Abstract
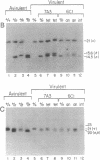
We attempted to generate homozygous dhfr-ts (dihydrofolate reductase-thymidylate synthase) knockouts in virulent Leishmania major, an asexual diploid protozoan parasite. Transfection of a neo (neomycin phosphotransferase) targeting fragment yielded heterozygous replacement lines with high efficiency. However, second transfections with a hyg (hygromycin B phosphotransferase) targeting fragment in the presence of metabolites shown to rescue homozygous knockouts in attenuated Leishmania did not yield the expected dhfr-ts- thymidine auxotrophs obtained previously with attenuated lines. Molecular karyotype, Southern blot, and flow cytometric DNA content analysis of clonal transfectants revealed three classes: (i) genomic tetraploids, containing two wild-type dhfr-ts chromosomes and one neo and one hyg replacement chromosome; (ii) aneuploid trisomic lines with one wild-type dhfr-ts and one neo and one hyg replacement chromosome; (iii) diploids bearing homologous integration of the targeting fragment without replacement. Aneuploid and tetraploid lines predominated. This confirms the common impression that natural populations of Leishmania are often aneuploid. The remarkable ability of these parasites to undergo and tolerate changes in chromosome number suggests a general method for testing whether genes are essential for growth in vitro, as the ability of Leishmania to simultaneously undergo homologous gene replacement while retaining wild-type genes by increasing chromosome number provides a diagnostic and positive experimental result. Our results show that virulent Leishmania require at least one copy of dhfr-ts and argue that DHFR-TS plays an unanticipated role in addition to its role in the de novo synthesis of thymidine. These results also have implications for genetic tests of the organization of Leishmania populations.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achterberg V., Gercken G. Cytotoxicity of ester and ether lysophospholipids on Leishmania donovani promastigotes. Mol Biochem Parasitol. 1987 Mar;23(2):117–122. doi: 10.1016/0166-6851(87)90146-0. [DOI] [PubMed] [Google Scholar]
- Bastien P., Blaineau C., Pages M. Leishmania: sex, lies and karyotype. Parasitol Today. 1992 May;8(5):174–177. doi: 10.1016/0169-4758(92)90016-u. [DOI] [PubMed] [Google Scholar]
- Beck J. T., Ullman B. Biopterin conversion to reduced folates by Leishmania donovani promastigotes. Mol Biochem Parasitol. 1991 Nov;49(1):21–28. doi: 10.1016/0166-6851(91)90126-q. [DOI] [PubMed] [Google Scholar]
- Beck J. T., Ullman B. Nutritional requirements of wild-type and folate transport-deficient Leishmania donovani for pterins and folates. Mol Biochem Parasitol. 1990 Dec;43(2):221–230. doi: 10.1016/0166-6851(90)90147-e. [DOI] [PubMed] [Google Scholar]
- Cruz A., Beverley S. M. Gene replacement in parasitic protozoa. Nature. 1990 Nov 8;348(6297):171–173. doi: 10.1038/348171a0. [DOI] [PubMed] [Google Scholar]
- Cruz A., Coburn C. M., Beverley S. M. Double targeted gene replacement for creating null mutants. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7170–7174. doi: 10.1073/pnas.88.16.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhay M., Kelleher M., Bacic A., McConville M. J., Tolson D. L., Pearson T. W., Handman E. Lipophosphoglycan expression and virulence in ricin-resistant variants of Leishmania major. Mol Biochem Parasitol. 1990 May;40(2):255–267. doi: 10.1016/0166-6851(90)90047-p. [DOI] [PubMed] [Google Scholar]
- Evans D. A., Kennedy W. P., Elbihari S., Chapman C. J., Smith V., Peters W. Hybrid formation within the genus Leishmania? Parassitologia. 1987 May-Dec;29(2-3):165–173. [PubMed] [Google Scholar]
- Gibson W., Garside L., Bailey M. Trisomy and chromosome size changes in hybrid trypanosomes from a genetic cross between Trypanosoma brucei rhodesiense and T. b. brucei. Mol Biochem Parasitol. 1992 Apr;51(2):189–199. doi: 10.1016/0166-6851(92)90069-v. [DOI] [PubMed] [Google Scholar]
- Grögl M., Oduola A. M., Cordero L. D., Kyle D. E. Leishmania spp.: development of pentostam-resistant clones in vitro by discontinuous drug exposure. Exp Parasitol. 1989 Jul;69(1):78–90. doi: 10.1016/0014-4894(89)90173-2. [DOI] [PubMed] [Google Scholar]
- Herrmann H. O., Gercken G. Metabolism of 1-0-[1'-14C]octadecyl-sn-glycerol in Leishmania donovani promastigotes. Ether lipid synthesis and degradation of the ether bond. Mol Biochem Parasitol. 1982 Feb;5(2):65–76. doi: 10.1016/0166-6851(82)90042-1. [DOI] [PubMed] [Google Scholar]
- Howell E. E., Foster P. G., Foster L. M. Construction of a dihydrofolate reductase-deficient mutant of Escherichia coli by gene replacement. J Bacteriol. 1988 Jul;170(7):3040–3045. doi: 10.1128/jb.170.7.3040-3045.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovannisci D. M., Beverley S. M. Structural alterations of chromosome 2 in Leishmania major as evidence for diploidy, including spontaneous amplification of the mini-exon array. Mol Biochem Parasitol. 1989 May 1;34(2):177–188. doi: 10.1016/0166-6851(89)90009-1. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Imai Y. Solubilization and partial characterization of alkylglycerol monooxygenase from rat liver microsomes. Eur J Biochem. 1983 Apr 15;132(1):23–27. doi: 10.1111/j.1432-1033.1983.tb07320.x. [DOI] [PubMed] [Google Scholar]
- Kapler G. M., Beverley S. M. Transcriptional mapping of the amplified region encoding the dihydrofolate reductase-thymidylate synthase of Leishmania major reveals a high density of transcripts, including overlapping and antisense RNAs. Mol Cell Biol. 1989 Sep;9(9):3959–3972. doi: 10.1128/mcb.9.9.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapler G. M., Coburn C. M., Beverley S. M. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol. 1990 Mar;10(3):1084–1094. doi: 10.1128/mcb.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. M., Law J. M., Chapman C. J., Van Eys G. J., Evans D. A. Evidence of genetic recombination in Leishmania. Mol Biochem Parasitol. 1991 Jun;46(2):253–263. doi: 10.1016/0166-6851(91)90049-c. [DOI] [PubMed] [Google Scholar]
- King D. L., Turco S. J. A ricin agglutinin-resistant clone of Leishmania donovani deficient in lipophosphoglycan. Mol Biochem Parasitol. 1988 Apr;28(3):285–293. doi: 10.1016/0166-6851(88)90013-8. [DOI] [PubMed] [Google Scholar]
- Lanotte G., Rioux J. A. Fusion cellulaire chez les Leishmania (Kinetoplastida, Trypanosomatidae). C R Acad Sci III. 1990;310(7):285–288. [PubMed] [Google Scholar]
- McConville M. J., Bacic A. A family of glycoinositol phospholipids from Leishmania major. Isolation, characterization, and antigenicity. J Biol Chem. 1989 Jan 15;264(2):757–766. [PubMed] [Google Scholar]
- Panton L. J., Tesh R. B., Nadeau K. C., Beverley S. M. A test for genetic exchange in mixed infections of Leishmania major in the sand fly Phlebotomus papatasi. J Protozool. 1991 May-Jun;38(3):224–228. doi: 10.1111/j.1550-7408.1991.tb04433.x. [DOI] [PubMed] [Google Scholar]
- Peixoto M. P., Beverley S. M. In vitro activity of sulfonamides and sulfones against Leishmania major promastigotes. Antimicrob Agents Chemother. 1987 Oct;31(10):1575–1578. doi: 10.1128/aac.31.10.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S. Z., Naessens J., Liesegang B., Moloo S. K., Magondu J. Analysis by flow cytometry of DNA synthesis during the life cycle of African trypanosomes. Acta Trop. 1984 Dec;41(4):313–323. [PubMed] [Google Scholar]
- Sternberg J., Tait A. Genetic exchange in African trypanosomes. Trends Genet. 1990 Oct;6(10):317–322. doi: 10.1016/0168-9525(90)90252-2. [DOI] [PubMed] [Google Scholar]
- TIETZ A., LINDBERG M., KENNEDY E. P. A NEW PTERIDINE-REQUIRING ENZYME SYSTEM FOR THE OXIDATION OF GLYCERYL ETHERS. J Biol Chem. 1964 Dec;239:4081–4090. [PubMed] [Google Scholar]
- Tait A. Sexual processes in the kinetoplastida. Parasitology. 1983 Apr;86(Pt 4):29–57. doi: 10.1017/s0031182000050836. [DOI] [PubMed] [Google Scholar]
- Tibayrenc M., Kjellberg F., Ayala F. J. A clonal theory of parasitic protozoa: the population structures of Entamoeba, Giardia, Leishmania, Naegleria, Plasmodium, Trichomonas, and Trypanosoma and their medical and taxonomical consequences. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2414–2418. doi: 10.1073/pnas.87.7.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Müller I., Kimsey P., Cerny A., Behin R., Zinkernagel R. M., Louis J. A. Exacerbation of experimental murine cutaneous leishmaniasis with CD4+ Leishmania major-specific T cell lines or clones which secrete interferon-gamma and mediate parasite-specific delayed-type hypersensitivity. Eur J Immunol. 1991 Mar;21(3):559–567. doi: 10.1002/eji.1830210305. [DOI] [PubMed] [Google Scholar]
- Turco S. J., Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- Urlaub G., Käs E., Carothers A. M., Chasin L. A. Deletion of the diploid dihydrofolate reductase locus from cultured mammalian cells. Cell. 1983 Jun;33(2):405–412. doi: 10.1016/0092-8674(83)90422-1. [DOI] [PubMed] [Google Scholar]