Abstract
Background
Clinical practice guidelines recommend 40–60 mg of prednisone equivalent for 10–14 days for patients with acute exacerbations of chronic obstructive pulmonary disease (COPD). However, the amount of corticosteroid prescribed varies widely in clinical practice. Using the electronic health record (EHR), we implemented an evidence based orderset to standardize treatment of patients hospitalized with acute exacerbations of COPD.
Methods
This is a pre- and post- intervention study on patients hospitalized between January 1, 2009 and September 30, 2012 with primary discharge diagnosis of COPD (ICD-9 code: 491.xx, 492.xx and 496) and receipt of at least one dose of corticosteroid at our tertiary care hospital. Data on baseline demographics, dose of corticosteroid in prednisone equivalent administered during the first 48 hours and during the entire hospitalizations were collected from the electronic health record. Evidence-based guidelines were used to build and implement acute exacerbations of COPD management electronic ordersets in our electronic health record, Epic®. We divided the study into two time periods (January 1, 2009 through February 28, 2011 as pre- (n= 203) and March 1, 2011 through September 30, 2012 as post-intervention periods (n=217)). The primary outcome measure was corticosteroid dose administered in the first 48 hours. Secondary outcome measures were corticosteroid dosage during the entire hospitalization, length of stay, hospital follow-up rates, and 30-day readmission rates.
Results
A total of 420 patients with acute exacerbations of COPD were included in the study. In the post-intervention period, the median amount of corticosteroid used in the first 48 hours was significantly reduced (306.2 mg vs. 156.25 mg, p<0.0001), as was that used during the entire hospitalization (352.5 mg vs. 175 mg, p<0.0001). There was no difference in hospital follow-up rates, length of stay, or 30-day readmission rates between the two periods.
Conclusions
Evidence-based electronic ordersets improve compliance with clinical practice guidelines and reduce the total dose of corticosteroid administered in patients hospitalized with acute exacerbations of COPD.
Keywords: COPD, clinical practice guidelines, Health IT, readmission, corticosteroid, follow-up
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is the third leading cause of death in the United States (US), and the only leading cause for which the morbidity and mortality rates are increasing.1,2 An estimated 6.2% of the adult United States population has chronic obstructive pulmonary disease. In 2008 there were over 600,000 hospitalizations due to an acute exacerbation of COPD3 with an average length of stay (LOS) of 4.5 days that accounted for 54 billion dollars in direct healthcare costs.4
Acute exacerbations are part of the natural course of the disease (the worsening of symptoms or respiratory failure) which requires interaction with a care provider via a telephone consult, clinic visit, emergency department (ED) visit or hospitalization. The mainstays of hospital care for patients with acute exacerbations of COPD include the provision of supplemental oxygen, short-acting bronchodilators, systemic corticosteroids and usually antibiotics. Despite available evidence-based guidelines, a large study conducted in the United States showed that only 33% of patients received ideal care, defined as receiving all the recommended treatment and not receiving any non-beneficial therapies.5 Additionally, the majority of hospitalized patients were found to receive parenteral steroids despite data suggesting equal efficacy for low and high dose corticosteroid usage.6 Parenteral steroid use by physicians may partly be driven by justification to meet requirement for inpatient care for insurance providers.
The implementation of clinical practice guidelines can be a useful tool to ensure standardization of care and to improve the overall quality of care. Many barriers exist to the uniform implementation of practice guidelines. Some commonly cited reasons include: reimbursement structures; lack of information technology (IT) systems; physician culture, beliefs and habits; and the guideline development process. Studies show that access to information technology alone is not sufficient to increase physician adherence to practice guidelines; one must ensure access to the guideline at the point of care, information technology support and resources for staff training.7 The published literature on implementation of evidence-based care, while growing, is still limited.8, 9, 10
In consideration of the above, we implemented evidence-based guidelines into our electronic health record (EHR) to standardize the inpatient management of patients hospitalized for acute exacerbations of COPD. We hypothesized that standardizing treatment would reduce variation and total dose of corticosteroid administered in patients hospitalized with COPD exacerbation.
Methods
STUDY SETTING
The University of Texas Medical Branch (UTMB) in Galveston, Texas is a tertiary care academic institution. All patients over the age of 40 years who were discharged with a primary diagnosis of acute exacerbations of COPD between January 1, 2009 and September 30, 2012 and who had received a least one dose of systemic corticosteroids during hospitalization were included in this study. The pre-intervention period was from January 1, 2009 through February 28, 2011 (25 months). The post-intervention period was from March 1, 2011 through September 30, 2012 (18 months). The Institutional Review Board of UTMB approved the study and waived the requirement for informed consent.
INTERVENTION
The standardized acute exacerbations of COPD orderset was developed internally and embedded into the institutional electronic health record; The University of Texas Medical Branch at Galveston (UTMB) utilizes Epic® software. Content was driven by the 2007 Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines1. The orderset was developed with input from pulmonary and primary care physicians for use upon admission of patients admitted with a possible diagnosis of COPD exacerbation. It included management options during the inpatient encounter, as well as orders to schedule a 14-day hospital follow up with the patient’s listed primary care provider (PCP). The hospital follow up order was electronically routed to the central access center (central scheduling center). They schedule the follow up appointment, or route to the primary care provider clinic for an overbook, if no appointment existed. Prior to the intervention, there were no ordersets or clinical protocols available for the management of acute exacerbation of COPD.
IMPLEMENTATION
The orderset was released on March 1, 2011. An information sharing campaign was launched and all primary users (defined as residents and faculty in the departments of Internal Medicine and Family Medicine) were educated on the use of the admission ordersets. The orderset usage was monitored monthly and averaged approximately 40% for the first 6 months post-implementation.
In order to increase the orderset usage, we reviewed the symptoms and medication received by patients in the emergency department who subsequently had a primary discharge diagnosis of COPD. Based on the symptoms and medication received, a best practice advisory (BPA) was then implemented in the electronic health record. This implementation flagged the provider if the patient presented to the emergency department with a chief complaint of shortness of breath, increased sputum production or cough, and received a bronchodilator and a corticosteroid prior to hospital admission. When a patient fulfilled these criteria, the electronic health record would suggest the acute exacerbations of COPD orderset to the admitting provider. Figure 1 details the timeline and methodology of the intervention.
Figure 1.
Corticosteroid usage in the first 48 hours of hospitalization.
Since implementation of the best practice advisory, acute exacerbations of COPD orderset usage rates have exceeded 70% for this patient population. Orderset usage and outcomes data were presented every 6 months to the providers at the Internal Medicine and Family Medicine noon conferences. Feedback on issues related to the best practice advisory and ordersets were encouraged from the end users and optimized as necessary throughout the intervention period.
We validated our case definition for acute exacerbations of COPD by randomly selecting 20 cases during the pre and post-intervention periods. The data were reviewed by two independent physicians; 19/20 (95%) of the selected cases who had primary discharge diagnosis of COPD and had received one dose of corticosteroid; meeting the GOLD criteria for acute exacerbations of COPD during each of the two periods.1
DATA COLLECTION
Age, gender, race/ethnicity, admission source, insurance status, marital status, habitual uses, comorbidities and baseline pulmonary function tests were abstracted from the electronic health record for each patient. All electronic health record reports are validated by sampling reporting results and performing a manual chart review. Orderset usage was generated from the electronic health record by tracking orders used from the orderset. (each order placed from the orderset was tagged as associated with the orderset.) Laboratory and pharmacy services are completely integrated within the electronic health record. During the implementation of Epic® manual processes were applied to ensure validation of pharmacy and laboratory information. Pharmacy data is directly built in Epic and was validated for accuracy by the clinical pharmacists at the time of its release in 2007. Laboratory data is on Cerner® and is brought into Epic® via an interface. Length of stay and 30-day readmission data was collected from the hospital discharge database. Clinic follow-up rates were collected from the electronic health record.
Process of Care Measures
The two primary processes of care measures related to corticosteroid usage were (1) during the first 48 hours of admission and (2) during the entire hospitalization. This outcome was measured as prednisone equivalent in mg. The primary outcome measure was corticosteroid dose administered in the first 48 hours. Other process of care metrics evaluated included binary assessments on: the administration of any antibiotics; inhaled corticosteroid usage; long-acting beta agonist usage, long-acting muscarinic antagonist usage at the time of discharge, intensive care use, use of invasive or mechanical ventilation, and discharge on supplemental oxygen. A total of 162 patients were required in each group to see a 30% reduction in corticosteroid dose in the first 48hrs at 95% power with two sided alpha of 0.05.
Outcome Measures
Secondary outcome measures tracked were length of stay (measured in days), 30-day readmissions (related to COPD and all cause) and clinic follow-up appointments kept during the baseline and post-intervention periods. Clinic follow-up appointments were evaluated for occurrence in either the first 15 days or within the 30 days of discharge, and were defined as the first clinic appointment kept after hospital discharge recorded within the UTMB system.
STATISTICAL ANALYSIS
Two-sample tests of the means and/or variance were conducted on the collected variables to compare patients in the baseline group and the intervention group. In the comparison of means, the unpaired t-test was used for continuous variables such as age and corticosteroid use, and the Chi-squared test was used for categorical variables such as gender and marital status. The Wilcoxon signed-rank test was used to compare medians. The F test was used to compare variance in corticosteroid use. For all tests, a p-value <0.05 was considered statistically significant. The analysis was performed using R statistical software version 2.15.3 (www.r-project.org).
Results
Between January 1, 2009 and September 30, 2012, a total of 420 patients met the study criteria. Of these, 203 were discharged in the pre-intervention period and 217 discharged during the post-intervention period. Table 1 summarizes the baseline characteristics and demographic data for both groups. As shown, the baseline characteristics and demographic data during the two periods were comparable and no significant differences were noted in gender, race, insurance status, marital status, comorbidities or tobacco use. Overall, the majority of patients with COPD exacerbation were admitted via the emergency department. In the post-intervention period, a significantly larger percentage of patients were admitted via the emergency department(83% vs. 74%) (p<0.0001). Pulmonary function tests were available in 98 and 70 subjects during the pre- and post- intervention period, respectively. Subjects in post intervention period had a lower % predicted forced expiratory volume in 1 sec compared to pre-intervention period (41.17% vs. 46.8%, p-0.05).
Table 1.
Baseline characteristics of patients hospitalized with acute exacerbations of COPD between January 1, 2009 and September 30, 2012a
| Characteristics | Pre-intervention (N=203) N (%) |
Post-intervention (N=217) N (%) |
p-value |
|---|---|---|---|
| Age, mean (SD), yearb | 66.03(10.81) | 67.03(11.63) | 0.3649 |
|
| |||
| Gender | |||
| Male | 104(51%) | 108(50%) | 0.8401 |
| Female | 99(49%) | 109(50%) | |
|
| |||
| Admission Source | |||
| Emergency Room | 150(74%) | 180(83%) | <0.0001 |
| Outpatient | 37(18%) | 9(4%) | |
| Othersc | 16(8%) | 28(13%) | |
|
| |||
| Race | |||
| Caucasian/White | 145(71%) | 162(75%) | 0.6975 |
| Black/African | 46(23%) | 42(19%) | |
| Hispanic/Latino | 12(6%) | 12(6%) | |
|
| |||
| Financial Group | |||
| Medicare | 134(66%) | 153(71%) | 0.0949 |
| Medicaid | 17(8%) | 27(12%) | |
| Commercial Insurance | 33(16%) | 20(9%) | |
| Self-Pay | 19(9%) | 17(8%) | |
|
| |||
| Marital Status | |||
| Divorced | 65(32%) | 64(29%) | 0.2931 |
| Married | 58(29%) | 48(22%) | |
| Separated | 10(5%) | 10(5%) | |
| Single | 34(17%) | 40(18%) | |
| Widowed | 16(18%) | 55(25%) | |
|
| |||
| Comorbidities | |||
| Depression | 39(19%) | 51(24%) | 0.3412 |
| Anxiety | 33(16%) | 26(12%) | 0.2630 |
| Lung cancer | 4(2%) | 7(3%) | 0.6176 |
| Diabetes | 47(23%) | 57(26%) | 0.5314 |
| Hypertension | 145(71%) | 147(68%) | 0.4751 |
| Congestive heart failure | 48(24%) | 46(21%) | 0.6283 |
| Coronary artery disease | 46(23%) | 40(18%) | 0.3412 |
| Osteoporosis | 17(8%) | 29(13%) | 0.1797 |
|
| |||
| Habitual | |||
| Smoker | 112(55%) | 113(52%) | 0.5903 |
| Alcohol use | 100(49%) | 102(47%) | 0.7153 |
| Drug use | 28(14%) | 42(19%) | 0.1623 |
|
| |||
| Pulmonary Function Testd mean (SD) | |||
| FEV-1 | 1.23 (0.58) | 1.02 (0.48) | 0.01 |
| FVC | 2.31 (0.81) | 2.07 (0.78) | 0.06 |
| FEV-1/FVC | 53.68 (14.9) | 49.51 (14.4) | 0.07 |
| FEV-1 (% predicted) | 46.8 (18.8) | 41.17 (18.06) | 0.05 |
COPD: Chronic Obstructive Pulmonary Disease.
SD: standard deviation.
Other admission sources include direct admissions from home and transfers from other facilities
Pulmonary Function Test data were available for 98 patients in Pre-intervention and 70 patients in Post-intervention
FEV-1: Forced vital capacity in 1 second
FVC: Forced vital capacity
Process of care
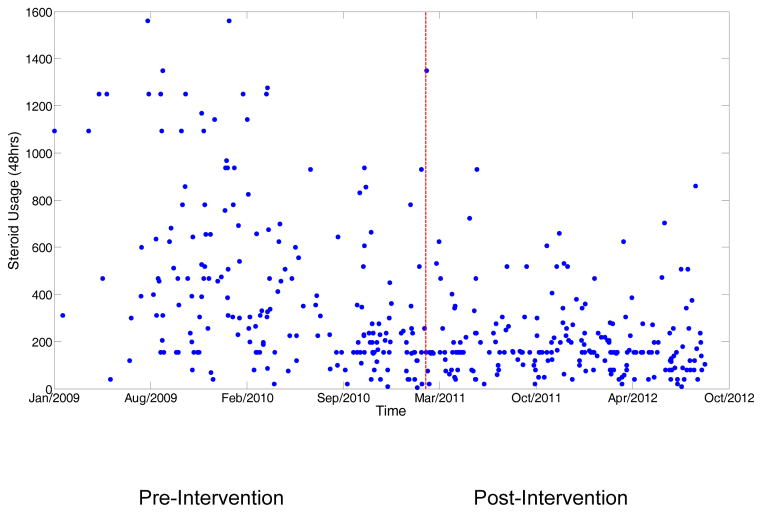
Table 2 presents the process of care during hospitalization. The two groups did not differ significantly in use of inhaled corticosteroids or inhaled long-acting beta agonists during hospitalization. The pneumococcal vaccination rates were higher in post intervention period compared to pre-intervention period (57% vs. 45%, p-value 0.02). There was an increase in long acting muscarinic antagonist usage during hospitalization in the post intervention group (13% vs. 25%, p=0.002). Overall use of antibiotics was high in these patients and did not differ significantly between groups (88% vs. 90%, p=0.81). Intensive care use, non-invasive/mechanical ventilation were more common in post intervention period. More patients were discharged on supplement oxygen in post-intervention period compared to pre-intervention period (57% vs.42.9%, p-value-0.005). Primary outcome of corticosteroid use in the first 48hrs. was dramatically lower in the post-intervention period. Pre-intervention median corticosteroid usage was 306.2 mg of prednisone equivalents. In the post-intervention period, this amount decreased by 49 % to 156.25 mg of prednisone equivalents (p<0.0001). The median corticosteroid usage during the entire hospitalization decreased from a baseline of 352.5 mg of prednisone equivalents to 175.0 mg of prednisone equivalents (p<0.001). Usage of parenteral steroids decreased from 86.2% (pre-intervention period) to 72.8% in the post-intervention group. Figure 2 shows the scatterplot of corticosteroid usage during the first 48 hours of hospitalization and Figure 3 shows the scatterplot during the entire hospitalization. The post-intervention period had both lower dosage of corticosteroid and less variation in the use of corticosteroid than in the pre-intervention period (F-test for variation p<0.001). As shown in the figure the reduction in dose of corticosteroid started before the orderset release, as most of the physicians participated in the orderset build and thus were aware of these changes prior to the implementation.
Table 2.
Process of care of patients hospitalized with acute exacerbations of COPDa
| Variable | Pre-intervention N (%) |
Post-intervention N (%) |
p-value |
|---|---|---|---|
| Corticosteroid use in the first 48 hrs. of hospitalization, in mg median (IQR) b | 306.2(156.3– 601.6) | 156.25(100.0– 236.3) | <0.0001 |
| Corticosteroid use during entire hospitalization, in mg median (IQR) b | 352.5(156.3– 698.7) | 175(120.0– 30.6.0) | <0.0001 |
| Antibiotics received | 180(88%) | 195(90%) | 0.82 |
| Inhaled corticosteroid | 31(15%) | 32(15%) | 0.99 |
| Long acting beta agonist | 7(3%) | 13(6%) | 0.32 |
| Long acting muscarinic antagonist | 26(13%) | 54(25%) | 0.002 |
| Pneumococcal vaccination | 91(45%) | 123(57%) | 0.019 |
| Influenza vaccination | 112(55%) | 138(64%) | 0.097 |
| Intensive Care Use | 7(3.4%) | 21 (9.7%) | 0.018 |
| Non-invasive Ventilation use | 3 (1.5%) | 10 (4.6%) | 0.12 |
| Mechanical Ventilation | 0 | 8(3.7%) | 0.02 |
| Discharged on Oxygen therapyd | 87 (42.9%) | 127 (57.1%) | 0.005 |
COPD: Chronic Obstructive Pulmonary Disease
Corticosteroid administered is calculated as prednisone equivalent.
IQR: interquartile range
Not all patients were new oxygen therapy users
Figure 2.

Corticosteroid usage during the entire hospitalization.
Figure 3.
Corticosteroid usage during the entire hospitalization.
Outcomes
Secondary outcomes data for the baseline and intervention groups are displayed in Table 3. Median length of stay was 3 days with no statistically significant differences between the two groups. The 15-day and 30-day outpatient follow-up kept rates also did not differ. However, 90% of the patients in the post intervention period had a follow up appointment made at the time of discharge. All-cause 30-readmission rates were 22% during the baseline period and 20% during the intervention period (p=0.72). COPD-related 30-day readmission rates were 9% and 10% (p=0.91) for the baseline and intervention groups, respectively. The mortality rate was unchanged between the two groups.
Table 3.
Secondary outcomes of patients hospitalized with acute exacerbations of COPDa
| Outcomes | Pre-intervention N (%) |
Post-intervention N (%) |
p-value |
|---|---|---|---|
| Length of Stay in days median (IQR)b | 3(2–4) | 3(2–4) | 0.9909 |
| 15-day outpatient follow up post dischargec | 95(47%) | 105(48%) | 0.8196 |
| 30-day outpatient follow up post dischargec | 129(64%) | 123(57%) | 0.1817 |
| 30-day readmission (all cause) | 45(22%) | 44(20%) | 0.7230 |
| 30-day readmission (COPD) | 19(9%) | 22(10%) | 0.9170 |
COPD: Chronic Obstructive Pulmonary Disease
IQR: interquartile range
During the post-intervention period 90% of patients had a follow up appointment with their outpatient provider made at the time of discharge. % shown is the number of patients who actually kept the appointment.
Discussion
Our study showed a proof of concept of the use of health information technology to translate clinical practice guidelines into standardized care and reduce the variation in care for patients hospitalized with acute exacerbations of COPD.
Corticosteroids remain the mainstay of treatment in patients with COPD exacerbation. However, the actual dose and duration of corticosteroids have remained largely unknown. In general, shorter courses of corticosteroids had a slightly higher risk of treatment failure and increased rate of hyperglycemia.1111 Similar results were seen in terms of reduction in length of stay when a lower dose of prednisone (i.e., 30mg) was used compared to a placebo.12 2011 GOLD guidelines recommend 40 mg of prednisone daily for 10–14 days; however, a recent multicenter randomized controlled trial confirmed that shorter duration of low dose prednisone (40 mg of prednisone for 5 days) is equivalent to using 40mg of prednisone for longer duration (i.e., 14 days). The rates of re-exacerbation over the next 6 weeks were similar. However, in this study, all patients were treated with long-acting beta agonist, long-acting muscarinic agonist and inhaled corticosteroid during the 6 month study period.13 New 2014 GOLD guidelines are changed and recommend using 40 mg of prednisone for 5 days for treatment of acute exacerbation of COPD (Ref.). The risk of hyperglycemia, cataract, diabetes and osteopenia with corticosteroid usage are all well-established; recently, thromboembolic disease was added to the list.14 This risk was pronounced in recent users cautioning clinicians to use corticosteroids dose and duration diligently.
Our study did not show reduction in hospital length of stay. This is likely because our length of stay during the pre- and post- intervention period is shorter than the median length of stay of 4.5 days seen in a recent study of national discharge database of patients hospitalized for acute exacerbations of COPD4. Length of stay is associated with readmission rates and shorter length of stays can be associated with increase readmission rates. It was reassuring in our study that we did not see an increase in readmission rates with these shorter stays. These results are similar to a recent VA study showing length of stay was not tied to increase risk of readmissions.15 Antibiotic usage in our cohort was high, a pattern consistent with recent study.5 Antibiotics are not routinely recommended in all patients with COPD exacerbation; observational studies showed patients who received antibiotics had a lower length of stay and decreased risk of relapse than those who did not.16
Certain aspects of our implementation helped drive our success. Lack of awareness is often cited as a reason for not adopting clinical practice guidelines10, and we overcame this by launching an information sharing campaign aimed at end users (in our case, residents and interns). We also solicited feedback from end users when developing the orderset, to ensure that it seamlessly integrated into their current workflow.
Orderset usage rates have typically been problematic with the integration into health information technology.7, 17, 18, 19 This issue was overcome by developing a best practice advisory to prompt the provider to use the admission orderset. This orderset was developed based on modeling data and only suggested the usage of the orderset when the patient met certain criteria, thus limiting alert fatigue for providers.
Our study has several limitations. Firstly, the orderset was implemented in a university hospital setting with an integrated Health information technology system and may not be applicable to hospitals with limited information technology resources. Secondly, our use of corticosteroids, although reduced by 51% from baseline, is still higher than the recommended dose. This is because most of our patients were admitted through the emergency department and received first a dose of 125 mg methylprednisolone, the only dose stocked in our Pyxis® system. Spirometry data was available in less than 50% of the subjects and is consistent with prior studies of underutilization of spirometry to diagnose COPD (Ref.). Our study did not show reduction in length of stay or 30-day readmission rates due to the small sample size; it is notable that our length of stay is already shorter than the national average. We were successful in making follow-up appointments in 90% of the patients during post intervention period. Only two-thirds of patients with follow appointments actually kept the appointment. Post-discharge follow-up has been shown to reduce readmission rates in patients with COPD20 and future studies should explore reasons for no show rates, as well as expand the care transition model to home visits and telephone calls for patients with limited mobility or reduced resources for travel.
In summary, implementation of clinical practice guidelines embedded in the electronic health record helps standardize care and reduce variation in the care of hospitalized patients with acute exacerbations of COPD.
Acknowledgments
The authors thank Sarah Toombs Smith PhD for help in preparation of the manuscript; and Laura Grady RN, Rick Trevino and Daran Gray for their help in building the ordersets, Best Practice Advisor and reporting tools in the electronic health record. Regina Ramirez Pharm D for her help with data collection.
Funding Source: This work was supported by grants K-08 AG 031583 from the National Institutes of Health and UT system Health IT and system engineering grant.
All authors had access to the data and a role in writing the manuscript.
Footnotes
Prior Presentation: American Thoracic Society Meeting on May 22nd, 2012, Denver, CO
Conflict of interest Statement: Lindsay Sonstein, MD, Carlos Clark DO, Susan Seidensticker, Li Zeng, PhD and Gulshan Sharma, MD do not have a financial relationship with any commercial entity that has an interest in the content of this manuscript.
References
- 1.Global initiative on obstructive lung disease. [February 18th, 2012];Diagnosis and management Guidelines. 2011 Available: http://www.goldcopd.org/international-news.html.
- 2.Decramer M, Janssens W. Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med. 2013 Mar;1(1):73–83. doi: 10.1016/S2213-2600(12)70060-7. Epub 2013 Jan 14. [DOI] [PubMed] [Google Scholar]
- 3.Wier LM, Elixhauser A, Pfuntner A, et al. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet] Rockville (MD): Agency for Health Care Policy and Research (US); 2006. Feb, Overview of Hospitalizations among Patients with COPD, 2008: Statistical Brief #106. 2011 Feb. Available from: http://www.ncbi.nlm.nih.gov/books/NBK53969/ [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services. National Institutes of Health. National Heart Lung and Blood Institute. Morbidity and Mortality: 2009 Chartbook on Cardiovascular, Lung and Blood Diseases. [Accessed June 25, 2013]. [Google Scholar]
- 5.Lindenauer PK, Pekow P, Gao S, Crawford AS, Gutierrez B, Benjamin EM. Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2006 Jun 20;144(12):894–903. doi: 10.7326/0003-4819-144-12-200606200-00006. [DOI] [PubMed] [Google Scholar]
- 6.Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010 Jun 16;303(23):2359–67. doi: 10.1001/jama.2010.796. [DOI] [PubMed] [Google Scholar]
- 7.Kenefick H, Lee J, Fleishman V. Improving physician adherence to clinical practice guidelines: barriers and strategies fo change. Cambridge (MA): New england healthcare Institute; 2008. [Google Scholar]
- 8.Ballard DJ, Ogola G, Fleming NS, Stauffer BD, Leonard BM, Khetan R, Yancy CW. Impact of a standardized heart failure order set on mortality, readmission, and quality and costs of care. Int J Qual Health Care. 2010 Dec;22(6):437–44. doi: 10.1093/intqhc/mzq051. Epub 2010 Oct 8. [DOI] [PubMed] [Google Scholar]
- 9.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, Rubin HR. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999 Oct 20;282(15):1458–65. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 10.Ban A, Ismail A, Harun R, Abdul Rahman A, Sulung S, Syed Mohamed A. Impact of clinical pathway on clinical outcomes in the management of COPD exacerbation. BMC Pulm Med. 2012 Jun 22;12:27. doi: 10.1186/1471-2466-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niewoehner DE, Erbland ML, Deupree RH, Collins D, Gross NJ, Light RW, Anderson P, Morgan NA. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999 Jun 24;340(25):1941–7. doi: 10.1056/NEJM199906243402502. [DOI] [PubMed] [Google Scholar]
- 12.Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomized controlled trial. Lancet. 1999 Aug 7;354(9177):456–60. doi: 10.1016/s0140-6736(98)11326-0. [DOI] [PubMed] [Google Scholar]
- 13.Leuppi JD, Schuetz P, Bingisser R, Bodmer M, Briel M, Drescher T, Duerring U, Henzen C, Leibbrandt Y, Maier S, Miedinger D, Müller B, Scherr A, Schindler C, Stoeckli R, Viatte S, von Garnier C, Tamm M, Rutishauser J. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013 Jun 5;309(21):2223–31. doi: 10.1001/jama.2013.5023. [DOI] [PubMed] [Google Scholar]
- 14.Johannesdottir SA, Horváth-Puhó E, Dekkers OM, Cannegieter SC, Jørgensen JO, Ehrenstein V, Vandenbroucke JP, Pedersen L, Sørensen HT. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med. 2013 May 13;173(9):743–52. doi: 10.1001/jamainternmed.2013.122. [DOI] [PubMed] [Google Scholar]
- 15.Kaboli PJ, Go JT, Hockenberry J, Glasgow JM, Johnson SR, Rosenthal GE, Jones MP, Vaughan-Sarrazin M. Associations between reduced hospital length of stay and 30-day readmission rate and mortality: 14-year experience in 129 Veterans Affairs hospitals. Ann Intern Med. 2012 Dec 18;157(12):837–45. doi: 10.7326/0003-4819-157-12-201212180-00003. [DOI] [PubMed] [Google Scholar]
- 16.Stefan MS, Rothberg MB, Shieh MS, Pekow PS, Lindenauer PK. Association between antibiotic treatment and outcomes in patients hospitalized with acute exacerbation of COPD treated with systemic steroids. Chest. 2013 Jan;143(1):82–90. doi: 10.1378/chest.12-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright A, Feblowitz JC, Pang JE, Carpenter JD, Krall MA, Middleton B, Sittig DF. Use of order sets in inpatient computerized provider order entry systems: a comparative analysis of usage patterns at seven sites. Int J Med Inform. 2012 Nov;81(11):733–45. doi: 10.1016/j.ijmedinf.2012.04.003. Epub 2012 Jul 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kijsirichareanchai K, Ngamruengphong S, Rakvit A, Nugent K, Parupudi S. The utilization of standardized order sets using AASLD guidelines for patients with suspected cirrhosis and acute gastrointestinal bleeding. Qual Manag Health Care. 2013 Apr-Jun;22(2):146–51. doi: 10.1097/QMH.0b013e31828bc328. [DOI] [PubMed] [Google Scholar]
- 19.Walker KA, Nachreiner D, Patel J, Mayo RL, Kearney CD. Impact of standardized palliative care order set on end-of-life care in a community teaching hospital. J Palliat Med. 2011 Mar;14(3):281–6. doi: 10.1089/jpm.2010.0398. [DOI] [PubMed] [Google Scholar]
- 20.Sharma G, Kuo YF, Freeman JL, Zhang DD, Goodwin JS. Outpatient follow-up visit and 30-day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med. 2010 Oct 11;170(18):1664–70. doi: 10.1001/archinternmed.2010.345. [DOI] [PMC free article] [PubMed] [Google Scholar]