tran TV, et al. An intestinal epithelial defect conferring ER stress results in inflammation involving both innate and adaptive immunity. Mucosal Immunol. 2011;4:354–364. doi: 10.1038/mi.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brandl K, Rutschmann S, Li X, et al. Enhanced sensitivity to DSS colitis caused by a hypomorphic Mbtps1 mutation disrupting the ATF6-driven unfolded protein response. Proc Natl Acad Sci U S A. 2009;106:3300–3305. doi: 10.1073/pnas.0813036106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Llarena M, Bailey D, Curtis H, et al. Different mechanisms of recognition and ER retention by transmembrane transcription factors CREB-H and ATF6. Traffic. 2010;11:48–69. doi: 10.1111/j.1600-0854.2009.00997.x. [DOI] [PubMed] [Google Scholar]
- 43.Asada R, Saito A, Kawasaki N, et al. The endoplasmic reticulum stress transducer OASIS is involved in the terminal differentiation of goblet cells in the large intestine. J Biol Chem. 2008;287:8144–8153. doi: 10.1074/jbc.M111.332593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hino K, Saito A, Asada R, et al. Increased susceptibility to dextran sulfate sodium-induced colitis in the endoplasmic reticulum stress transducer OASIS deficient mice. PLoS One. 2014;9:e88048. doi: 10.1371/journal.pone.0088048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grootjans J, Hodin CM, de Haan J-J, et al. Level of activation of the unfolded protein response correlates with Paneth cell apoptosis in human small intestine exposed to ischemia/reperfusion. Gastroenterology. 2011;140:529–539. e3. doi: 10.1053/j.gastro.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 46.Hart LS, Cunningham JT, Datta T, et al. ER stress-mediated autophagy promotes Myc-dependent transformation and tumor growth. J Clin Invest. 2012;122:4621–4634. doi: 10.1172/JCI62973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Avivar-valderas A, Salas E, Diehl JA, et al. PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol Cell Biol. 2011;31:3616–3629. doi: 10.1128/MCB.05164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knodler LA, Celli J. Eating the strangers within: host control of intracellular bacteria via xenophagy. Cell Microbiol. 2011;13:1319–1327. doi: 10.1111/j.1462-5822.2011.01632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hampe J, Franke A, Rosenstiel P, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39:207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- 50.Parkes M, Barrett JC, Prescott NJ, et al. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn’s disease susceptibility. Nat Genet. 2007;39:830–832. doi: 10.1038/ng2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cadwell K, Patel KK, Maloney NS, et al. Virus-plus-susceptibility gene interaction determines Crohn’s disease gene Atg16L1 phenotypes in intestine. Cell. 2010;141:1135–1145. doi: 10.1016/j.cell.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cadwell K, Liu JY, Brown SL, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murthy A, Li Y, Peng I, et al. A Crohn’s disease variant in Atg16l1 enhances its degradation by caspase 3. Nature. 2014;506:456–462. doi: 10.1038/nature13044. [DOI] [PubMed] [Google Scholar]
- 54.Ogata M, Hino S, Saito A, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimodaira Y, Takahashi S, Kinouchi Y, et al. Modulation of endoplasmic reticulum (ER) stress-induced autophagy by C/EBP homologous protein (CHOP) and inositol-requiring enzyme 1alpha (IRE1alpha) in human colon cancer cells. Biochem Biophys Res Commun. 2014;445:524–533. doi: 10.1016/j.bbrc.2014.02.054. [DOI] [PubMed] [Google Scholar]
- 56.Li J, Ni M, Lee B, et al. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008;15:1460–1471. doi: 10.1038/cdd.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57▪▪.Deuring JJ, Fuhler GM, Konstantinov SR, et al. Genomic ATG16L1 risk allele restricted Paneth cell ER stress in quiescent Crohn’s disease. Gut. 2013;63:1081–1091. doi: 10.1136/gutjnl-2012-303527. This study shows a link between genomic ATG16L1 risk allele and ER stress on human Crohn’s disease. Paneth cells of patients with ATG16L1 risk allele express increased ER stress markers GRP78 and peIF2α. [DOI] [PubMed] [Google Scholar]
- 58.Barrett JC, Hansoul S, Nicolae DL, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40:955–962. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGovern DP, Gardet A, Torkvist L, et al. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet. 2010;42:332–337. doi: 10.1038/ng.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shkoda A, Ruiz Pa, Daniel H, et al. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology. 2007;132:190–207. doi: 10.1053/j.gastro.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 61.Deuring JJ, de Haar C, Koelewijn CL, et al. Absence of ABCG2-mediated mucosal detoxification in patients with active inflammatory bowel disease is due to impeded protein folding. Biochem J. 2012;441:87–93. doi: 10.1042/BJ20111281. [DOI] [PubMed] [Google Scholar]
- 62.Treton X, Pedruzzi E, Cazals-Hatem D, et al. Altered endoplasmic reticulum stress affects translation in inactive colon tissue from patients with ulcerative colitis. Gastroenterology. 2011;141:1024–1035. doi: 10.1053/j.gastro.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 63.Hu S, Ciancio MJ, Lahav M, et al. Translational inhibition of colonic epithelial heat shock proteins by IFN-gamma and TNF-alpha in intestinal inflammation. Gastroenterology. 2007;133:1893–1904. doi: 10.1053/j.gastro.2007.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu B, Gulati AS, Cantillana V, et al. Irgm1-deficient mice exhibit Paneth cell abnormalities and increased susceptibility to acute intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2014;305:G573–G584. doi: 10.1152/ajpgi.00071.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Werner T, Wagner SJ, Martinez I, et al. Depletion of luminal iron alters the gut microbiota and prevents Crohn’s disease-like ileitis. Gut. 2011;60:325–333. doi: 10.1136/gut.2010.216929. [DOI] [PubMed] [Google Scholar]