Abstract
Background
There are many treatment options for metastatic colorectal cancer (CRC). However, national treatment patterns for metastatic CRC, and the stability of hospital treatment patterns over time, have not been well described.
Methods
We used data from the 2006–2011 National Cancer Data Base to study adults with newly diagnosed metastatic CRC (N=84,161 patients in 1051 hospitals). Using hierarchical models, we characterized hospital volume in the use of different treatment modalities (primary site resection, metastatic site resection, chemotherapy, and palliative care). We then assessed variation in the receipt of treatment according to hospitals’ relative volume of services used. Finally, we examined the extent to which hospital treatment patterns changed over the past decade.
Results
Overall use of volume of services varied widely (5.0% in the hospitals with low volumes of service (HLVS) to 22.3% in the hospitals with high volumes of service (HHVS)). As hospitals’ volumes of services increased, adjusted rates of metastatic site surgery (6.6% to 30.8%, p < 0.001) and multi-agent chemotherapy (37.8% to 57.4%; p < 0.001) utilization increased, but primary site resection varied little (56.8% vs. 59.5%; p= 0.024). Interestingly, utilization of palliative care also increased (8.1% to 11.3%; p=0.002). Hospital treatment patterns did not change over time, with HHVS consistently utilizing more metastatic site resection and multi-agent chemotherapy than HLVS.
Conclusion
There is wide variation in hospital treatment patterns for metastatic CRC and these patterns have been stable over time. It appears that much of the approach for metastatic CRC treatment depends upon the hospital where the patient presents.
INTRODUCTION
Nearly 20% of patients with colorectal cancer (CRC) present with metastasis at the time of diagnosis.1–3 Historically, liver metastasis portended a dismal prognosis because of limited therapeutic options.2,4 Over the last 20 years, however, there have been expansions in both systemic treatment options and liver-directed interventions, including improved results with liver surgery because of better techniques and more specialty-trained surgeons.5–12 With contemporary treatment modalities, recent reports cite a nearly 30-month survival with metastatic CRC, compared to historic survival rates of 8 months without treatment.13–15
Nevertheless, there is little consensus regarding optimal treatments for this patient population,16,17 and increasing concerns that patients are not being appropriately referred for chemotherapy and surgical resection.18,19 To date, national patterns of care for patients with metastatic CRC have not been well described. While decisions for any given patient with metastatic CRC depend on clinicopathologic characteristics and individual preferences, it is possible that treatments are influenced by where a patient presents for care. In addition, how quickly clinical advances for metastatic CRC diffuse across the country has not previously been fully examined.
In the present study, we used national clinical registry data to assess treatment patterns for patients with metastatic CRC. Our goal was to determine the degree to which different treatment modalities such as metastatic site surgery or multi-agent chemotherapy can characterize a hospital’s overall “aggressiveness” in treatment. We also assessed what hospital attributes were associated with treatment, and to what extent treatment patterns have changed over the past decade.
METHODS
Data source and study population
We utilized data from the National Cancer Data Base (NCDB) Participant User File (PUF). The NCDB is a joint project of the American College of Surgeons Commission on Cancer (CoC) and the American Cancer Society. Data from over 1500 CoC-accredited hospitals represent nearly 70% of all newly diagnosed cancer patients in the United States and are collected using standardized coding schemata. Details of data abstraction have been previously described, and it is important to note that CoC programs are required to identify treatment that their patients received from all sources, even if treatment occurs at facilities other than theirs.20,21 To allow for attribution of one patient to one hospital, patients were assigned to a CoC-accredited hospital for analysis if they received their first treatment course at the reporting facility.
We identified all patients over age 17 who presented with metastatic cancer of the colon, rectosigmoid, or rectum from 2006–2011 using International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) topographical and histology codes. Patients with unknown treatment status or missing information regarding metastatic disease assessment were excluded from analysis. We also excluded patients who were treated at hospitals with less than 20% chemotherapy administration for stage III CRC, as this likely reflects incomplete documentation,22,23 and patients at hospitals with very low annual stage IV patient volume (< 5 stage patients with metastatic CRC per year) to reduce random variation caused by small sample sizes.
In order to stratify hospitals for analysis, we defined use of a high volume of services based on the proportion of patients in each hospital receiving all three of the following modalities for their disease: primary site resection, metastatic site resection, and chemotherapy (single- or multi-agent). Metastatic site resection was defined as resection of disease from distant organ sites and/or distant (non-regional) lymph node basins. Surgical treatment denoted in the PUF as palliative care treatment was not considered metastatic site resection. Other outcomes included receipt of single or multi-agent chemotherapy, primary site resection (excluding destruction, fulguration, and transanal excisions when used as a solitary treatment) and palliative care.
Statistical analysis
Variation in hospital volume of services
We first assessed variation in hospitals’ treatment strategies. To do this, we used random-intercept hierarchical logistic regression models specifying the hospital as the higher level, and adjusted all outcomes for patient age, sex, race, as well as their interactions, patient primary insurer, median ZIP-code income and education levels, Charlson/Deyo score24 and primary tumor location as covariates. Hierarchical models account for within- and between-hospital outcome variation and minimize the influence of random variation from small sample sizes on model-based estimates.25–27 Hospitals were grouped into equal quintiles of utilization (“volumes of service”) based on the adjusted proportions of patients receiving primary and metastatic site resections as well as chemotherapy.
Patient demographics and tumor characteristics as well as hospital attributes (CoC accreditation status and US census region) were compared across hospital quintiles, ranging from hospitals with low volumes of service (HLVS) to hospitals with high volumes of service (HHVS). In addition, we examined adjusted rates of chemotherapy use (single-agent and multi-agent), primary site resection, metastatic site resection, and use of palliative care services to determine the relative utilization of treatments across hospital quintiles.
Hospital treatment patterns over time
We assessed temporal changes in hospital treatment patterns. To do this, we used additional data from the NCDB PUF for years 2003 and 2004 in order to characterize hospitals’ volume of services in 2003–2004 and grouped them into quintiles using the same methods described above. We then used hospitals’ volume of services in 2003–2004 as a benchmark to compare their treatment rates over three subsequent time periods: 2005–2006, 2007–2008, and 2009–2011. The earlier data points were not used for the primary analysis. Specifically, we assessed adjusted rates of overall volume of services, as well as use of primary site resection, metastatic site resection, multi-agent chemotherapy and palliative care for each time period.
Finally, to assess the robustness of our findings, we performed several sensitivity analyses. In one, we restricted the study cohort to patients with definitive T staging of their primary tumor, as these patients were more likely to have undergone resection of their primary site of disease. In another, we restricted the cohort to ‘average risk’ patients, defined a priori as those younger than 80 years, who did not refuse any treatment modality, and were not deemed high-risk by their treating physician. In another, we excluded treatment with single agent chemotherapy, therefore only defining aggressive treatment as multi-agent chemotherapy in addition to primary site and metastatic site resections.
We performed all statistical analyses using Stata Release 12 (StataCorp, College Station TX). All reported P-values are two-sided with alpha set at p=0.05. Model discrimination was evaluated using the c-statistic and calibration was assessed with the Hosmer-Lemeshow goodness of fit test.28,29 The study protocol was reviewed and deemed “not regulated” by the University of Michigan Institutional Review Board.
RESULTS
We initially identified 107,393 patients with metastatic CRC in the 2006–2011 NCDB PUF. After applying exclusion criteria, our cohort included 84,161 patients treated in 1051 hospitals, as shown in Figure 1. Over the study period, 48,525 (57.7%) underwent non-palliative primary site resection, 47,026 (55.9%) received chemotherapy (40,203 (47.7%) multi-agent and 6,823 (8.1%) single-agent treatments), and 15,847 (18.8%) had non-palliative resection of metastatic disease. All three therapies were utilized in 8,688 (10.3%) patients.
Figure 1.

Study cohort derivation
Patient and hospital characteristics, stratified by quintiles, ranging from HLVS to HHVS, are shown in Table 1. Receipt of treatments varied from 5.0% in the LHVS hospitals to 22.3% in the HHVS (trend p-value < 0.001). Compared with HLVS quintile, patients in the HHVS were, on average, slightly younger, more frequently Black, more likely to have private insurance and more likely to have multiple comorbid conditions as measured by a Charlson/Deyo score of at least 2. Hospitals in the HHVS quintile were more likely to have an Academic/Research classification (28.6% vs. 16.6%) and were more likely located in the Great Lakes/Midwest regions (35.7% vs. 19.9%) compared to those in the HLVS quintile. There was a relative decrease in the representation of Comprehensive Community Cancer centers in the HHVS quintile (52.9% vs. 61.1%). Patient volumes were higher in the HHVS quintile hospitals (18.9 vs 14.0 patients/yr).
TABLE 1.
Hospital and patient characteristics across quintiles of hospital volume of services*
| QUINTILE OF AGGRESSIVENESS | Q1 HLVS | Q2 | Q3 Average |
Q4 | Q5 HHVS |
|---|---|---|---|---|---|
| N hospitals` | 211 | 210 | 210 | 210 | 210 |
|
Adjusted receipt of high volume of services, % patients |
5.0 | 7.8 | 10.6 | 14.7 | 22.3 |
| HOSPITAL CHARACTERISTICS |
N (%) of hospitals |
||||
| Stage IV patients/y (mean, sd) a | 13.8 (8.4) | 13.3 (9.6) | 16.1 (11.7) | 16.0 (10.8) | 18.9 (15.6) |
| Facility type a,b | |||||
| Community CC | 45 (21.3) | 59 (28.1) | 43 (20.5) | 44 (21.0) | 39 (18.6) |
| Comprehensive Community CC | 129 (61.1) | 130 (61.9) | 122 (58.1) | 112 (53.3) | 111 (52.9) |
| Academic/Research CC | 35 (16.6) | 21 (10.0) | 44 (21.0) | 54 (25.7) | 60 (28.6) |
| Facility Census region a | |||||
| Atlantic | 27 (12.8) | 32 (15.2) | 37 (17.6) | 34 (16.2) | 27 (12.9) |
| Northeast | 8 (3.8) | 18 (8.6) | 14 (6.7) | 20 (9.5) | 11 (5.2) |
| Great Lakes/Midwest | 43 (20.4) | 43 (20.5) | 51 (24.3) | 70 (33.3) | 77 (36.7) |
| Mountain/West/Pacific | 71 (33.7) | 63 (30.0) | 54 (25.7) | 40 (19.1) | 30 (14.3) |
| South/Southeast | 62 (29.4) | 54 (25.7) | 53 (25.2) | 46 (21.9) | 65 (31.0) |
| PATIENT CHARACTERISTICS |
N (%) of patients |
||||
| Age, y (mean, sd) a | 65.9 (13.9) | 66.6 (14.1) | 66.3 (13.9) | 65.7 (14.2) | 64.5 (14.2) |
| Female sex | 7312 (47.9) | 6788 (47.9) | 8259 (47.9) | 8313 (48.4) | 9651 (47.6) |
| Race a | |||||
| White | 10339 (67.7) | 9662 (68.1) | 12054 (69.9) | 11897 (69.3) | 14259 (70.3) |
| Black | 2357 (15.4) | 1816 (12.8) | 2270 (13.2) | 2370 (13.8) | 3070 (15.1) |
| Hispanic | 970 (6.4) | 1176 (8.3) | 1094 (6.3) | 688 (4.0) | 612 (3.0) |
| Asian/Pacific Islander | 494 (3.2) | 470 (3.3) | 398 (2.3) | 406 (2.4) | 460 (2.3) |
| Other | 1107 (7.3) | 1056 (7.4) | 1437 (8.3) | 1805 (10.5) | 1894 (9.3) |
| High school degree (%) by ZIP codea | |||||
| >80% | 7107 (46.6) | 6673 (47.1) | 6910 (40.1) | 6529 (38.0) | 8350 (41.1) |
| 80% or less | 7412 (48.5) | 6691 (47.2) | 9200 (53.3) | 9725 (56.7) | 10811 (53.3) |
| Unknown | 748 (4.9) | 816 (5.8) | 1143 (6.6) | 912 (5.3) | 1134 (5.6) |
| ZIP-code median income a | |||||
| <$34,999 | 5858 (38.4) | 4578 (32.3) | 5706 (33.1) | 5385 (31.4) | 6712 (33.1) |
| >$35,000 | 8663 (56.7) | 8787 (62.0) | 10404 (60.3) | 10869 (63.3) | 12449 (61.3) |
| Unknown | 746 (4.9) | 815 (5.7) | 1143 (6.6) | 912 (5.3) | 1134 (5.6) |
| Primary insurer a | |||||
| Private | 4950 (32.4) | 4830 (34.1) | 5913 (34.3) | 6171 (35.9) | 7769 (38.3) |
| Medicare | 7486 (49.0) | 7215 (50.9) | 8749 (50.7) | 8377 (48.8) | 9749 (48.0) |
| Other | 1789 (11.7) | 1402 (9.9) | 1649 (9.6) | 1755 (10.2) | 1884 (9.3) |
| Not insured | 1042 (6.8) | 733 (5.2) | 942 (5.5) | 863 (5.0) | 893 (4.4) |
| Charlson/Deyo score ≥2 a | 1058 (6.9) | 985 (6.9) | 1282 (7.4) | 1350 (7.9) | 1563 (7.7) |
| Primary tumor location a | |||||
| Colon | 11436 (74.9) | 10650 (75.1) | 12793 (74.1) | 12732 (74.2) | 14983 (73.8) |
| Rectosigmoid junction | 1306 (8.6) | 1187 (8.4) | 1500 (8.7) | 1412 (8.2) | 1691 (8.3) |
| Rectum | 2525 (16.5) | 2343 (16.5) | 2960 (17.2) | 3022 (17.6) | 3621 (17.8) |
Models used to derive hospital-adjusted service volumes accounted for patient age, sex, race, insurance type, ZIP-code level income and education levels, comorbidities, and primary tumor location
Univariate P-value (from ANOVA/Pearson chi-squared test) <0.05;
CC: Cancer Center
Variation in treatment modalities as well as palliative care utilization across hospitals is shown in Figure 2. What constituted the services rendered for cancer treatment varied differently across hospitals. For example, metastatic site surgery rates varied the most across hospitals (6.6% in the HLVS to 30.8% in the HHVS; trend p-value < 0.001), while primary site resections varied least (56.8% vs. 59.5%, respective; trend p-value 0.024). Use of multi-agent chemotherapy increased as hospital overall aggressiveness increased (37.8% to 57.4%; trend p-value < 0.001), while single-agent chemotherapy use was similar across hospitals (8.1% to 8.3%; trend p-value 0.249). Adjusted rates of palliative care were lowest in the HLVS, and tended to increase as volumes of services increased (8.1% to 11.3%; trend p-value 0.002). The 2003–2011 study cohort used to assess temporal changes in treatment included 114,068 patients. In general, hospitals’ past treatment patterns were strong predictors of their future treatment patterns, and differences between hospitals in their rates of therapy utilization did not appreciably change over time, as shown in Table 2. HHVS (based on past treatment rates) had consistently higher rates of metastatic site resection, multi-agent chemotherapy and palliative care usage than HLVS over time. Table 2 displays the adjusted odds of receiving various treatments for patients treated in HHVS vs. HLVS based on past treatment patterns, after accounting for patient age, sex, race, insurance type, ZIP-code level income and education levels, comorbidities, and primary tumor location. For example, in 2005–06 a patient treated in a HHVS vs. HLVS had 2.85 the odds (95% CI 2.38-3.40) of having metastatic site surgery. In 2009–11, the same patient had 2.08 the odds (95% CI 1.75-2.47) of having metastatic site surgery. In contrast, primary site resection rates were similar across quintiles over time, and converged by the most recent time period evaluated.
Figure 2.
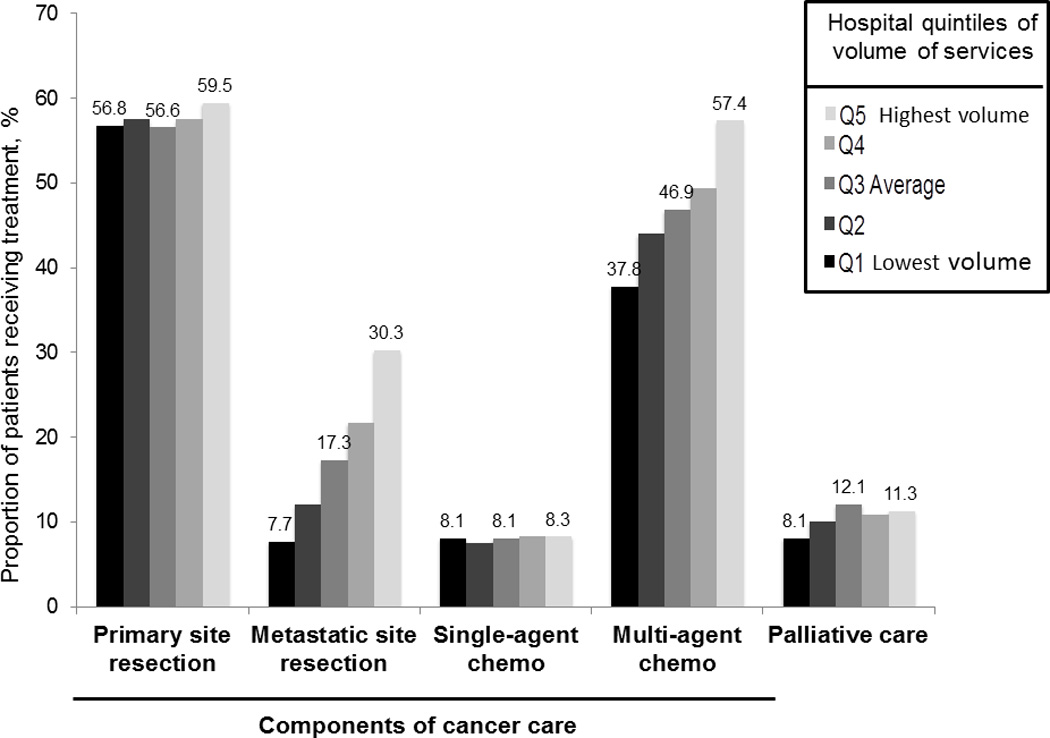
Treatment modality variation across levels of hospital volume of services
TABLE 2.
Effect of hospitals’ past use of a high volume of services on adjusted odds of receiving different treatments a
| Adjusted odds ratio of receiving treatment if diagnosed in a HHVS vs. HLVS based on 2003–2004 treatment patterns |
|||
|---|---|---|---|
| TREATMENT | 2005–2006 | 2007–2008 | 2009–2011 |
| Primary site surgery | 1.13 (1.01–1.27) | 1.02 (0.91–1.14) | 1.00 (0.89–1.13) |
| Metastatic site surgery | 2.85 (2.38–3.40) | 2.31 (1.94–2.76) | 2.08 (1.75–2.47) |
| Multi-agent chemotherapy | 2.05 (1.75–2.41) | 1.87 (1.59–2.19) | 1.73 (1.49–2.01) |
| Palliative care | 1.89 (1.38–2.57) | 1.52 (1.14–2.04) | 1.32 (0.99–1.74) |
Models used to derive hospital-adjusted service volumes accounted for patient age, sex, race, insurance type, ZIP-code level income and education levels, comorbidities, and primary tumor location
Patterns persisted in sensitivity analyses (excluding patients without definitive T staging or excluding elderly patients, those who refused treatment or were deemed too high-risk for any treatment
The sensitivity analyses confirmed the trends we observed. Variation in hospital relative volume of services and its components persisted when assessing ‘average-risk’ patients or those with liver-only metastases. When excluding patients older than 80 years and without high comorbidity burden, hospital treatment rates were generally higher, but the stability of treatment patterns persisted in the same fashion as above. When assessing patients with definitive T staging, rates of multi-agent chemotherapy and metastatic site surgery were increased overall, but with persistent variation across hospitals and the same overall temporal trends as in the primary analysis. Differences in hospital characteristics across the quintiles showed the same trends as in the primary analysis.
DISCUSSION
Using national clinical registry data, we have demonstrated broad variation in hospital treatment patterns for metastatic CRC. There were wide differences in the volume of services rendered, driven in large part by differential rates of metastatic site resection and chemotherapy use across hospitals, rather than differential rates of primary site resection. Interestingly, hospitals’ volume of cancer-directed treatments paralleled their use of palliative care services. Higher volumes of services were notably seen in hospitals with higher levels of cancer center accreditation. Moreover, hospitals’ treatment patterns were stable over the past decade. These findings imply that to a large degree, metastatic CRC treatment is dictated not by the disease itself, but by the cancer program in which the patient is treated.
Patterns in metastatic CRC treatment have been difficult to define. In this study, we found structural and regional differences across volume of services, suggesting that both area and hospital-level attributes contribute to differences in treatment. Regional differences in resource utilization and intensity of care are well described, both from a cost and payment perspective.30–32 Similar variation in volume of services at a hospital level are seen in this large database. These findings imply that while clinicopathologic factors certainly influence treatment, much of the approach towards metastatic CRC depends upon the hospital where the patient is treated. Interestingly, those hospitals with the highest volume of services for the treatment of metastatic colorectal cancer also had generally higher use of palliative care. This finding may reflect nuanced differences in those hospitals’ philosophy towards overall cancer care in general, rather than simply offering “aggressive” care to every patient. Variation within any given hospital could certainly reflect patient preferences, but also could reflect how physicians within a certain hospital approach treatments differently and whether that is more highly influenced by patient or provider characteristics is unknown.
In addition to displaying broad variability, hospital treatment patterns were largely unchanged over the past decade. For other cancers with poor prognoses (e.g., esophagus, pancreas), there has been a trend towards offering more surgical treatments to older and sicker patients.33 Whether patients with metastatic CRC are treated similarly has not been as extensively investigated on a national basis. The complement of efficacious therapies for metastatic disease, including chemotherapy, biological agents, surgical resection, and non-surgical liver directed interventions, continues to expand. 6,7,13,16,34 At the same time, there are more specialty-trained liver surgeons and overall decreases in morbidity and mortality associated with liver surgery.12,35 Despite this, it seems that these advances have not been promulgated across the country and may be limited to only a subset of hospitals which may have already demonstrated an approach toward overall cancer care that is demonstrated by not only a generally higher volume of services but a higher use of palliative care.
It is also possible that differential knowledge and understanding of metastatic CRC treatment options breeds variation in care patterns. It has been demonstrated that rates of adherence to recommended treatment measures for cancer are highly correlated with the level of evidence supporting them.36,37 In contrast to other stages of CRC, there are fewer standardized treatments for metastatic disease that carry strong guideline endorsement and there are few guidelines for use of palliative services.17,31,36,37 Further, the indications and timing for resection, both for the primary tumor and metastases, continue to evolve.9,13,16,38,39 Finally, emerging evidence suggests substantial heterogeneity in physicians’ understanding of what constitutes “resectable” disease.18,40,41
There are important limitations to this study. First, we were unable to assess the extent to which treatment represented appropriate care. However, our intent was to provide an assessment of national treatment pattern variation independent of inferences regarding appropriate care at the individual patient level. Similarly, due to limitations of the PUF data, we could not assess factors relevant to patient selection, surgical decision-making (e.g., size and location of metastases), and type and sequencing of multimodality treatments (e.g., choice of agents and use of agents pre- or post-operatively). However, the observed variation in resection rates was not substantially altered when focusing on “average-risk” patients or patients with liver-only metastases, and therefore is unlikely to be fully explained by differences in patients’ resectability. Second, we were unable to assess patients with metachronous metastatic disease due to lack of this type of data in the NCDB PUF. As such, our results should be interpreted in the context of patients with metastatic disease at presentation. Third, we could not assess utilization of certain liver-directed interventions (such as radiofrequency ablation or trans-arterial chemoembolization) if they were not included as a part of a surgical procedure. Finally, these data represent a group of hospitals meeting stringent structural, process and patient volume accreditation requirements by the CoC, which tend to be generally larger, more frequently urban, and have more cancer-related services available to patients than other hospitals.42 Our results may not be generalizable to all hospitals, but these data are representative of the vast majority of hospitals in the United States most likely to treat this patient population though referral patterns are not able to be defined.
There is broad variation in how hospitals approach metastatic CRC, including the use of palliative care services. Moreover, the stability of hospitals’ treatment patterns implies that a particular hospital’s generalized approach to cancer care may have as strong an influence on patients’ treatment as their disease status. Why such differences in treatment patterns persist are unclear since improved outcomes following certain treatment strategies are widely reported in the literature. Improvement of care may involve improved knowledge dissemination, increased multidisciplinary collaboration between medical and surgical oncologists in treatment decisions, and, when appropriate, increased referral to specialty centers for metastatic CRC.
Acknowledgments
Funding: This work was supported by the National Institutes of Health (5T32CA009672-22 to RWK) and the Agency for Healthcare Research and Quality (1K08 HS20937-01 to SLW) and the American Cancer Society (RSG-12-269-01-CPHPS to SLW).
Footnotes
Disclosures: RWK received payment from Blue Cross/Blue Shield of Michigan for data entry, unrelated to this work. There are no other financial disclosures or conflicts of interest to report.
The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators.
REFERENCES
- 1.Leporrier J, Maurel J, Chiche L, et al. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg. 2006;93:465–474. doi: 10.1002/bjs.5278. [DOI] [PubMed] [Google Scholar]
- 2.Manfredi S, Lepage C, Hatem C, et al. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–259. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R, MA J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer. 2007;109:718–726. doi: 10.1002/cncr.22448. [DOI] [PubMed] [Google Scholar]
- 5.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aloia TA, Vauthey JN. Management of colorectal liver metastases: past, present, and future. Updates Surg. 2011;63:1–3. doi: 10.1007/s13304-011-0054-y. [DOI] [PubMed] [Google Scholar]
- 7.Andreou A, Aloia TA, Brouquet A, et al. Recent advances in the curative treatment of colorectal liver metastases. Gastrointestinal cancer research : GCR. 2011;4:S2–S8. [PMC free article] [PubMed] [Google Scholar]
- 8.Mayo SC, Heckman JE, Shore AD, et al. Shifting trends in liver-directed management of patients with colorectal liver metastasis: a population-based analysis. Surgery. 2011;150:204–216. doi: 10.1016/j.surg.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCahill LE, Yothers G, Sharif S, et al. Primary mFOLFOX6 plus bevacizumab without resection of the primary tumor for patients presenting with surgically unresectable metastatic colon cancer and an intact asymptomatic colon cancer: definitive analysis of NSABP trial C-10. J Clin Oncol. 2012;30:3223–3228. doi: 10.1200/JCO.2012.42.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208–1215. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 11.Nordlinger B, Van Cutsem E, Gruenberger T, et al. Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann Oncol. 2009;20:985–992. doi: 10.1093/annonc/mdn735. [DOI] [PubMed] [Google Scholar]
- 12.Scarborough JE, Pietrobon R, Bennett KM, et al. Workforce projections for hepato-pancreato-biliary surgery. J Am Coll Surg. 2008;206:678–684. doi: 10.1016/j.jamcollsurg.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrag D. The price tag on progress--chemotherapy for colorectal cancer. N Engl J Med. 2004;351:317–319. doi: 10.1056/NEJMp048143. [DOI] [PubMed] [Google Scholar]
- 15.Venook AP, Niedzwiecki D, Heinz-Josef L, et al. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC) J Clin Oncol. 2014;32:5s. (suppl; abstr LBA3) [Google Scholar]
- 16.Adams RB, Aloia TA, Loyer E, et al. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB. 2013;15:91–103. doi: 10.1111/j.1477-2574.2012.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benson AB, Bekaii-Saab T, Chan E, et al. Metastatic colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:141–152. doi: 10.6004/jnccn.2013.0022. [DOI] [PubMed] [Google Scholar]
- 18.Ksienski D, Woods R, Speers C, et al. Patterns of referral and resection among patients with liver-only metastatic colorectal cancer (MCRC) Ann Surg Oncol. 2010;17:3085–3093. doi: 10.1245/s10434-010-1304-9. [DOI] [PubMed] [Google Scholar]
- 19.Sjovall A, Jarv V, Blomqvist L, et al. The potential for improved outcome in patients with hepatic metastases from colon cancer: a population-based study. Eur J Surg Oncol. 2004;30:834–841. doi: 10.1016/j.ejso.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winchester DP, Stewart AK, Phillips JL, et al. The national cancer data base: past, present, and future. Ann Surg Oncol. 2010;17:4–7. doi: 10.1245/s10434-009-0771-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boland GM, Chang GJ, Haynes AB, et al. Association between adherence to National Comprehensive Cancer Network treatment guidelines and improved survival in patients with colon cancer. Cancer. 2012;119:1593–1601. doi: 10.1002/cncr.27935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chagpar R, Xing Y, Chiang YJ, et al. Adherence to stage-specific treatment guidelines for patients with colon cancer. J Clin Oncol. 2012;30:972–979. doi: 10.1200/JCO.2011.39.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 25.Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217:336–346. doi: 10.1016/j.jamcollsurg.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 26.Dimick JB, Staiger DO, Birkmeyer JD. Ranking hospitals on surgical mortality: the importance of reliability adjustment. Health Serv Res. 2010;45:1614–1629. doi: 10.1111/j.1475-6773.2010.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krell RW, Hozain A, Kao LS, et al. Reliability of risk-adjusted outcomes for profiling hospital surgical quality. JAMA Surg. 2014;149:467–474. doi: 10.1001/jamasurg.2013.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hosmer DW, Lemeshow S. A goodness-of-fit test for the multiple logistic regression model. Communications in Statistics. 1980;A10:1043–1069. [Google Scholar]
- 29.Merkow RP, Hall BL, Cohen ME, et al. Relevance of the c-statistic when evaluating risk-adjustment models in surgery. J Am Coll Surg. 2012;214:822–830. doi: 10.1016/j.jamcollsurg.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 30.Hollenbeck BK, Ye Z, Dunn RL, et al. Provider treatment intensity and outcomes for patients with early-stage bladder cancer. J Natl Cancer Inst. 2009;101:571–580. doi: 10.1093/jnci/djp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keating NL, Landrum MB, Lamont EB, et al. Area-level variations in cancer care and outcomes. Med Care. 2012;50:366–373. doi: 10.1097/MLR.0b013e31824d74c0. [DOI] [PubMed] [Google Scholar]
- 32.Kwok AC, Semel ME, Lipsitz SR, et al. The intensity and variation of surgical care at the end of life: a retrospective cohort study. Lancet. 2011;378:1408–1413. doi: 10.1016/S0140-6736(11)61268-3. [DOI] [PubMed] [Google Scholar]
- 33.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:2128–2137. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aslam MI, Kelkar A, Sharpe D, et al. Ten years experience of managing the primary tumours in patients with stage IV colorectal cancers. Int J Surg. 2010;8:305–313. doi: 10.1016/j.ijsu.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Dixon E, Vollmer CM, Jr., Bathe O, et al. Training, practice, and referral patterns in hepatobiliary and pancreatic surgery: survey of general surgeons. J Gastrointest Surg. 2005;9:109–114. doi: 10.1016/j.gassur.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg CC, Lipsitz SR, Neville B, et al. Receipt of appropriate surgical care for Medicare beneficiaries with cancer. Arch Surg. 2011;146:1128–1134. doi: 10.1001/archsurg.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.In H, Neville BA, Lipsitz SR, et al. The role of National Cancer Institute-designated cancer center status: observed variation in surgical care depends on the level of evidence. Ann Surg. 2012;255:890–895. doi: 10.1097/SLA.0b013e31824deae6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jafari MD, Jafari F, Halabi WJ, et al. Colorectal Cancer Resections in the Aging US Population: A Trend Toward Decreasing Rates and Improved Outcomes. JAMA Surg. 2014 doi: 10.1001/jamasurg.2013.4930. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto T, Hasegawa S, Matsumoto S, et al. Overcoming the challenges of primary tumor management in patients with metastatic colorectal cancer unresectable for cure and an asymptomatic primary tumor. Dis Colon Rectum. 2014;57:679–686. doi: 10.1097/DCR.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 40.Nathan H, Bridges JF, Cosgrove DP, et al. Treating patients with colon cancer liver metastasis: a nationwide analysis of therapeutic decision making. Ann Surg Oncol. 2012;19:3668–3676. doi: 10.1245/s10434-012-2564-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei AC, Sandhu L, Devitt KS, et al. Practice Patterns for the Management of Hepatic Metastases from Colorectal Cancer: A Mixed Methods Analysis. Ann Surg Oncol. 2012;20:1567–1574. doi: 10.1245/s10434-012-2698-3. [DOI] [PubMed] [Google Scholar]
- 42.Bilimoria KY, Bentrem DJ, Stewart AK, et al. Comparison of commission on cancer-approved and -nonapproved hospitals in the United States: implications for studies that use the National Cancer Data Base. J Clin Oncol. 2009;27:4177–4181. doi: 10.1200/JCO.2008.21.7018. [DOI] [PubMed] [Google Scholar]


