To the editor:
Dysregulation in platelet production and abnormal platelet counts have been well known as a predictor of adverse outcomes in patients with infection, cancer, and end-stage renal disease.1-3 The prevalence rates of thrombocytopenia and thrombocytosis among people older than 64 years of age are 6% and 0.9%, respectively.4 Aging plays an important role in the rising prevalence of chronic diseases and results in high mortality and morbidity among older people. However, few studies have assessed the impact of platelet counts on clinical outcomes among healthy populations, particularly among older people.5-7 Therefore, we aimed to examine the association between platelet count and mortality in a large-scale community-dwelling elderly cohort based on the records of the Taipei City Elderly Health Examination Database, which was designed to provide annual health examinations for citizens ≥65 years of age supported by the Taipei City government. All Taipei citizens who met the age criteria were encouraged to participate in this voluntary health examination program and gave written informed consent so that their medical records could be used for research purposes. In addition, the Taipei City government contracted with hospitals to ensure that all qualified hospitals used an identical standardized examination protocol. Detailed information on the health examination and data offered by the Taipei City Elderly Health Examination Database has been described previously.8 To protect privacy, the Department of Health of the Taipei City government provided deidentified data of every participant; this database can be accessed by the public for research purposes. The institutional review board of the Taipei City Hospital approved this study (TCHIRB-1010323-E and TCHIRB-1030601-W).
The present study consisted of participants ≥65 years of age without a history of end-stage renal disease or kidney transplantation due to interference with platelet function by immunosuppressant and uremia per se. All had undergone at least 1 platelet count measurement between 2001 and 2010. Eligible individuals were categorized into 5 strata according to the platelet counts of <100, 100 to <200, 200 to <300, 300 to <400, and ≥400 × 109/L. The primary outcome was all-cause mortality, and the cause of death was classified into 4 categories, including cardiovascular mortality, cancer-related mortality, mortality due to severe sepsis or septic shock, and mortality due to other causes. Cox proportional hazard models were used to assess the association between each study end point and different categories of platelet count. Any variables with P values of <.1 in univariate analysis, as well as conventional risk factors of a poor outcome in the general population, were considered as potential confounding factors and were entered into the multivariate analysis. Because platelet counts may be associated with the subsequent development of cardiovascular disease, we excluded participants with a history of cardiovascular diseases at baseline for the analysis of cardiovascular death. Information on mortality of these participants was collected by linking our database with the Taiwan National Death Registry through a unique personal identification number. All of the participants were followed until death or until December 31, 2010.
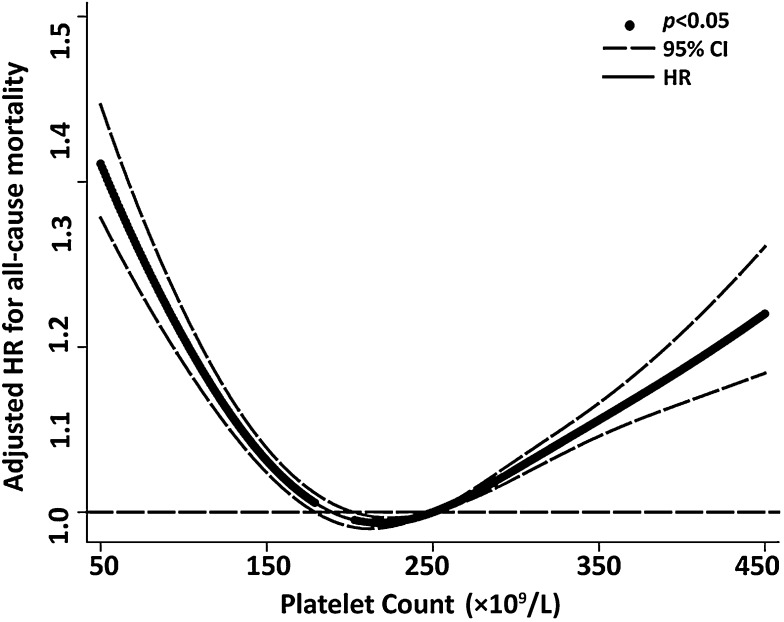
Baseline characteristics of the cohort are shown in supplemental Table 1 (see supplemental Data available on the Blood Web site). In total, 131 308 older people with a mean age of 72.6 ± 6.3 years enrolled in this study. During a mean follow-up of 5.8 ± 2.9 years, there were 17 759 all-cause deaths (13.5%). The incidence rates and HR of all-cause and cause-specific mortality among the participants in the 5 platelet count strata are shown in Table 1. Compared with older people with platelet counts between 200 and <300 × 109/L (reference group), adjusted HRs of all-cause mortality in those with platelet counts of <100, 100 to <200, 300 to <400, and ≥400 × 109/L were 1.93 (95% CI, 1.77-2.11), 1.08 (1.05-1.12), 1.18 (1.11-1.25), and 1.67 (1.49-1.87), respectively. A cubic spline was plotted to show this U-shaped relationship between platelet counts and overall mortality (Figure 1). The U-shaped mortality curve associated with platelet counts was also observed for all cause-specific deaths among older people.
Table 1.
Incidence and risks of all-cause and cause-specific mortality in older people
| Platelet count, ×109/L | No. of events | No. of person-years | Incidence rate* | Cox proportional hazard models | |||
|---|---|---|---|---|---|---|---|
| Crude HR (95% CI) | P | Adjusted HR† (95% CI) | P | ||||
| All-cause mortality | |||||||
| <100 | 602 | 10 730 | 56.10 | 2.80 (2.58-3.04) | <.001 | 1.93 (1.77-2.11) | <.001 |
| 100 to <200 | 7598 | 304 486 | 24.95 | 1.25 (1.21-1.29) | <.001 | 1.08 (1.05-1.12) | <.001 |
| 200 to <300 | 7827 | 393 530 | 19.89 | 1 | 1 | ||
| 300 to <400 | 1384 | 52 724 | 26.25 | 1.33 (1.26-1.41) | <.001 | 1.18 (1.11-1.25) | <.001 |
| ≥400 | 348 | 5 987 | 58.13 | 3.00 (2.69-3.33) | <.001 | 1.67 (1.49-1.87) | <.001 |
| Cardiovascular mortality‡ | |||||||
| <100 | 72 | 9 256 | 7.78 | 1.78 (1.41-2.26) | <.001 | 1.24 (0.97-1.59) | .086 |
| 100 to <200 | 1405 | 267 048 | 5.26 | 1.20 (1.12-1.29) | <.001 | 1.06 (0.98-1.14) | .151 |
| 200 to <300 | 1529 | 350 235 | 4.37 | 1 | 1 | ||
| 300 to <400 | 215 | 47 125 | 4.56 | 1.05 (0.91-1.21) | .511 | 0.96 (0.82-1.11) | .568 |
| ≥400 | 50 | 5 233 | 9.55 | 2.17 (1.63-2.89) | <.001 | 1.38 (1.02-1.86) | .037 |
| Thrombotic CV mortality‡ | |||||||
| <100 | 57 | 9 256 | 6.16 | 1.59 (1.22-2.07) | .001 | 1.10 (0.84-1.45) | .494 |
| 100 to <200 | 1223 | 267 048 | 4.58 | 1.18 (1.09-1.28) | <.001 | 1.04 (0.95-1.12) | .406 |
| 200 to <300 | 1355 | 350 235 | 3.87 | 1 | 1 | ||
| 300 to <400 | 191 | 47 125 | 4.05 | 1.05 (0.90-1.22) | .518 | 0.94 (0.80-1.11) | .487 |
| ≥400 | 47 | 5 233 | 8.98 | 2.30 (1.71-3.09) | <.001 | 1.44 (1.06-1.97) | .021 |
| Hemorrhagic CV mortality‡ | |||||||
| <100 | 15 | 9 256 | 1.62 | 3.28 (1.94-5.56) | <.001 | 2.32 (1.33-4.04) | .003 |
| 100 to <200 | 182 | 267 048 | 0.68 | 1.36 (1.11-1.68) | .004 | 1.21 (0.97-1.52) | .094 |
| 200 to <300 | 174 | 350 235 | 0.50 | 1 | 1 | ||
| 300 to <400 | 24 | 47 125 | 0.51 | 1.03 (0.67-1.58) | .884 | 1.08 (0.71-1.67) | .720 |
| ≥400 | 3 | 5 233 | 0.57 | 1.17 (0.37-3.66) | .790 | 0.91 (0.29-2.89) | .875 |
| Cancer mortality | |||||||
| <100 | 233 | 10 730 | 21.71 | 3.47 (3.03-3.97) | <.001 | 2.66 (2.31-3.05) | <.001 |
| 100 to <200 | 2392 | 304 486 | 7.86 | 1.26 (1.19-1.33) | <.001 | 1.10 (1.03-1.16) | .003 |
| 200 to <300 | 2448 | 393 530 | 6.22 | 1 | 1 | ||
| 300 to <400 | 457 | 52 724 | 8.67 | 1.40 (1.26-1.54) | <.001 | 1.31 (1.18-1.45) | <.001 |
| ≥400 | 106 | 5 987 | 17.71 | 2.86 (2.35-3.48) | <.001 | 1.91 (1.55-2.35) | <.001 |
| Sepsis mortality | |||||||
| <100 | 61 | 10 730 | 5.68 | 2.75 (2.12-3.57) | <.001 | 1.74 (1.32-2.29) | <.001 |
| 100 to <200 | 780 | 304 486 | 2.56 | 1.25 (1.13-1.38) | <.001 | 1.06 (0.95-1.18) | .276 |
| 200 to <300 | 806 | 393 530 | 2.05 | 1 | 1 | ||
| 300 to <400 | 142 | 52 724 | 2.69 | 1.29 (1.08-1.55) | <.001 | 1.06 (0.87-1.28) | .583 |
| ≥400 | 52 | 5 987 | 8.69 | 4.31 (3.25-5.70) | <.001 | 1.85 (1.37-2.49) | <.001 |
| Mortality due to other causes | |||||||
| <100 | 207 | 10 730 | 19.29 | 2.82 (2.45-3.25) | <.001 | 1.84 (1.59-2.13) | <.001 |
| 100 to <200 | 2585 | 304 486 | 8.49 | 1.25 (1.18-1.32) | <.001 | 1.08 (1.02-1.14) | .009 |
| 200 to <300 | 2665 | 393 530 | 6.77 | 1 | 1 | ||
| 300 to <400 | 500 | 52 724 | 9.48 | 1.43 (1.30-1.57) | <.001 | 1.22 (1.10-1.34) | <.001 |
| ≥400 | 129 | 5 987 | 21.55 | 3.32 (2.78-3.95) | <.001 | 1.56 (1.30-1.89) | <.001 |
CI, confidence interval; CV, cardiovascular; HR, hazard ratio.
Per 1000 person-years.
Adjusted for age, sex, smoking, alcohol use, systolic and diastolic pressure, pulse rate, estimated glomerular filtration rate, diabetes mellitus, hypertension, coronary artery disease, cerebrovascular disease, total serum cholesterol, triglyceride, high-density lipoprotein cholesterol, hemoglobin, fasting glucose, serum albumin, body mass index, white blood cell count, and uric acid.
For the analysis of cardiovascular death, we excluded older people with a history of cardiovascular disease at baseline. Finally, 114 944 participants without cardiovascular disease entered this model.
Figure 1.
Adjusted HR for all-cause mortality according to platelet counts in all 131 308 older people. A cubic spline curve was plotted to show a U-shaped curve relationship between platelet counts and overall mortality.
The causes of cardiovascular mortality were further divided into thrombotic (eg, myocardial infarction, peripheral artery disease, and ischemic stroke) and hemorrhagic (eg, intracerebral hemorrhage and subarachnoid hemorrhage) categories. When compared with participants with normal platelet counts, those with platelet counts ≥400 × 109/L (HR, 1.44; 95% CI, 1.06-1.97) and below 100 × 109/L (HR, 2.32; 95% CI, 1.33-4.04) were associated with significantly higher risks of thrombotic and hemorrhagic cardiovascular mortality, respectively.
To our knowledge, this is the largest cohort to be examined for the association between abnormal platelet count and mortality among older people. We demonstrated that the platelet count exhibits a U-shaped association with all-cause and cause-specific mortality independent of other risk factors. Moreover, thrombocytopenia is associated with hemorrhagic cardiovascular deaths, whereas a platelet count ≥400 × 109/L is related to increased risk of cardiovascular mortality due to thrombotic events. These results are consistent with previous data, which demonstrated that intracerebral hemorrhage was more common in elderly people with chronic immune thrombocytopenia, whereas interactions between platelets and other cells within the microvasculature may contribute to the development of atherosclerosis.9,10
Some limitations in this study should be acknowledged. First, information on the use of antiplatelet drugs was not available in this analysis. Second, we did not measure platelet function. Third, all participants in this study are ethnically Han Chinese and thus the results may not be generalizable to other populations. Finally, our observational study may suffer from residual confounding. Therefore, it is altogether unlikely that platelet count would have a causal effect on noncardiovascular mortality, and the results found that platelet counts could be used as a prognostic factor to predict mortality among community-dwelling older people.
Footnotes
The online version of this article contains a data supplement.
Authorship
Acknowledgments: This study was based in part on the data from the Taipei City Public Health Database, provided by the Department of Health of the Taipei City government and managed by the Databank for Public Health Analysis. The interpretations and conclusions contained herein do not represent those of the Taipei City government.
This study was supported by grants from the Ministry of Science and Technology (MOST 102-2314-B-010-004-MY3), Taipei Veterans General Hospital (V102C-129, V103C-024), and Taipei City Hospital (10102-62-083) to D.-C.T.; the Foundation for Poison Control; and the Ministry of Education’s Aim for the Top University Plan.
Contribution: D.-C.T. designed the research; M.-T.T. performed the research and drafted the manuscript; Y.-T.C. performed the research and analyzed the data; C.-H.L. and T.-P.H. carried out the data management; and all authors read, critically reviewed, and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Der-Cherng Tarng, Division of Nephrology, Department of Medicine, Taipei Veterans General Hospital, 201, Section 2, Shih-Pai Rd, Taipei 11217, Taiwan; e-mail: dctarng@vghtpe.gov.tw.
References
- 1.Mirsaeidi M, Peyrani P, Aliberti S, et al. Thrombocytopenia and thrombocytosis at time of hospitalization predict mortality in patients with community-acquired pneumonia. Chest. 2010;137(2):416–420. doi: 10.1378/chest.09-0998. [DOI] [PubMed] [Google Scholar]
- 2.Suppiah R, Shaheen PE, Elson P, et al. Thrombocytosis as a prognostic factor for survival in patients with metastatic renal cell carcinoma. Cancer. 2006;107(8):1793–1800. doi: 10.1002/cncr.22237. [DOI] [PubMed] [Google Scholar]
- 3.Molnar MZ, Streja E, Kovesdy CP, et al. High platelet count as a link between renal cachexia and cardiovascular mortality in end-stage renal disease patients. Am J Clin Nutr. 2011;94(3):945–954. doi: 10.3945/ajcn.111.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biino G, Balduini CL, Casula L, et al. Analysis of 12,517 inhabitants of a Sardinian geographic isolate reveals that predispositions to thrombocytopenia and thrombocytosis are inherited traits. Haematologica. 2011;96(1):96–101. doi: 10.3324/haematol.2010.029934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Bom JG, Heckbert SR, Lumley T, et al. Platelet count and the risk for thrombosis and death in the elderly. J Thromb Haemost. 2009;7(3):399–405. doi: 10.1111/j.1538-7836.2008.03267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thaulow E, Erikssen J, Sandvik L, Stormorken H, Cohn PF. Blood platelet count and function are related to total and cardiovascular death in apparently healthy men. Circulation. 1991;84(2):613–617. doi: 10.1161/01.cir.84.2.613. [DOI] [PubMed] [Google Scholar]
- 7.Msaouel P, Lam AP, Gundabolu K, et al. Abnormal platelet count is an independent predictor of mortality in the elderly and is influenced by ethnicity. Haematologica. 2014;99(5):930–936. doi: 10.3324/haematol.2013.101949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen YT, Shih CJ, Ou SM, Hung SC, Lin CH, Tarng DC Taiwan Geriatric Kidney Disease (TGKD) Research Group. Periodontal disease and risks of kidney function decline and mortality in older people: a community-based cohort study. Am J Kidney Dis. 2015;66(2):223–230. doi: 10.1053/j.ajkd.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Neunert C, Noroozi N, Norman G, et al. Severe bleeding events in adults and children with primary immune thrombocytopenia: a systematic review. J Thromb Haemost. 2015;13(3):457–464. doi: 10.1111/jth.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan ZS, Jackson SP. The role of platelets in atherothrombosis. Hematology Am Soc Hematol Educ Program. 2011. 2011:51-61. [DOI] [PubMed]