Abstract
Background
Central lymph node metastasis (CLNM) is common in papillary thyroid carcinoma (PTC). Prophylactic central lymph node dissection (PCLND) for patients with clinically negative central compartment lymph nodes (CN0) remains controversial. The phrase “clinically negative” is used to indicate that patients exhibited no clinical evidence of CLNM by ultrasonography (US) or computerized tomography (CT) preoperatively. In this study, we analyze the risk factors for CLNM in CN0 patients.
Methods
The PUBMED and SCIE databases were systematically searched for works published through January 31, 2015. All of the patients included in this study underwent thyroidectomy+PCLND. Revman 5.3 software was used to analyze the data.
Results
Twenty studies and 9084 patients were included in this meta-analysis. The following variables were associated with an increased risk of CLNM in CN0 patients: age < 45 years (OR = 1.59, 95% CI = 1.42–1.78, p<0.00001), male sex (OR = 1.95, 95% CI = 1.63–2.32, p<0.00001), multifocality (OR = 1.43, 95% CI = 1.22–1.67, p<0.00001), tumor size > 2 cm for PTC patients (OR = 2.98, 95% CI 2.08–4.28, p<0.00001) or tumor size > 0.5 cm for papillary thyroid microcarcinoma (PTMC) patients (OR = 2.30, 95% CI = 1.71–3.09, p<0.00001), location of the primary tumor in the central area and low pole (OR = 1.86, 95% CI = 1.48–2.33, p<0.00001), lymphovascular invasion (OR = 4.35, 95% CI = 2.24–8.46, p<0.0001), extrathyroidal extension (OR = 2.27, 95% CI = 1.76–2.94, p<0.00001), and capsular invasion (OR = 1.72, 95% CI = 1.39–2.41, p<0.00001). PTC (tumor size>1cm) exhibited a higher risk factor associated with CLNM than PTMC (tumor size<1cm) (OR = 2.83, 95% CI = 2.15–3.72, p<0.00001). Bilateral tumors (OR = 1.21, 95% CI = 0.92–1.58, p = 0.17) and lymphocytic thyroiditis (OR = 0.88, 95% CI = 0.71–1.09, p = 0.25) had no association with CLNM in CN0 patients.
Conclusions
Our systematic review identified several clinical features associated with CLNM in CN0 patients, including age, sex, multifocality, size, location, lymphovascular invasion, capsular invasion, and extrathyroidal extension. These factors should guide the application of PCLND in CN0 patients.
Introduction
Thyroid cancer, the most prevalent endocrine malignancy, accounts for 1% of all malignant neoplasms and 2.7% of all malignant tumors in females, representing the ninth-most common cancer in women[1,2]. Papillary thyroid carcinoma (PTC) is the most common histological subtype of thyroid cancer, comprising 80–85% of all thyroid malignancies[1]. The prognosis of PTC is favorable, with a 10-year survival >91% and 15-year survival >87%[3,4]. Central lymph node metastasis (CLNM) is observed in 20%-90% of patients[5,6]. The significance of lymph node metastasis in PTC patients remains controversial. Previous studies reported that lymph node metastasis may only impact recurrence but not survival[7]. However, recent emerging evidence from a large-scale nested case-control study indicated that lymph node metastasis and incomplete surgical excision are the two primary characteristics associated with higher morbidity[1]. Furthermore, some scholars considered the presence of central lymph nodes to be as important as primary tumors[8].
Prophylactic central lymph node dissection (PCLND) is frequently performed in patients with clinically negative central lymph nodes (CN0). It remains controversial whether elective or prophylactic CLND should be applied in patients with T1 and T2 cancer[9]. The benefits of PCLND for CN0 patients are manifold. First, PCLND can facilitate the diagnosis of an accurate TNM stage and may contribute to the decision to use radioactive iodine (RAI) therapy or thyroid stimulating hormone (TSH) suppressive therapy[10,11]. Second, PCLND may decrease recurrence, increase disease-specific survival, and reduce the Tg levels during postoperative follow-up[7]. In addition, PCLND can help to avoid a reoperation and any associated complications[11,12]. PCLND may be significant for therapy as well as the prognosis of PTC, especially for patients older than 45 years. Because patients older than 45 years exhibit a decreasing capacity to uptake RAI, RAI therapy may be less effective[13]. However, no overwhelming evidence has proven that PCLND definitively improves patient prognosis[14–16]. Although some studies indicate that there is no difference between the outcomes of thyroidectomy and thyroidectomy+PCLND for experienced surgeons[17,18], many authors believe that this statement does not represent the true morbidity rates of patients in whom PCLND is performed. Not all the surgeons are experienced thyroid surgeons, and there is indeed an association between PCLND and postoperative complications[19,20].
Neck ultrasound (US) and contrast enhanced computed tomography (CT) are widely used for preoperative imaging to visualize the CLNM lesions. However, both US and contrast-enhanced CT are not particularly accurate, with low sensitivities of 23%-53.2% and 41%-66.7%, respectively[21,22]. The high incidence of CLNM and low sensitivity of US and CT make it challenging to determine which factors are associated with subclinical CLNM. The phrase “subclinical CLNM” indicates CN0 patients who are proved to have CLNM that is pathologically confirmed intraoperatively or postoperatively. Although many risk factors have been analyzed in many studies for CN0 patients, the results are inconsistent. Thus, we performed a systematic review to assess the clinical features of CLNM in CN0 patients.
Materials and Methods
Our systematic review was conducted according to the guidelines proposed by the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement[23]. (S1 PRISMA Checklist.)
Search strategy
A comprehensive literature research was performed using the PubMed and Web of Science databases for studies published through January 31, 2015 using the key words ((“thyroid cancer or thyroid carcinoma or thyroid neoplasm” AND “papillary”) OR (“PTC”) AND (“central or central compartment or level IV” AND “lymph nodes”)). Two authors (Sun W and Lan XB) performed the selection process independently, and the discrepancies were resolved by discussion.
Selection criteria
In this systematic review, we included studies that met the following criteria: a) prospective or retrospective original literature; b) English language; c) none of the patients had clinical evidence of CLNM preoperatively; d) all of the patients underwent thyroidectomy plus unilateral or bilateral PCLND, and PTC was pathologically confirmed intraoperatively or postoperatively; and e) completed medical records were available for data extraction. The following exclusion criteria were used to eliminate studies from our meta-analysis: a) patients who had undergone prior head and neck irradiation or oncological surgery; b) reviews, letters to the editor, abstracts or meeting proceedings; and c) patients with a family history of thyroid cancer.
Data extraction and quality assessment
Two authors abstracted the relevant data from the included articles in accordance with the prepared standardized form. Authors, publication years, countries of study, study design, PTC or papillary thyroid microcarcinoma (PTMC), case number, surgery intervention, 10 possible risk factors and the corresponding numbers of patients were recorded independently. The risk factors included the following: age, sex, multifocality, size, location, lymphovascular invasion, capsular invasion, extrathyroidal extension, bilateral tumors, and lymphocytic thyroiditis. Central lymph nodes were divided into three or four subgroups in some articles: ipsilateral paratracheal, pretracheal, contralateral paratracheal, and prelaryngeal. We included only the data on ipsilateral paratracheal nodes. The Newcastle-Ottawa quality assessment scale was used to assess the quality of the studies.
Statistical analysis
All of the statistical analyses were performed using Revman software (5.3). The results are presented as odds ratios (ORs) with a 95% confidence interval (CI), and a p value< 0.05 was considered statistically significant, except where otherwise specified. Moreover, heterogeneity was quantified using the Q-test and the I2 statistic. When p>0.1 and I2<50%, a fixed-effect model was used; otherwise, a random-effects model was applied. Possible publication bias was tested by Begg’s funnel plot.
Results
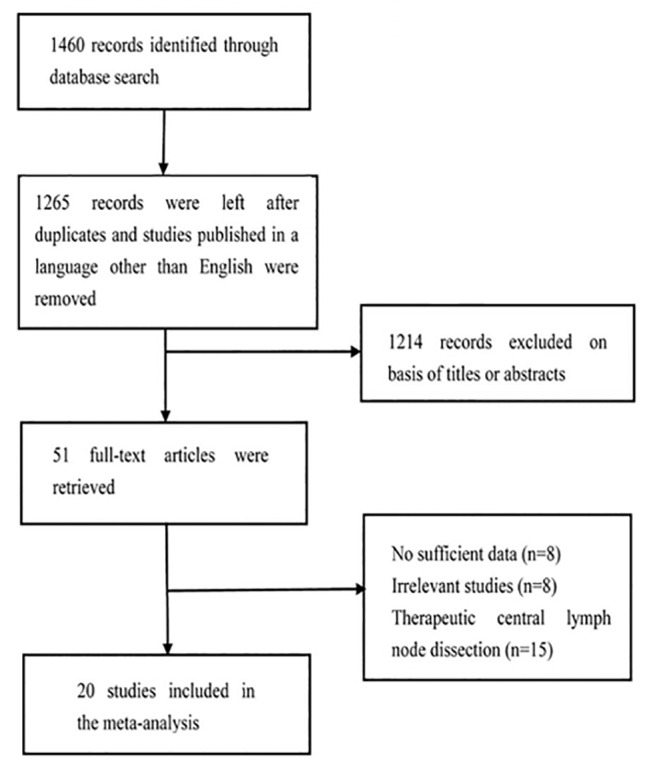
After screening for studies, 1460 studies were initially considered for inclusion in this meta-analysis. A total of 195 studies were excluded because of duplication and language. Approximately 1214 reviews, case reports, and irrelevant studies were excluded after carefully scanning titles and abstracts. The remaining 51 articles were subjected to an evaluation of the full text. A total of 20 studies and 9084 patients were included in our meta-analysis after applying the inclusion criteria; 3 studies were prospective, and 17 studies were retrospective. The basic characteristics of the papers are summarized in Table 1 and the original data is presented in S1 Table. Begg’s funnel plots are presented in the supporting information section. A flow chart of the selection process for the studies included in the meta-analysis is presented in Fig 1.
Table 1. Basic characteristics of included studies.
| Author | Year | Country | Study design | PTC/PTMC | Case number | Surgical intervention | Quality assessment |
|---|---|---|---|---|---|---|---|
| Lim, Y. C[24] | 2009 | Korea | retrospective study | PTMC | 86 | TT+bilateral CLND | 8 |
| Ying Jie Wu [25] | 2013 | China | retrospective study | PTC | 228 | TT +ipsilateral CLND | 6 |
| Jong-Lyel Roh [26] | 2011 | Korea | prospective study | PTC | 184 | TT+bilateral CLND | 8 |
| Q Wang B [27] | 2014 | China | retrospective study | PTC | 188 | TT+bilateral CLND or lobectomy plus isthmusectomy+ipsilateral CLND | 7 |
| Lie-Hao Jiang [28] | 2014 | China | retrospective study | PTC | 916 | TT+bilateral CLND or lobectomy plus isthmusectomy+ipsilateral CLND | 7 |
| Wendong Wang [29] | 2013 | China | retrospective study | PTC | 276 | TT+bilateral CLND or lobectomy plus isthmusectomy+ipsilateral CLND | 7 |
| Yi-Li Zhou [30] | 2012 | China | Retrospective study | PTMC | 122 | TT+bilateral CLND | 9 |
| Bo-Yeon Kim [31] | 2012 | Korea | retrospective study | PTMC | 160 | TT+bilateral CLND | 9 |
| Yinlong Yang [32] | 2014 | China | retrospective study | PTMC | 291 | TT+bilateral CLND | 9 |
| SuSheng Miao[33] | 2013 | China | prospective study. | PTC | 184 | TT+bilateral CLND | 8 |
| Bon Seok Koo [34] | 2009 | Korea | prospectively study | PTC | 111 | TT+bilateral CLND | 9 |
| Park J.P. [35] | 2014 | Korea | retrospective study | PTMC | 193 | TT+bilateral CLND or lobectomy plus isthmusectomy+ipsilateral CLND | 7 |
| Keke Liang [36] | 2014 | China | retrospective study | PTC | 529 | TT+bilateral CLND or lobectomy plus isthmusectomy+ipsilateral CLND | 7 |
| Yoon Kyoung So [37] | 2010 | Korea | retrospective study | PTMC | 551 | TT+bilateral CLND | 8 |
| Gilberto Teixeira[38] | 2011 | Brazil | retrospective study | PTC | 72 | TT+ipsilateral CLND | 7 |
| Yasuhiro Ito [39] | 2013 | Japan | retrospective study | PTC | 3219 | TT/NTT+ipsilateral or bilateral CLND | 7 |
| Lee K.E. [40] | 2012 | Korea | retrospective study | PTC | 161 | TT+bilateral CLND | 8 |
| LING-NA MAO [41] | 2015 | China | retrospective study | PTC | 389 | TT+ipsilateral or bilateral CLND | 7 |
| PTMC | 332 | TT+ipsilateral or bilateral CLND | |||||
| Mujgan Caliskan [42] | 2012 | Korea | retrospective study | PTMC | 842 | TT/NTT+ipsilateral CLND | 7 |
| Sang-Hyuk Lee [43] | 2008 | Korea | retrospective study | PTMC | 50 | TT+bilateral CLND | 9 |
TT: Total thyroidectomy. NTT: Nearly total thyroidectomy PTMC: papillary thyroid microcarcinoma
Fig 1. Flow chart of the study selection process.
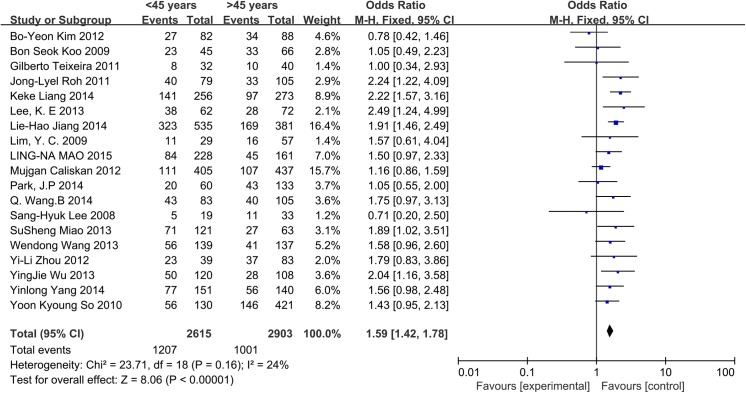
Age
A fixed-effects model was applied in this analysis (p = 0.16, I2 = 24%). Among CN0 patients, the rate of CLNM was 46.16% in patients <45 years and 34.48% in the patients >45 years. The results indicate that age <45 years was associated with an increased rate of CLNM in CN0 patients (OR = 1.59, 95% CI = 1.42–1.78, p<0.00001) (Fig 2).
Fig 2. Forest plots of the association between age and CLNM in CN0 patients.
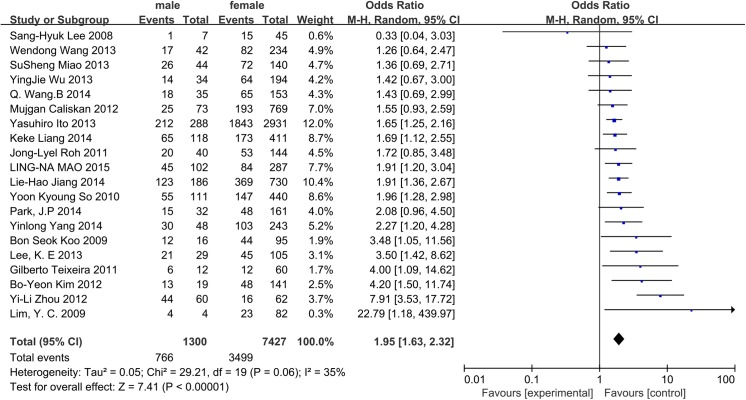
Sex
A random-effects model was adopted to analyze the data (p = 0.06, I2 = 35%). The incidence of CLNM was 58.92% in men and 47.11% in women. Male CN0 patients exhibited a significantly higher incidence of CLNM (OR = 1.95, 95% CI = 1.63–2.32, p<0.00001) (Fig 3).
Fig 3. Forest plots of the association between sex and CLNM in CN0 patients.
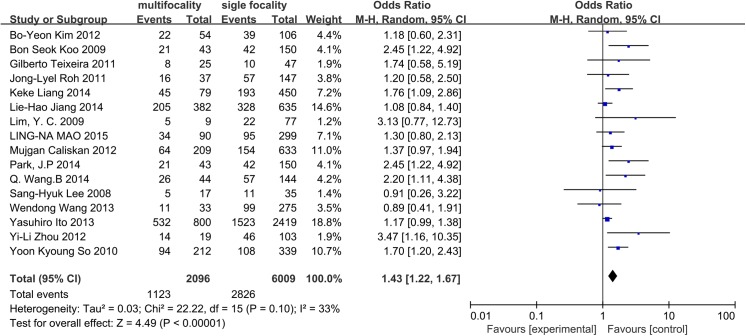
Multifocality
A random-effects model was applied to assess heterogeneity (p = 0.10, I2 = 33%). Sixteen studies were included in the analysis of tumor multifocality. We observed a positive correlation between the number of foci and the incidence of CLNM in CN0 patients (OR = 1.43, 95% CI = 1.22–1.67, p<0.0001) (Fig 4).
Fig 4. Forest plots of the association between multifocality and CLNM in CN0 patients.
Size
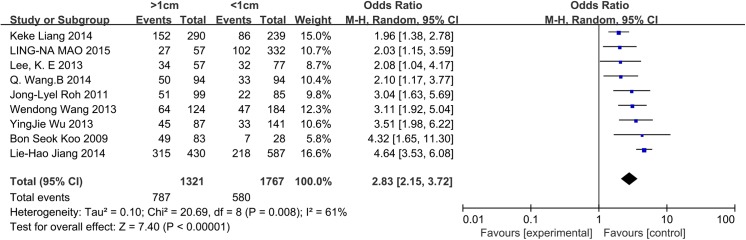
Seven and eight studies were included in the analysis of the influence of tumor size in patients with PTC and PTMC, respectively. For patients with PTC, a random-effects model was used (p = 0.001, I2 = 72%). Tumor size >2 cm was significantly associated with CLNM in CN0 PTC patients (OR = 2.98, 95% CI = 2.08–4.28, p<0.00001). For patients with PTMC, 0.5 cm was established as the cut-off value. A random-effects model was used (p = 0.06, I2 = 48%). Tumor size >0.5 cm was identified as a risk factor associated with CLNM in CN0 patients (OR = 2.30, 95% CI = 1.71–3.09, p<0.00001) (Figs 5 and 6). We also analyzed whether the prevalence of CLNM was different between PTC and PTMC. A random-effects model was applied (p = 0.008, I2 = 61%). PTC (tumor size>1cm) exhibited a higher risk factor associated with CLNM than PTMC (tumor size<1cm) (OR = 2.83, 95% CI = 2.15–3.72, p<0.00001) (Fig 7).
Fig 5. Forest plots of the association between size (PTC) and CLNM in CN0 patients.
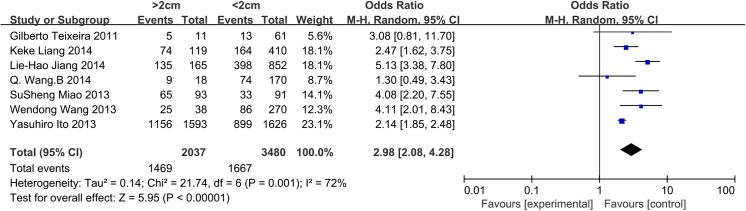
Fig 6. Forest plots of the association between size (PTMC) and CLNM in CN0 patients.
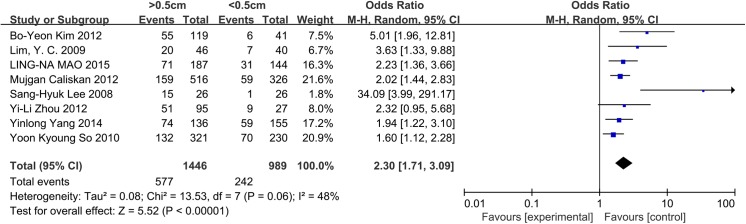
Fig 7. Forest plots of the different prevalence of CLNM between PTC (tumor size>1cm) and PTMC (tumor size<1cm) in CN0 patients.
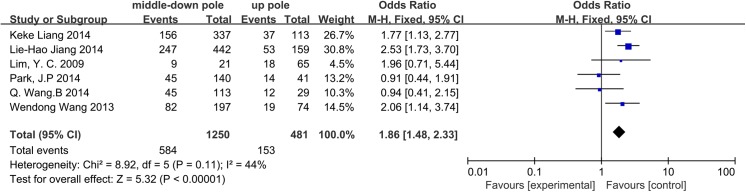
Location
The influence of tumor location on CLNM in CN0 patients was assessed in 6 studies. We divided the thyroid into three areas: upper pole, central area, and lower pole. Approximately 31.81%, 46.77%, and 46.65% located in the upper pole, central area, and lower pole, respectively, exhibited CLNM. When pooling the central area and lower pole for comparison with the upper pole, we observed that tumors located in the central area and lower pole of the thyroid lobe were associated with a high rate of CLNM (OR = 1.86, 95% CI = 1.48–2.33, p<0.00001). A fixed-effects model was used to analyze the data (I2 = 44%, p = 0.11) (Fig 8).
Fig 8. Forest plots of the association between location and CLNM in CN0 patients.
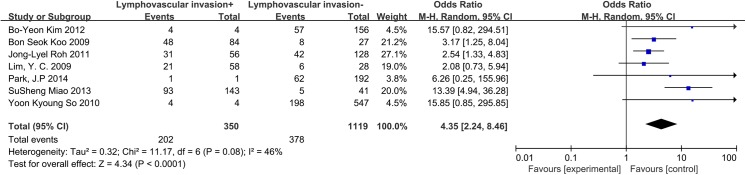
Lymphovascular invasion
A random-effects model was applied to analyze these data (I2 = 46%, p = 0.08). Seven included studies were investigated. Lymphovascular invasion exhibited a 4.35-fold increased risk of CLNM in CN0 patients (OR = 4.35, 95% CI = 2.24–8.46, p<0.0001) (Fig 9).
Fig 9. Forest plots of the association between lymphovascular invasion and CLNM in CN0 patients.
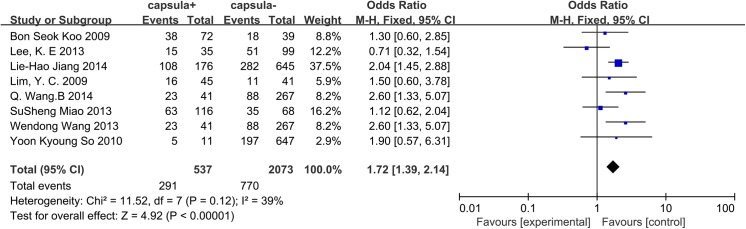
Capsular invasion
A fixed-effects model was applied to our data (p = 0.12, I2 = 39%), and 8 studies were included in this analysis. We concluded that for CN0 patients, capsular invasion was associated with CLNM (OR = 1.72, 95% CI = 1.39–2.14, p = 0.001) (Fig 10).
Fig 10. Forest plots of the association between capsular invasion and CLNM in CN0 patients.
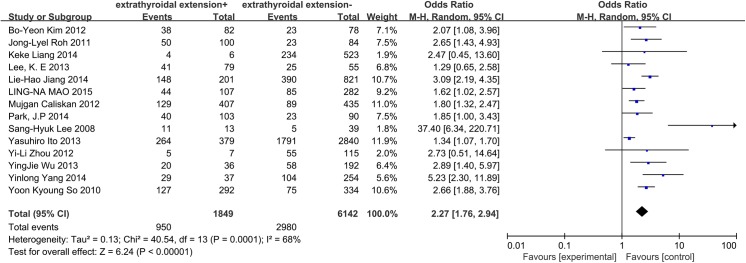
Extrathyroidal extension
A random-effects model determined that the heterogeneity of the data on extrathyroidal extension was significant (p = 0.0001, I2 = 68%). A total of 14 papers and 7991 patients were included in this analysis. Extrathyroidal extension exhibited a high propensity to spread to central lymph nodes in CN0 patients (OR = 2.27, 95% CI = 1.76–2.94, p<0.00001) (Fig 11).
Fig 11. Forest plots of the association between extrathyroidal extension and CLNM in CN0 patients.
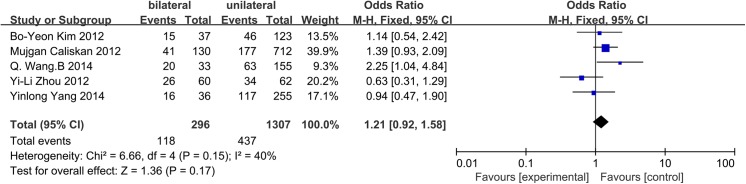
Bilateral tumors
A fixed-effects model was applied due to insignificant heterogeneity (p = 0.15, I2 = 40%). Unilateral or bilateral tumors were evaluated in 5 studies. However, neither unilateral tumors nor bilateral tumors were associated with CLNM (OR = 1.21, 95% CI = 0.92–1.58, p = 0.17) (Fig 12).
Fig 12. Forest plots of the association between bilateral tumors and CLNM in CN0 patients.
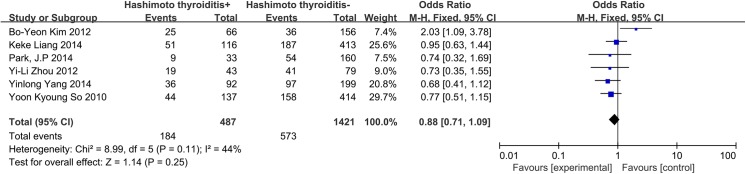
Hashimoto’s thyroiditis
A fixed-effects model was applied to analyze the data involving Hashimoto’s thyroiditis (I2 = 44%, p = 0.11). In our database, a total of 6 studies were included, and CLNM was not associated with the coexistence of chronic Hashimoto’s thyroiditis (OR = 0.88, 95% CI = 0.71–1.09, p = 0.25) (Fig 13).
Fig 13. Forest plots of the association between Hashimoto’s thyroiditis and CLNM in CN0 patients.
Discussion
PTC exhibits low malignancy, relatively good prognosis and a generally positive response to surgery[3,4]. However, PTC also exhibits a high propensity to spread to regional lymph nodes. The central neck compartment is the primary zone of lymphatic involvement in PTC (20%-90% of patients), and CLNM is highly associated with recurrence and overall survival [5,6,44,45]. Patients with papillary thyroid microcarcinoma were also included in our review. PTMC is defined as a tumor that is 10 mm or less along its greatest dimension in accordance with the histologic classification of thyroid tumors by the World Health Organization[31]. The reasons to explore and finally operate on these patients are based on malignant findings from preoperative ultrasonography and fine-needle aspiration biopsy. For PTMC, CLNM appears to affect recurrence but not disease-free survival[15,16,46]. The prevalence of ipsilateral and contralateral CLNM in the included studies in our analysis was 25%-63.83% and 9.24%-30.6%, respectively. The prevalence of ipsilateral CLNM was 25%-49.18% for PTMC (tumor size<1cm) CN0 patients and 47.37%-73.26% for PTC (tumor size>1cm) CN0 patients in our included studies. In the present meta-analysis, PTC (tumor size>1cm) was associated with a higher risk factor than PTMC (tumor size<1cm) in CN0 patients (OR = 2.83, 95% CI = 2.15–3.72, p<0.00001). Preoperative US and contrast-enhanced CT play important roles in screening for CLNM. However, the sensitivities of these techniques are inconsistent, ranging from 23%-53.2% and 41%-66.7% for preoperative US and contrast-enhanced CT, respectively[21,22]. The low sensitivity of US and CT may be due to the following reasons: First, central metastatic lymph nodes are so small that they may not be accurately detected by US and CT. Second, the existence of an intact thyroid gland increases the difficulty of examining posterior lymph nodes around the recurrent laryngeal nerve by US. Third, positive criteria for US and CT in the central neck have not yet been established[47]. In contrast to patients with clinically positive central neck metastasis, there remains controversy concerning how to manage PTC patients with clinically negative CLNM[48].
The leading treatment guidelines are not concordant when compared in relation to their recommendations regarding the application of PCLND. On one hand, the American Thyroid Association (ATA) guidelines recommend that routine PCLND should be performed only in patients with advanced T3 and T4 primary tumors[9]. However, this may not be appropriate, as many T1 or T2 tumors also exhibit CLNM, and different clinicopathologic parameters may play crucial roles in CLNM[43]. The ATA restricts PCLND due to the limitations of its influence on prognosis and because the majority of thyroid surgeries in the United States are performed by surgeons who, according to National Inpatient Sample from 1988 through 2000, perform few such surgeries[49]. Currently, the number of surgeries performed by high-volume surgeons in USA is growing. Cases performed by high-volume surgeons increased from 12% in 1993 through 2000 to 25% in 2001 through 2008, in contrast cases performed by very-low volume surgeons decreased from 51% to 34%[50]. Inexperienced surgeons are associated with a greater rate of complications[51]. However, the Japanese Association of Endocrine Surgeons (JAES) routinely recommends PCLND. This may be attributed to the fact that the use of RAI is limited due to legal restrictions[52]. In conclusion, to more appropriately select patients for PCLND, a careful preoperative evaluation of the risk factors of CLNM in CN0 patients is required.
Many studies have focused on the risk factors of CLND for CN0 patients. However, the results are inconsistent. Age has been reported to be a risk factor in many studies. The cut-off age of 45 years is widely used as a clinical marker for prognosis[5]. Traditionally, patients older than 45 years are associated with poor prognosis and increased recurrence[53]. In our study, we observed that age younger than 45 years is a significant risk factor for CLNM in CN0 patients. Some authors have reported a significant correlation between a higher risk of thyroid cancer and age younger than 45 years[54,55]. Do BA et al. reported that well-differentiated thyroid cancer and lymph node metastasis occurred more often in patients younger than 50 years[56].
Although the incidence of thyroid cancer is higher in women, the rates of malignancy and mortality due to thyroid cancer are higher in men[57,58]. Male sex was identified as a risk factor when patients with thyroid nodules were evaluated, and this factor may be suggestive of thyroid carcinoma[59]. Male patients are also more prone to unhealthy lifestyles and harmful environmental factors, such as smoking and drinking. Several studies have reported that men exhibited a poorer prognosis than women [60,61]. We concluded that male sex is a significant risk factor for CLNM in CN0 patients.
As always, tumor size is an important factor in TNM staging, and large tumors are more prone to be aggressive[62]. In our data, we observed that tumor size >2 cm exhibited a 2.98-fold increased risk of CLNM in CN0 patients. For PTMC, most studies consider 0.5 cm to be the cut-off value. In the present meta-analysis, we observed that tumors larger than 0.5 cm constitute an apparent risk factor. Other studies have reported that tumor sizes smaller than 0.5 cm exhibit no micrometastases or contralateral CLNM in CN0 patients[18,32].
Some studies have reported that multifocal PTC increases the risk of locoregional recurrence as well as lymph node metastasis and distant metastasis[63–65]. For years, multifocal PTC was considered the intraglandular spread of the primary tumor. Thus, multifocal PTC may be more aggressive than unifocal PTC[66]. Ning Qu et al. reported that an increasing number of tumors was associated with a tendency toward more aggressive features and predicted poor prognosis in PTC[43]. Whether multifocality is related to CLNM in CN0 patients remains controversial. In our study, we concluded that multifocality is a risk factor for CLNM.
The association between Hashimoto’s thyroiditis (HT) and PTC was first proposed by Dailey et al. in 1955[67]. It remains controversial whether HT is associated with CLNM in PTC patients. Some studies suggest that the coexistence of HT is not associated with CLNM in PTC or PTMC patients[31,68]. However, another meta-analysis reported that PTCs with coexistent HT were significantly related to the absence of lymph node metastasis[69]. Our data indicated that there was no association between HT and CLNM in CN0 patients. The differences between our findings and previous conclusions may be related to different selection criteria and different study designs.
There are several limitations of this study. First, the included studies were not randomized case-control trials. Second, the types of thyroidectomies performed on the CN0 patients were not consistent and included total thyroidectomy, nearly total thyroidectomy, and lobectomy plus isthmusectomy. Third, we only recorded ipsilateral CLNM from the studies in which level VI was divided into three or four groups. This recording method could have led to some unavoidable bias, as some patients only exhibited contralateral CLNM. Fourth, most of the patients from the included studies were from Asia.
In conclusion, this meta-analysis identified the following significant risk factors for central lymph node metastasis in CN0 patients: age, sex, multifocality, tumor size, tumor location, lymphovascular invasion, capsular invasion, and extrathyroidal extension. Bilateral tumors and Hashimoto’s thyroiditis do not appear to be correlated with CLNM in CN0 patients.
Supporting Information
(DOC)
(EPS)
(EPS)
(EPS)
(EPS)
(EPS)
(EPS)
(EPS)
(EPS)
(EPS)
(EPS)
(EPS)
(EPS)
(XLS)
Acknowledgments
This work was supported by the Liaoning BaiQianWan Talents Program (No. 2014921033). The authors declare that no conflicts of interest exist with respect to the publication of this study. We thank American Journal Experts (AJE) for assisting in the language usage, spelling, and grammar of our manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Liaoning BaiQianWan Talents Program (No. 2014921033), URLs: www.ln.hrss.gov.cn/ln/99/10/2014/11/i45652.shtml.
References
- 1. Lundgren CI, Hall P, Dickman PW, Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer. 2006;106: 524–531. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127: 2893–2917. 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 3. Sciuto R, Romano L, Rea S, Marandino F, Sperduti I, Maini CL. Natural history and clinical outcome of differentiated thyroid carcinoma: a retrospective analysis of 1503 patients treated at a single institution. Ann Oncol. 2009;20: 1728–1735. 10.1093/annonc/mdp050 [DOI] [PubMed] [Google Scholar]
- 4. Toniato A, Boschin I, Casara D, Mazzarotto R, Rubello D, Pelizzo M. Papillary thyroid carcinoma: factors influencing recurrence and survival. Ann Surg Oncol. 2008;15: 1518–1522. 10.1245/s10434-008-9859-4 [DOI] [PubMed] [Google Scholar]
- 5. Grebe SK, Hay ID. Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am. 1996;5: 43–63. [PubMed] [Google Scholar]
- 6. Kouvaraki MA, Shapiro SE, Fornage BD, Edeiken-Monro BS, Sherman SI, Vassilopoulou-Sellin R, et al. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery. 2003;134: 946–954; discussion 954–945. [DOI] [PubMed] [Google Scholar]
- 7. White ML, Gauger PG, Doherty GM. Central lymph node dissection in differentiated thyroid cancer. World J Surg. 2007;31: 895–904. [DOI] [PubMed] [Google Scholar]
- 8. Guerrero MA, Clark OH. Controversies in the management of papillary thyroid cancer revisited. ISRN oncology. 2011:303128 10.5402/2011/303128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19: 1167–1214. 10.1089/thy.2009.0110 [DOI] [PubMed] [Google Scholar]
- 10. Moo TA, McGill J, Allendorf J, Lee J, Fahey T 3rd, Zarnegar R. Impact of prophylactic central neck lymph node dissection on early recurrence in papillary thyroid carcinoma. World J Surg. 2010;34: 1187–1191. 10.1007/s00268-010-0418-3 [DOI] [PubMed] [Google Scholar]
- 11. Mazzaferri EL, Doherty GM, Steward DL. The pros and cons of prophylactic central compartment lymph node dissection for papillary thyroid carcinoma. Thyroid. 2009;19: 683–689. 10.1089/thy.2009.1578 [DOI] [PubMed] [Google Scholar]
- 12. Shindo M, Wu JC, Park EE, Tanzella F. The importance of central compartment elective lymph node excision in the staging and treatment of papillary thyroid cancer. Arch Otolaryngol Head Neck Surg. 2006;132: 650–654. [DOI] [PubMed] [Google Scholar]
- 13. Vini L, Hyer SL, Marshall J, A'Hern R, Harmer C. Long-term results in elderly patients with differentiated thyroid carcinoma. Cancer. 2003;97: 2736–2742. [DOI] [PubMed] [Google Scholar]
- 14. Qu N, Zhang L, Ji QH, Zhu YX, Wang ZY, Shen Q, et al. Number of tumor foci predicts prognosis in papillary thyroid cancer. BMC Cancer. 2014;14: 914 10.1186/1471-2407-14-914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003;237: 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Noguchi S, Yamashita H, Uchino S, Watanabe S. Papillary microcarcinoma. World J Surg. 2008;32: 747–753. 10.1007/s00268-007-9453-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fritz e D, Doherty GM. Surgical management of cervical lymph nodes in differentiated thyroid cancer. Otolaryngol Clin North Am. 2010;43: 285–300, viii. 10.1016/j.otc.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 18. Carty SE, Cooper DS, Doherty GM, Duh QY, Kloos RT, Mandel SJ, et al. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid. 2009;19: 1153–1158. 10.1089/thy.2009.0159 [DOI] [PubMed] [Google Scholar]
- 19. Roh JL, Park JY, Park CI. Total thyroidectomy plus neck dissection in differentiated papillary thyroid carcinoma patients: pattern of nodal metastasis, morbidity, recurrence, and postoperative levels of serum parathyroid hormone. Ann Surg. 2007;245: 604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cavicchi O, Piccin O, Caliceti U, De Cataldis A, Pasquali R, Ceroni AR. Transient hypoparathyroidism following thyroidectomy: a prospective study and multivariate analysis of 604 consecutive patients. Otolaryngol Head Neck Surg. 2007;137: 654–658. [DOI] [PubMed] [Google Scholar]
- 21. Choi JS, Kim J, Kwak JY, Kim MJ, Chang HS, Kim EK. Preoperative staging of papillary thyroid carcinoma: comparison of ultrasound imaging and CT. AJR Am J Roentgenol. 2009;193: 871–878. 10.2214/AJR.09.2386 [DOI] [PubMed] [Google Scholar]
- 22. Hwang HS, Orloff LA. Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer. Laryngoscope. 2011;121: 487–491. 10.1002/lary.21227 [DOI] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8: 336–341. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 24. Lim YC, Choi EC, Yoon YH, Kim EH, Koo BS. Central lymph node metastases in unilateral papillary thyroid microcarcinoma. Br J Surg. 2009;96: 253–257. 10.1002/bjs.6484 [DOI] [PubMed] [Google Scholar]
- 25. Wu Y, Gu J, Shang J, Wang W, Wang K. Central lymph node metastasis in cN0 papillary thyroid carcinoma. J BUON. 2013;18: 733–738. [PubMed] [Google Scholar]
- 26. Roh JL, Kim JM, Park CI. Central lymph node metastasis of unilateral papillary thyroid carcinoma: patterns and factors predictive of nodal metastasis, morbidity, and recurrence. Ann Surg Oncol. 2011;18: 2245–2250. 10.1245/s10434-011-1600-z [DOI] [PubMed] [Google Scholar]
- 27. Wang Q, Chu B, Zhu J, Zhang S, Liu Y, Zhuang M, et al. Clinical analysis of prophylactic central neck dissection for papillary thyroid carcinoma. Clin Transl Oncol. 2014;16: 44–48. 10.1007/s12094-013-1038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jiang LH, Chen C, Tan Z, Lu XX, Hu SS, Wang QL, et al. Clinical Characteristics Related to Central Lymph Node Metastasis in cN0 Papillary Thyroid Carcinoma: A Retrospective Study of 916 Patients. Int J Endocrinol. 2014;2014: 385787 10.1155/2014/385787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang W, Gu J, Shang J, Wang K. Correlation analysis on central lymph node metastasis in 276 patients with cN0 papillary thyroid carcinoma. Int J Clin Exp Pathol. 2013;6: 510–515. [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou YL, Gao EL, Zhang W, Yang H, Guo GL, Zhang XH, et al. Factors predictive of papillary thyroid micro-carcinoma with bilateral involvement and central lymph node metastasis: a retrospective study. World J Surg Oncol. 2012;10: 67 10.1186/1477-7819-10-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim BY, Jung CH, Kim JW, Lee SW, Kim CH, Kang SK, et al. Impact of clinicopathologic factors on subclinical central lymph node metastasis in papillary thyroid microcarcinoma. Yonsei Med J. 2012;53: 924–930. 10.3349/ymj.2012.53.5.924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang Y, Chen C, Chen Z, Jiang J, Chen Y, Jin L, et al. Prediction of central compartment lymph node metastasis in papillary thyroid microcarcinoma. Clin Endocrinol (Oxf). 2014;81: 282–288. [DOI] [PubMed] [Google Scholar]
- 33. Miao S, Mao X, Pei R, Xiang C, Lv Y, Shi Q, et al. Predictive factors for different subgroups of central lymph node metastasis in unilateral papillary thyroid carcinoma. ORL J Otorhinolaryngol Relat Spec. 2013;75: 265–273. 10.1159/000354267 [DOI] [PubMed] [Google Scholar]
- 34. Koo BS, Choi EC, Yoon YH, Kim DH, Kim EH, Lim YC. Predictive factors for ipsilateral or contralateral central lymph node metastasis in unilateral papillary thyroid carcinoma. Ann Surg. 2009;249: 840–844. 10.1097/SLA.0b013e3181a40919 [DOI] [PubMed] [Google Scholar]
- 35. Park JP, Roh JL, Lee JH, Baek JH, Gong G, Cho KJ, et al. Risk factors for central neck lymph node metastasis of clinically noninvasive, node-negative papillary thyroid microcarcinoma. Am J Surg. 2014;208: 412–418. 10.1016/j.amjsurg.2013.10.032 [DOI] [PubMed] [Google Scholar]
- 36. Liang K, He L, Dong W, Zhang H. Risk factors of central lymph node metastasis in cN0 papillary thyroid carcinoma: a study of 529 patients. Med Sci Monit. 2014;20: 807–811. 10.12659/MSM.890182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. So YK, Son YI, Hong SD, Seo MY, Baek CH, Jeong HS, et al. Subclinical lymph node metastasis in papillary thyroid microcarcinoma: a study of 551 resections. Surgery. 2010;148: 526–531. 10.1016/j.surg.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 38. Teixeira G, Teixeira T, Gubert F, Chikota H, Tufano R. The incidence of central neck micrometastatic disease in patients with papillary thyroid cancer staged preoperatively and intraoperatively as N0. Surgery. 2011;150: 1161–1167. 10.1016/j.surg.2011.09.019 [DOI] [PubMed] [Google Scholar]
- 39. Ito Y, Fukushima M, Higashiyama T, Kihara M, Takamura Y, Kobayashi K, et al. Tumor size is the strongest predictor of microscopic lymph node metastasis and lymph node recurrence of N0 papillary thyroid carcinoma. Endocr J. 2013;60: 113–117. [DOI] [PubMed] [Google Scholar]
- 40. Lee KE, Chung IY, Kang E, Koo do H, Kim KH, Kim SW, et al. Ipsilateral and contralateral central lymph node metastasis in papillary thyroid cancer: patterns and predictive factors of nodal metastasis. Head Neck. 2013;35: 672–676. 10.1002/hed.23016 [DOI] [PubMed] [Google Scholar]
- 41. Mao LN, Wang P, Li ZY, Wang Y, Song ZY. Risk factor analysis for central nodal metastasis in papillary thyroid carcinoma. Oncol Lett. 2015;9: 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Caliskan M, Park JH, Jeong JS, Lee CR, Park SK, Kang SW, et al. Role of prophylactic ipsilateral central compartment lymph node dissection in papillary thyroid microcarcinoma. Endocr J. 2012;59: 305–311. [DOI] [PubMed] [Google Scholar]
- 43. Lee SH, Lee SS, Jin SM, Kim JH, Rho YS. Predictive factors for central compartment lymph node metastasis in thyroid papillary microcarcinoma. Laryngoscope. 2008;118: 659–662. 10.1097/MLG.0b013e318161f9d1 [DOI] [PubMed] [Google Scholar]
- 44. Pelizzo MR, Boschin IM, Toniato A, Piotto A, Pagetta C, Gross MD, et al. Papillary thyroid carcinoma: 35-year outcome and prognostic factors in 1858 patients. Clin Nucl Med.2007;32: 440–444. [DOI] [PubMed] [Google Scholar]
- 45. Zaydfudim V, Feurer ID, Griffin MR, Phay JE. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery. 2008;144: 1070–1077; discussion 1077–1078. 10.1016/j.surg.2008.08.034 [DOI] [PubMed] [Google Scholar]
- 46. Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Clinical significance of metastasis to the central compartment from papillary microcarcinoma of the thyroid. World J Surg. 2006;30: 91–99. [DOI] [PubMed] [Google Scholar]
- 47. Lee DW, Ji YB, Sung ES, Park JS, Lee YJ, Park DW, et al. Roles of ultrasonography and computed tomography in the surgical management of cervical lymph node metastases in papillary thyroid carcinoma. Eur J Surg Oncol. 2013;39: 191–196. 10.1016/j.ejso.2012.07.119 [DOI] [PubMed] [Google Scholar]
- 48. Machens A, Hinze R, Thomusch O, Dralle H. Pattern of nodal metastasis for primary and reoperative thyroid cancer. World J Surg. 2002;26: 22–28. [DOI] [PubMed] [Google Scholar]
- 49. Saunders BD, Wainess RM, Dimick JB, Doherty GM, Upchurch GR, Gauger PG. Who performs endocrine operations in the United States? Surgery. 2003;134: 924–931; discussion 931. [DOI] [PubMed] [Google Scholar]
- 50. Loyo M, Tufano RP, Gourin CG. National trends in thyroid surgery and the effect of volume on short-term outcomes. Laryngoscope. 2013;123: 2056–2063. 10.1002/lary.23923 [DOI] [PubMed] [Google Scholar]
- 51. Sosa JA, Bowman HM, Tielsch JM, Powe NR, Gordon TA, Udelsman R. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg. 1998;228: 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takami H, Ito Y, Okamoto T, Yoshida A. Therapeutic strategy for differentiated thyroid carcinoma in Japan based on a newly established guideline managed by Japanese Society of Thyroid Surgeons and Japanese Association of Endocrine Surgeons. World J Surg. 2011;35: 111–121. 10.1007/s00268-010-0832-6 [DOI] [PubMed] [Google Scholar]
- 53. Londero SC, Krogdahl A, Bastholt L, Overgaard J, Pedersen HB, Hahn CH, et al. Papillary thyroid carcinoma in Denmark, 1996–2008: outcome and evaluation of established prognostic scoring systems in a prospective national cohort. Thyroid. 2015;25: 78–84. 10.1089/thy.2014.0294 [DOI] [PubMed] [Google Scholar]
- 54. Raza SN, Shah MD, Palme CE, Hall FT, Eski S, Freeman JL. Risk factors for well-differentiated thyroid carcinoma in patients with thyroid nodular disease. Otolaryngol Head Neck Surg. 2008;139: 21–26. 10.1016/j.otohns.2007.10.021 [DOI] [PubMed] [Google Scholar]
- 55. Obregon-Guerrero G, Martinez-Ordaz JL, Pena-Garcia JF, Ramirez-Martinez ME, Perez-Alvarez C, Hernandez-Avendano CV. Factors associated with malignancy in patients with thyroid nodules. Cir Cir. 2010;78: 479–484. [PubMed] [Google Scholar]
- 56. Do BA, Payne RJ, Bastianelli M, Mlynarek AM, Tamilia M, Hier M, et al. Is age associated with risk of malignancy in thyroid cancer? Otolaryngol Head Neck Surg. 2014;151: 746–750. 10.1177/0194599814547503 [DOI] [PubMed] [Google Scholar]
- 57. Rahbari R, Zhang L, Kebebew E. Thyroid cancer gender disparity. Future Oncol. 2010;6: 1771–1779. 10.2217/fon.10.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Libutti SK. Understanding the role of gender in the incidence of thyroid cancer. Cancer J. 2005;11: 104–105. [DOI] [PubMed] [Google Scholar]
- 59. Hegedus L. Clinical practice. The thyroid nodule. N Engl J Med. 2004;351: 1764–1771. [DOI] [PubMed] [Google Scholar]
- 60. Shaha AR, Shah JP, Loree TR. Risk group stratification and prognostic factors in papillary carcinoma of thyroid. Ann Surg Oncol. 1996;3: 534–538. [DOI] [PubMed] [Google Scholar]
- 61. Besic N, Pilko G, Petric R, Hocevar M, Zgajnar J. Papillary thyroid microcarcinoma: prognostic factors and treatment. J Surg Oncol. 2008;97: 221–225. [DOI] [PubMed] [Google Scholar]
- 62. Tuttle RM, Haddad RI, Ball DW, Byrd D, Dickson P, Duh QY, et al. Thyroid carcinoma, version 2.2014. J Natl Compr Canc Netw. 2014;12: 1671–80. [DOI] [PubMed] [Google Scholar]
- 63. Tscholl-Ducommun J, Hedinger CE. Papillary thyroid carcinomas. Morphology and prognosis. Virchows Arch A Pathol Anat Histol. 1982;396: 19–39. [DOI] [PubMed] [Google Scholar]
- 64. Katoh R, Sasaki J, Kurihara H, Suzuki K, Iida Y, Kawaoi A. Multiple thyroid involvement (intraglandular metastasis) in papillary thyroid carcinoma. A clinicopathologic study of 105 consecutive patients. Cancer. 1992;70: 1585–1590. [DOI] [PubMed] [Google Scholar]
- 65. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97: 418–428. [DOI] [PubMed] [Google Scholar]
- 66. Mazeh H, Samet Y, Hochstein D, Mizrahi I, Ariel I, Eid A, et al. Multifocality in well-differentiated thyroid carcinomas calls for total thyroidectomy. Am J Surg. 2011;201: 770–775. 10.1016/j.amjsurg.2010.03.004 [DOI] [PubMed] [Google Scholar]
- 67. Dailey ME, Lindsay S, Skahen R. Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg. 195;70: 291–297. [DOI] [PubMed] [Google Scholar]
- 68. Kim EY, Kim WG, Kim WB, Kim TY, Kim JM, Ryu JS, et al. Coexistence of chronic lymphocytic thyroiditis is associated with lower recurrence rates in patients with papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2009;71: 581–586. [DOI] [PubMed] [Google Scholar]
- 69. Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven Hashimoto's thyroiditis: a meta-analysis. Eur J Endocrinol. 2013;168: 343–349. 10.1530/EJE-12-0903 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(EPS)
(EPS)
(EPS)
(EPS)
(EPS)
(EPS)
(EPS)
(EPS)
(EPS)
(EPS)
(EPS)
(EPS)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.