Abstract
Background
Triple-A syndrome (Allgrove syndrome) is an autosomal recessive disorder characterized by adrenal insufficiency, alacrima, achalasia, and – occasionally – autonomic instability. Mutations have been found in the AAAS gene on 12q13.
Case presentation
We present the case of a 12 year-old boy with classic systemic features of triple-A syndrome and several prominent ophthalmic features, including: accommodative spasm, dry eye, superficial punctate keratopathy, and pupillary hypersensitivity to dilute pilocarpine. MRI showed small lacrimal glands bilaterally. DNA sequencing of PCR-amplified fragments from the 16 exons of the AAAS gene revealed compound heterozygosity for a new, out-of-frame 5-bp deletion in exon 15, c1368-1372delGCTCA, and a previously-described nonsense mutation in exon 9, c938C>T, R286X.
Conclusions
In addition to known ophthalmic manifestations, triple-A syndrome can present with accommodative dysregulation and ocular signs of autonomic dysfunction.
Background
Triple-A syndrome (also known as Allgrove syndrome, OMIM #231550) is a rare, autosomal recessive disease characterized by alacrima, achalasia, ACTH-resistant adrenal insufficiency, autonomic dysfunction and neurodegeneration. [1,2] Ophthalmic manifestations include alacrima and keratoconjunctivitis sicca, sometimes resulting in corneal melt; lacrimal gland atrophy; pupillary abnormalities, including sluggish pupils, tonic pupils, and hypersensitivity to dilute miotics; and optic atrophy. [3-8] The phenotype in triple-A syndrome patients is highly variable. [9] Mutations in the AAAS gene on chromosome 12q13 have been described in several pedigrees with triple-A syndrome (Table 1). [7,9-19] Molecular data coupled with a detailed ophthalmic examination have not be reported in the literature.
Table 1.
Reported mutations in the AAAS gene in subjects with triple A syndrome. The numbering of nucleotides corresponds to the conventions of Dunnen and Antonarakis. [26] The most commonly-reported mutation is IVS 14+1G>A.
| Position | Protein | cDNA Mutation | References |
| IVS 4 | IVS 4-2A>G | Tullio-Pelet et al. (2000) | |
| Ex 9 | R312X | 934C>T | Tullio-Pelet et al. (2000) |
| Ex10 | S328fsX363 | 981_982insT | Tullio-Pelet et al. (2000) |
| IVS 14 | IVS 14+1G>A | Tullio-Pelet et al. (2000), Sandrini et al. (2001); Reshmi-Skarja et al (2003); Roubergue A et al (2004) | |
| Ex 16 | R478X | 1432C>T | Tullio-Pelet et al. (2000); Yuksel et al. (2004) |
| Ex 1 | Q15K | 43C>A | Handschug et al. (2001), Sandrini et al. (2001), Houlden et al. (2002); Prpic et al. (2003) |
| Ex 2 | W84X | 251G>A | Handschug et al. (2001) |
| Ex 6 | H160R | 479A>G | Handschug et al. (2001) |
| Ex 6 | F157fsX171 | 470_471delTT | Handschug et al. (2001) |
| Ex 8 | S263P | 787T>C | Handschug et al. (2001), Houlden et al. (2002); Prpic et al. (2003) |
| Ex 15 | S463fsX549 | 1389delC | Handschug et al. (2001) |
| Ex 9 | R286X | 856C>T | Handschug et al. (2001), this report |
| Ex 11 | R342X | 1024C>T | Handschug et al. (2001) |
| Ex 9 | W295X | 884G>A | Schmittmann-Ohters (2001) |
| IVS 11 | IVS 11+1G>A | Sandrini et al. (2001) | |
| Ex 16 | R478X | 1432C>T | Goizet et al. (2002), Yuksel et al. (2004) |
| Ex 8 | Q237X | 709C>T | Katsumata et al. (2002) |
| Ex 2 | I70fsX92 | 210delC | Houlden et al. (2002) |
| Ex 7 | R230X | 678C>T | Houlden et al. (2002), Reshmi-Skarja et al. (2003) |
| Ex 10 | V313A | 1238T>C | Houlden et al. (2002) |
| Ex 11 | L356fsX362 | 1066-1067delCT | Houlden et al. (2002) |
| Ex 12 | S382fsX413 | 1144-1147delTCTG | Houlden et al. (2002) |
| Ex 12 | D368fsX382 | 1104-1105insC | Houlden et al. (2002) |
| Ex 13 | 397fxs27 | 1191insA | Houlden et al. (2002) |
| Ex 16 | W474X | 1421G>A | Houlden et al. (2002) |
| Ex 12 | Q387X | 1159C>T | Prpic et al. (2003) |
| Ex 2 | H71fsX92 | 211delC | Reshmi-Skarja et al. (2003) |
| Ex 4 | R119X | 355C>T | Reshmi-Skarja et al. (2003) |
| Ex 5 | Q145X | 433C>T | Reshmi-Skarja et al. (2003) |
| IVS 5 | IVS 5+3insT | Reshmi-Skarja et al. (2003) | |
| Ex 15 | Q456X | 1366C>T | Reshmi-Skarja et al. (2003) |
| Ex 15 | X492 | 1368-1372delGCTCA | this report |
We report a case and a novel mutation in the AAAS gene in a 12 year-old boy with triple-A syndrome who exhibited evidence of accommodative spasms, in addition to dry eye and pupillary hypersensitivity to 0.125% pilocarpine.
Case presentation
Clinical studies
The subject was examined at the National Eye Institute and the National Institute of Child Health and Human Development at the National Institutes of Health. Informed parental consent and subject assent were obtained prior to examination and molecular studies. This study was performed in accordance with the Declaration of Helsinki.
Pupillography was performed using a standard pupilometry eye tracking system (ISAN Co, Burlington, Massachusetts). Pupil diameter was digitized at 60 Hz; binocular 150 ms stimuli delivered by green LEDs were used to elicit the light reaction.
Genetic studies
Analysis of AAAS mutations was performed as follows: Genomic DNA was isolated from whole blood following standard procedures. Intronic primers (Invitrogen) were appropriately chosen in order to sequence both splice sites of each exon. Primer sequences are available upon request. PCR products of each exon were run on agarose gels, specific bands were cut and the DNA was isolated following standard procedures. This DNA was sequences in both directions using a Beckman Coulter CEQ8000 following the manufacturer's protocol.
History and examination
The subject was diagnosed with adrenocortical insufficiency with elevated plasma ACTH at age 1 after a hypoglycemic seizure and was subsequently pharmacologically supplemented with glucocorticoids. Mineralocorticoid supplementation was initially instituted, but was subsequently discontinued. He cried without tears since birth. At age 2 he developed achalasia, which was treated successfully with surgery. The diagnosis of triple-A syndrome was made at age 4. Pregnancy and birth history were unremarkable. The subject was previously diagnosed with accommodative esotropia and treated with hyperopic correction. The proband's parents and three siblings did not have similar symptoms and were healthy. The parents' grandparents were from the same town in Italy, but there was no known consanguinity. The subject had speech delay as a child and receives speech therapy. He is currently enrolled in a normal 5th grade class with special aids for English, social studies and math. Over the past few years, he has developed ataxia. Review of systems was notable for photophobia and dry mouth.
Physical examination was remarkable for a fissured, dry tongue; palmar and plantar keratosis; +4 upper- and lower-extremity reflexes; 3–4 beats of lower-extremity clonus; a positive Babinski sign; and ataxic responses to tandem gait and finger-to-nose testing. The subject did not have orthostatic hypotension. Nerve conduction studies were not performed. An echocardiogram and an electrocardiogram were normal. CT of the abdomen was remarkable for little to no adrenal gland tissue. MRI of the brain showed no abnormalities, but the main lacrimal glands appeared small bilaterally (Figure 1).
Figure 1.
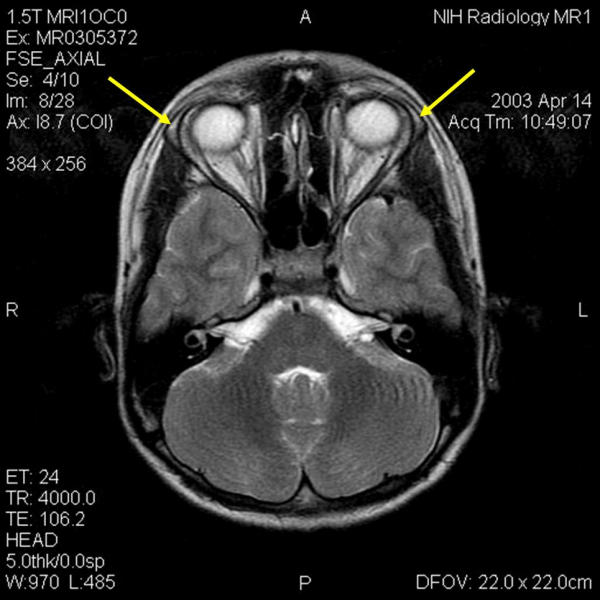
MRI of the brain of 12 year-old boy with triple-A syndrome showing hypoplastic lacrimal glands (yellow arrows.)
Visual acuity with his current spectacles (+3.25+1.50 × 120 OD, +3.25+1.50 × 100 OS) was 20/200 OD and 20/150 OS. Manifest refraction of -0.25+1.00 × 120 OD and +0.75 + 1.25 × 60 OS improved acuity to 20/50 and 20/70, respectively. Near vision at 14 inches was J7 OD and J3 OS. The subject had 400" of stereopsis on Titmus testing and had a red-green color deficiency (saw 3 of 15 Ishihara plates with each eye). Ocular motility was full and without nystagmus. Alternate cover testing with his current spectacles showed a 16–18 PD esotropia (ET) at 1/3 m and an 8 PD ET at 6 m. When allowed to wear a +3.00 D over his current refraction for several minutes, his ET decreased at near to 2–3 PD. Near point of accommodation was 11 cm OD and 9 cm OS. The patient had a somewhat variable, 35–40 ET at distance and near without correction. Clinically, his pupils were 5 mm OD and 5.5 mm OS and were somewhat sluggishly reactive to light. However, his light reaction to LED stimuli recorded with pupillography showed a relatively normal wave form. There was no afferent pupillary defect and constriction at near was not tested. The right pupil was tested with 0.125% pilocarpine and showed constriction to 2.5 mm in approximately 10 minutes. Schirmer testing results with anesthesia were 6 mmOD and 12 mmOS. Slit lamp examination was remarkable for trace injection, a decreased tear lake, and mild, bilateral superficial punctate keratopathy. Intraocular pressures were 11 mm Hg OD and 15 mmHg OS. His optic nerves, vessels, maculae, and retinal peripheries were normal on dilated examination. Cycloplegic refraction was +2.75 + 1.75 × 70 OD and +3.00 + 1.00 × 120. With this refraction, the subject could read 20/50 OD and 20/70 OS. After approximately 1 year of spectacle correction and amblyopia treatment, the subject's best-corrected vision improved to 20/30 OD and 20/40 OS. His stereopsis improved to 100 seconds of arc and his performance on Ishihara color-plate testing was stable.
Molecular testing
After informed consent, 5 cc of blood was drawn for research purposes. Sequencing of all 16 coding exons of the AAAS gene revealed compound heterozygosity for two mutations, both of which are predicted to premature stops in translation. In exon 9, the nonsense mutation leads to a shortened protein of 286, instead of 546, amino acids. In exon 15, and out-of-frame 5-bp deletion, c1368-1372delGCTCA, leads to a premature stop codon and a shortened protein of 492 amino acids (Figure 2.) Because both sequence changes are expected to result in premature truncation of the AAAS protein, they are likely pathological. This sequence alterations were not present in 100 control chromosomes from unaffected individuals.
Figure 2.
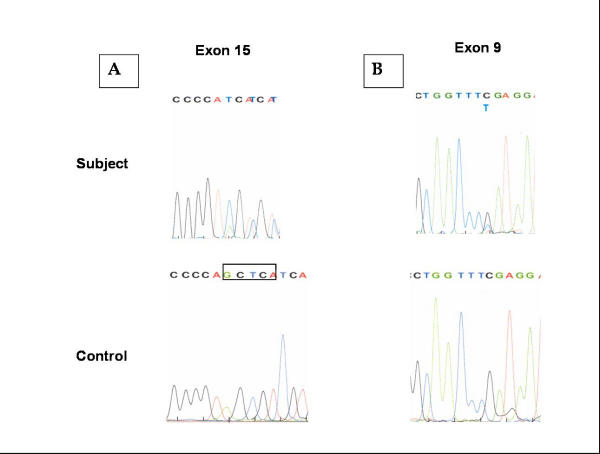
Compound heterozygous mutations in AAAS gene in subject with triple-A syndrome. The subject has a novel, five base-pair deletion in exon 15 (A, deletion boxed in control panel) predicted to cause a frameshift and a premature truncation of the Aladin protein. He also had a cytosine to thymidine mutation (B) in exon 9, predicted to cause a premature stop of protein translation.
Discussion
We present the case of an 12 year-old boy with classic triple-A syndrome who is a compound heterozygote for mutations in the AAAS gene. One mutation (R286X) has been previously reported and the other (c1368-1372delGCTCA) is novel. [11] The ophthalmic features consistent with the diagnosis of triple-A syndrome in this patient included: decreased tear production with a history of no spontaneous tearing; superficial punctate keratopathy; and increased pupillary sensitivity to 0.125% pilocarpine. In addition, the subject also appeared to inappropriately accommodate, as comparison of the uncyclopleged manifest refraction and the cycloplegic refraction suggested. This is not likely to be simply manifestation of his accommodative esotropia, per se. Although his esotropia improved with hyperopic correction, his vision did not until accommodation was pharmacologically blocked or until a compensatory refraction was given.
Accommodation results from contraction of the ciliary muscles, relaxation of the lens zonules, and an increase in the effective refracting power of the lens. This process is mediated via cholinergic, parasympathetic motor fibers. Patients with triple-A syndrome often manifest signs of autonomic dysregulation, including decreased lacrimation, pupillary abnormalities, orthostatic hypotension, sexual impotence, disturbances of heart rate, and abnormal reactions to intradermal histamine. [5,7] We hypothesize that the refractive difficulties experienced by our subject are related to autonomic dysfunction. Alternatively, because patients with triple-A syndrome have a neurodegenerative process that can affect cranial nerves, [14] the observed dysregulation may be due to central causes.
The decreased best-corrected visual acuity in this boy may have been due to superficial punctate keratopathy and/or amblyopia. Patients with triple-A syndrome sometimes develop optic atrophy. [7] Although our patient did not have clinical evidence of optic nerve dysfunction, we cannot rule out a subtle process that produced a moderate degree of visual impairment, despite the normal appearance of both the optic nerves and brain imaging. Similarly, it is possible that photoreceptor dysfunction in the retina – while never reported in triple-A syndrome – could explain some of the decreased best-corrected visual acuity and his red-green color deficiency. The patient was not available for follow-up testing with an electroretinogram, but his improvement with spectacle correction and amblyopia treatment over a year time argues against a progressive photoreceptor degeneration.
Lacrimation – both reflex and basal – is under parasympathetic control, as is pupillary miosis. [20-22] Autonomic dysfunction at the level of the lacrimal glands and the pupil presumably explain the abnormalities seen in this condition. Mullaney et al. noted small lacrimal glands on orbital imaging and a reduced number of serous-secreting cells on lacrimal gland biopsy of three patients with triple-A syndrome. [4] The hypersensitivity to dilute pilocarpine is likely due to up-regulation of post-synaptic muscarinic receptors on the iris sphincter. Frequent ocular lubrication was recommended to this subject.
The AAAS gene contains 16 exons and codes for a 546 amino acid protein called ALADIN. [10] ALADIN does not have major homology to any known proteins, but contains four WD motifs. WD proteins are a functionally diverse family whose members are involved in processes such as cell division, cell-fate determination, transcription, transmembrane signaling and intracellular trafficking. [23] Proteomic analysis of the mammalian nuclear pore complex (NPC) has shown that ALADIN is part of this structure, which is critical for communication between the nucleus and the cytoplasm of cells. [24] When mutated, ALADIN localizes to the cytoplasm, rather than the NPC. [25] However, cells from a subject with triple-A syndrome showed morphologically normal NPCs. These findings suggest that ALADIN is likely critical for nuclear pore function, rather than NPC structure. ALADIN may therefore be critical for the development of maintenance of the cell types affected in triple-A syndrome. Reshmi-Skarja et al. recently described chromosome and/or chromatid breaks, whole chromosome arm deletions, and marker chromosomes involving 9q12, a heterochromatic region know to be a fragile site, in subjects with triple-A syndrome [17].
List of Abbreviations
ACTH = adrenocorticotropic hormone; CT = computed tomography; D = diopter; DNA = deoxyribonucleic acid; MRI = magnetic resonance imaging; OMIM = Online Mendelian Inheritance in Man; PCR = polymerase chain reaction; PD = prism diopter
Competing Interests
No author has a competing interest relevant to this manuscript.
Author Contributions
BPB performed the ophthalmologic examination and composed this manuscript. RK and CS performed the molecular analysis on the AAAS gene. RCC performed and interpreted the pupillography. CAS performed the physical examination on the subject and made the diagnosis of triple-A syndrome. JL provided clinical examination and follow-up in the subject's home town.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
We would like to thank Stan Mackowiak, M.D. and Susan E. Stred, M.D., SUNY Upstate Medical University, for clinical care of the patient. We thank the subject's parents for consenting and the subject for assenting to the publication of this article.
Contributor Information
Brian P Brooks, Email: brooksb@mail.nih.gov.
Robert Kleta, Email: kletar@mail.nih.gov.
Rafael C Caruso, Email: rc21a@nih.gov.
Caroline Stuart, Email: cstuart@mail.nih.gov.
Jonathan Ludlow, Email: Jeepopto@aol.com.
Constantine A Stratakis, Email: stratakc@cc1.nichd.nih.gov.
References
- Allgrove J, Clayden GS, Grant DB, Macaulay JC. Familial glucocorticoid deficiency with achalasia of the cardia and deficient tear production. Lancet. 1978;1:1284–1286. doi: 10.1016/S0140-6736(78)91268-0. [DOI] [PubMed] [Google Scholar]
- Grant DB, Dunger DB, Smith I, Hyland K. Familial glucocorticoid deficiency with achalasia of the cardia associated with mixed neuropathy, long-tract degeneration and mild dementia. Eur J Pediatr. 1992;151:85–89. doi: 10.1007/BF01958948. [DOI] [PubMed] [Google Scholar]
- El-Rayyes Kalid, Hegab Samiha, Besisso Mohammed. A syndrome of alacrima, achlasia, and neuroloogic anomalies without adremocortical insufficiency. J Pediatr Ophthalmol Strabismus. 1991;28:35–37. [PubMed] [Google Scholar]
- Mullaney PB, Weatherhead R, Millar L, Ayyash II, Ayberk H, Cai F, Risco JM. Keratoconjunctivitis sicca associated with achalasia of the cardia, adrenocorticalinsufficiency, and lacrimal gland degeneration. Ophthalmology. 1998;105:643–650. doi: 10.1016/S0161-6420(98)94018-0. [DOI] [PubMed] [Google Scholar]
- Makari G, Hoffman WH, Carroll JE, Savage DR, Van Der Zalm T. Autonomic dysfunction and adrenocortical unresponsiveness. J Child Neurol. 1988;3:174–176. doi: 10.1177/088307388800300304. [DOI] [PubMed] [Google Scholar]
- Ehrich E, Aranoff G, Johnson WG. Familial achalasia associated with adrenocortical insufficiency, alacrima, and neurological abnormalities. Am J Med Genet. 1987;26:637–644. doi: 10.1002/ajmg.1320260319. [DOI] [PubMed] [Google Scholar]
- Houlden H, Smith S, de Carvalho M, Blake J, Mathias C, Wood NW, Reilly MM. Clinical and genetic characterization of families with triple A (Allgrove) syndrome. Brain. 2002;125:2681–2690. doi: 10.1093/brain/awf270. [DOI] [PubMed] [Google Scholar]
- Tsilou E, Stratakis CA, Rubin BI, Hay BN, Patronas N, Kaiser-Kupfer MI. Ophthalmic manifestations of Allgrove syndrome: report of a case. Clin Dysmorphol. 2001;10:231–233. doi: 10.1097/00019605-200107000-00016. [DOI] [PubMed] [Google Scholar]
- Prpic I, Huebner A, Persic M, Handschug K, Pavletic M. Triple A syndrome: genotype-phenotype assessment. Clin Genet. 2003;63:415–417. doi: 10.1034/j.1399-0004.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- Tullio-Pelet A, Salomon R, Hadj-Rabia S, Mugnier C, de Laet M, Chaouachi B, Bakiri F, Brottier P, Cattoloci L, Penet C, Begeot M, Naville D, Nicolino M, Chaussain JL, Weissenbach J, Munnich A, Lyonnet S. Mutant WD-repeat protein in triple-A syndrome. Nat Genet. 2000;26:332–335. doi: 10.1038/81642. [DOI] [PubMed] [Google Scholar]
- Handschug K, Sperling S, Yoon S-JK, Henning S, Clark AJL, Huebner A. Triple A syndrome is caused by mutations in AAAS, a new WD-repeat protein gene. Hum Mol Genet. 2001;10:283–290. doi: 10.1093/hmg/10.3.283. [DOI] [PubMed] [Google Scholar]
- Sandrini F, Farmakidis C, Kirschner LS, Wu SM, Tullio-Pelet A, Lyonnet S, Metzger DL, Bourdony CJ, Tiosano D, Chan WY, Stratakis CA. Spectrum of mutations of the AAAS gene in Allgrove syndrome: lack of mutations in six kindreds with isolated resistance to corticotropin. J Clin Endocrinol Metab. 2001;86:5433–5437. doi: 10.1210/jc.86.11.5433. [DOI] [PubMed] [Google Scholar]
- Schmittmann-Ohters K, Huebner A, Richter-Unruh A, Hauffa BP. Clinical and novel molecular findings in a 6.8-year-old Turkish boy with triple A syndrome. Horm Res. 2001;56:67–72. doi: 10.1159/000048093. [DOI] [PubMed] [Google Scholar]
- Goizet C, Catargi B, Tison F, Tullio-Pelet A, Hadj-Rabia S, Pujol F, Lagueny A, Lyonnet S, Lacombe D. Progressive bulbospinal amyotrophy in triple A syndrome with AAAS gene mutation. Neurology. 2002;58:962–965. doi: 10.1212/wnl.58.6.962. [DOI] [PubMed] [Google Scholar]
- Katsumata M, Hirose H, Kagami M, Tanaka T. Analysis of the AAAS gene in Japanese patients with triple A syndrome. Endocr J. 2002;49:49–53. doi: 10.1507/endocrj.49.49. [DOI] [PubMed] [Google Scholar]
- Huebner A, Kaindl A<, Braun R, Handschug K. New insights into the molecular basis of the triple A syndrome. Endocr Res. 2002;28:733–739. doi: 10.1081/ERC-120016998. [DOI] [PubMed] [Google Scholar]
- Reshmi-Skarja S, Huebner A, Handschug K, Finegold DN, Clark AJ, Gollin SM. Chromosomal fragility in patients with triple A syndrome. Am J Med Genet. 2003;117A:30–36. doi: 10.1002/ajmg.a.10846. [DOI] [PubMed] [Google Scholar]
- Roubergue A, Apartis E, Vidailhet M, Mignot C, Tullio-Pelet A, Lyonnet S, de Villemeur TB. Myoclonus and generalized digestive dysmotility in triple A syndrome with AAAS gene mutation. Mov Disord. 2004;19:344–346. doi: 10.1002/mds.10660. [DOI] [PubMed] [Google Scholar]
- Yuksel B, Braun R, Topaloglu AK, Mungan NO, Ozer G, Huebner A. Three children with triple A ayndrome due to a mutation (R478X) in the AAAS gene. Horm Res. 2004;61:3–6. doi: 10.1159/000075190. [DOI] [PubMed] [Google Scholar]
- Ruskell GL. Changes in nerve terminals and acini of the lacrimal gland and changes in secretion induced by autonomic denervation. Z Zellforsch Mikrosk Anat. 1969;94:261–281. doi: 10.1007/BF00339361. [DOI] [PubMed] [Google Scholar]
- Dunnington JH. Congenital alacrima in familial autonomic dysfunction. Trans Am Ophthalmol Soc. 1954;52:23–32. [PMC free article] [PubMed] [Google Scholar]
- Seifert P, Spitznas M. Demonstration of nerve fibers in human accessory lacrimal glands. Graefes Arch Clin Exp Ophthalmol. 1994;232:107–114. doi: 10.1007/BF00171672. [DOI] [PubMed] [Google Scholar]
- Smith TF, Gaitatzes C, Saxena K, Neer EJ. The WD repeat: a common architecture for diverse functions. Trends Biochem Sci. 1999;24:181–185. doi: 10.1016/S0968-0004(99)01384-5. [DOI] [PubMed] [Google Scholar]
- Cronshaw JM, Krutchinsky AN, Zhang W, Chait BT, Matunis MJ. Proteomic analysis of the mammalian nuclear pore complex. J Cell Biol. 2002;158:915–927. doi: 10.1083/jcb.200206106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronshaw JM, Matunis MJ. The nuclear pore complex protein ALADIN is mislocalized in triple A syndrome. Proc Natl Acad Sci U S A. 2003;100:5823–5827. doi: 10.1073/pnas.1031047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Dunnen JT, Antonarakis SE. Nomenclature for the description of human sequence variations. Hum Genet. 2001;109:121–124. doi: 10.1007/s004390100505. [DOI] [PubMed] [Google Scholar]


