Abstract
Background
The classical cadherins such as E- and N-cadherin are Ca2+-dependent cell adhesion molecules that play important roles in the development and maintenance of renal epithelial polarity. Recent studies have shown that a variety of cadherins are present in the kidney and are differentially expressed in various segments of the nephron. However, the interpretation of these findings has been complicated by the fact that the various studies focused on different panels of cadherins and utilized different species. Moreover, since only a few of the previous studies focused on the rat, information regarding the expression and localization of renal cadherins in this important species is lacking. In the present study, we have employed dual immunofluorescent labeling procedures that utilized specific antibodies against either E- or N-cadherin, along with antibodies that target markers for specific nephron segments, to characterize the patterns of cadherin expression in frozen sections of adult rat kidney.
Results
The results showed that N-cadherin is the predominant cadherin in the proximal tubule, but is essentially absent in other nephron segments. By contrast, E-cadherin is abundant in the distal tubule, collecting duct and most medullary segments, but is present only at very low levels in the proximal tubule. Additional results revealed different patterns of N-cadherin labeling along various segments of the proximal tubule. The S1 and S2 segments exhibit a fine threadlike pattern of labeling at the apical cell surface, whereas the S3 segment show intense labeling at the lateral cell-cell contacts.
Conclusions
These results indicate that E- and N-cadherin are differentially expressed in the proximal and distal tubules of rat kidney and they raise the possibility that differences in cadherin expression and localization may contribute to the differences in the susceptibility of various nephron segments to renal pathology or nephrotoxic injury.
Background
The tubular segments of the nephron can be thought of as a series of functionally distinct units each having unique permeability characteristics and fluid and electrolyte transport capabilities. The specific permeability and transport properties of the individual nephron segments are determined by the general cytoarchitecture of the epithelial cells and also by the manner in which the cells interact with each other [1]. These cell-cell interactions involve specialized junctional complexes that are necessary for the restriction of permeability, the establishment of epithelial polarity and the normal transport of materials across the cell monolayer (for reviews see [1-6]). These junctional complexes include: adherens junctions (zonulae adherens), occluding junctions (zonulae occludens or tight junctions), desmosomes and gap junctions. These complexes are composed of specific cell adhesion molecules and their associated scaffolding proteins that link the complexes to the cytoskeletal elements of the individual cells [1,5,7-9].
Of the many molecules that have been shown to be involved in renal epithelial cell-cell adhesion, the cadherins family of Ca2+-dependent cell adhesion molecules are among the most important. While over 50 cadherins have been described, the best characterized are the classical (Type I) cadherins such as E-cadherin and N-cadherin (for reviews see [10,11]). These classical cadherins are integral, transmembrane proteins that are usually localized at the adherens junctions of epithelial cells [9,12-14]. The extracellular domain of the cadherin contains the Ca2+-binding sites, as well as the adhesive regions of the molecule. The intracellular domain is bound to β-catenin which is bound to α-catenin, which in turn links the entire complex to the actin cytoskeleton [8,9,15-17]. In this context, the cadherin/catenin complex serves as a key structural component of adherens-type junctions. In addition, β-catenin functions as a component of the wingless or Wnt nuclear signaling pathway and plays an important role in the regulation of gene expression (for reviews see [18-21]).
Studies over the past 19 years have shown that a variety of cadherins, including E-, N-, P-, K-, R-, OB-, VE- and Ksp-cadherin are present in the kidney [22-32]. Several of these cadherins, such as OB-, R- and K-cadherin, are transiently expressed during different stages of development [29-31,33]. In adult kidney the most abundant cadherins appear to be the classical cadherins, N-, and E-cadherin [23,24,34-36], along with an atypical kidney-specific cadherin known as Ksp-cadherin [26,37]. The latter molecule differs from the classical cadherins in that it lacks the cytoplasmic catenin-binding domain [26,38,39]. At present, the functional significance of this atypical cadherin is unclear.
In terms of maintaining cell-cell adhesion along the nephron, the classical cadherins appear to be the key players, and a growing volume of evidence indicates that these classical cadherins are differentially expressed in various segments of the nephron. However, this issue is complicated by the fact that the various studies have focused on different panels of cadherins and utilized different species. In the first comprehensive studies to map the distribution of renal cadherins, Nouwen et al. [23] and Tani et al. [25] showed that in human kidney, N-cadherin is the predominant cadherin in proximal tubule, whereas E-cadherin is predominant in the distal tubule and other nephron segments. In a very elegant study of mouse kidney, Piepenhagen et al. [24] showed that E-cadherin is abundantly expressed in most segments of the nephron, including the proximal tubule, but they made no mention of N-cadherin. Cho et al. [29] also reported that E-cadherin is present in the proximal and distal tubules of newborn mice, but they, too, made no mention of N-cadherin. However, several more recent studies have shown that N-cadherin is present in the proximal tubule of the rat and mouse [34,35,40,41]. In a recent study examining the effects of the nephrotoxic metal Cd2+ on cadherin localization in rat kidney, we also observed that N-cadherin is present in the proximal tubule but is not expressed in other nephron segments [36]. In addition, we noticed what appeared to be different patterns of N-cadherin labeling along various segments of the proximal tubule; the S1 and S2 segments (proximal convoluted tubule) exhibited a fine, thread-like pattern of labeling near the apical cell surface, whereas the S3 segment (proximal straight tubule) showed intense labeling at the lateral cell-cell contacts. However, the specific identity of these tubular segments in that study was somewhat equivocal because their identification was based primarily on their general morphology and their location in sagittal cryosections. In the present study, we have employed dual immunofluorescence labeling procedures that utilized specific antibodies against either E- or N-cadherin, along with antibodies that target markers for specific nephron segments, to characterize the patterns of cadherin expression in cryosections of adult rat kidney. The results show that N-cadherin is, in fact, the predominant cadherin in the proximal tubule and that the convoluted and straight segments of the proximal tubule exhibit different patterns of N-cadherin labeling. By contrast, E-cadherin is abundant in the distal tubule, collecting duct and most medullary segments, but is present only at very low levels in the proximal tubule. The localization of the cadherin-binding protein β-catenin parallels that of both N- and E-cadherin.
Results
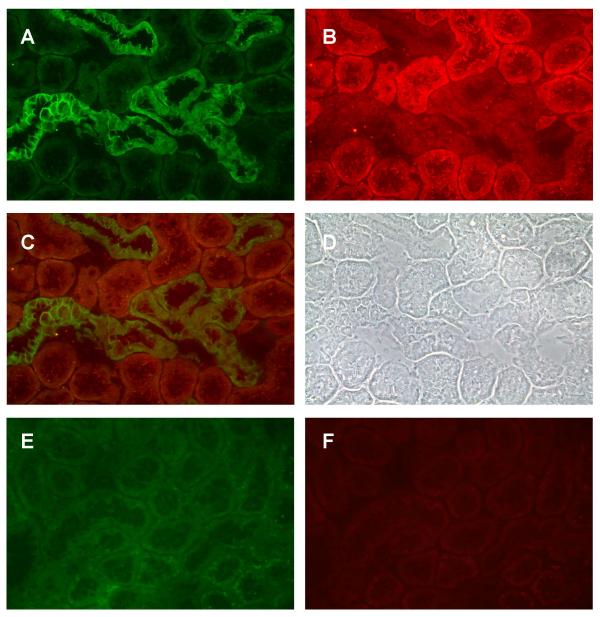
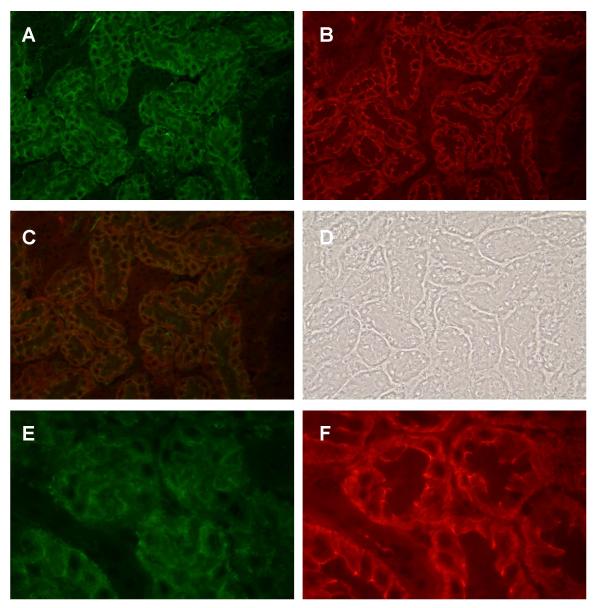
Results of dual labeling experiments showed that E-cadherin and N-cadherin are differentially localized in the kidney. Figure 1 shows the dual labeling of E- and N-cadherin in a field from the outer cortex. Note that the green E-cadherin labeling is concentrated at the lateral cell-cell contacts in a specific subpopulation of tubules. By contrast, the red N-cadherin labeling is concentrated in a different subpopulation of tubules. Moreover, the pattern of N-cadherin labeling differs from that of E-cadherin. The N-cadherin-labeled tubules exhibit a diffuse pattern of labeling on the basolateral cell surface and a fine, thread-like band of labeling near the apical cell surface. From their location and general morphology, the E-cadherin labeled structures were tentatively identified as mainly distal tubules and collecting ducts, whereas the N-cadherin labeled tubules were tentatively identified as the S1 and S2 segments of the proximal tubule (i.e., proximal convoluted tubule). It should be noted that neither E- nor N-cadherin was detected in the glomeruli (not shown). As may be seen in the photos of the controls, blank samples that were incubated in the presence of non-immune serum from the species in which the primary antibodies were generated showed a complete absence of labeling.
Figure 1.
Dual labeling of E-cadherin and N-cadherin in the outer cortex. Samples were processed for the dual labeling of E-cadherin and N-cadherin as described in the Methods and then viewed using a 40× objective. A: E-cadherin; B: N-cadherin; C: Overlay of images A and B; D: Phase contrast image of the same field; E and F show the lack of E- and N-cadherin labeling in a control sample that was incubated without the primary antibodies. Original magnification = 174×.
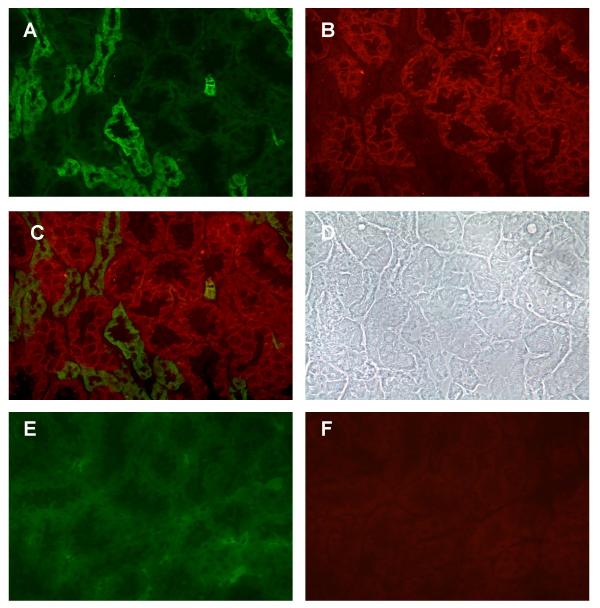
Figure 2 shows the dual labeling of E- and N-cadherin in a field from the inner cortex, near the outer stripe of the medulla. As in the outer cortex, the E-cadherin and N-cadherin labeling are concentrated in different populations of the tubules. The pattern of E-cadherin labeling in this region is also similar to that in the outer cortex, with the labeling concentrated at the lateral cell-cell contacts. Based on their morphology and location, these E-cadherin labeled structures most likely are distal tubules, collecting ducts and possibly portions of the loop of Henle. By contrast, the N-cadherin labeling is concentrated in a different population of tubules. Based on their location and morphology, these N-cadherin labeled structures were tentatively identified as the S3 segment of the proximal tubule (proximal straight tubule). Interestingly, the pattern of N-cadherin labeling in the inner cortex is somewhat different from that observed in the outer cortex. The N-cadherin labeling in the inner cortex is highly concentrated along the basolateral cell surface and at the lateral cell-cell contacts. None of the apical threadlike labeling that was observed in the outer cortex is evident in this region.
Figure 2.
Dual labeling of E-cadherin and N-cadherin in the inner cortex. Samples were processed for the dual labeling of E-cadherin and N-cadherin as described in the Methods and then viewed using a 40× objective. A: E-cadherin; B: N-cadherin; C: Overlay of images A and B; D: Phase contrast image of the same field; E and F show the lack of E- and N-cadherin labeling in a control sample that was incubated without the primary antibodies. Original magnification = 174×.
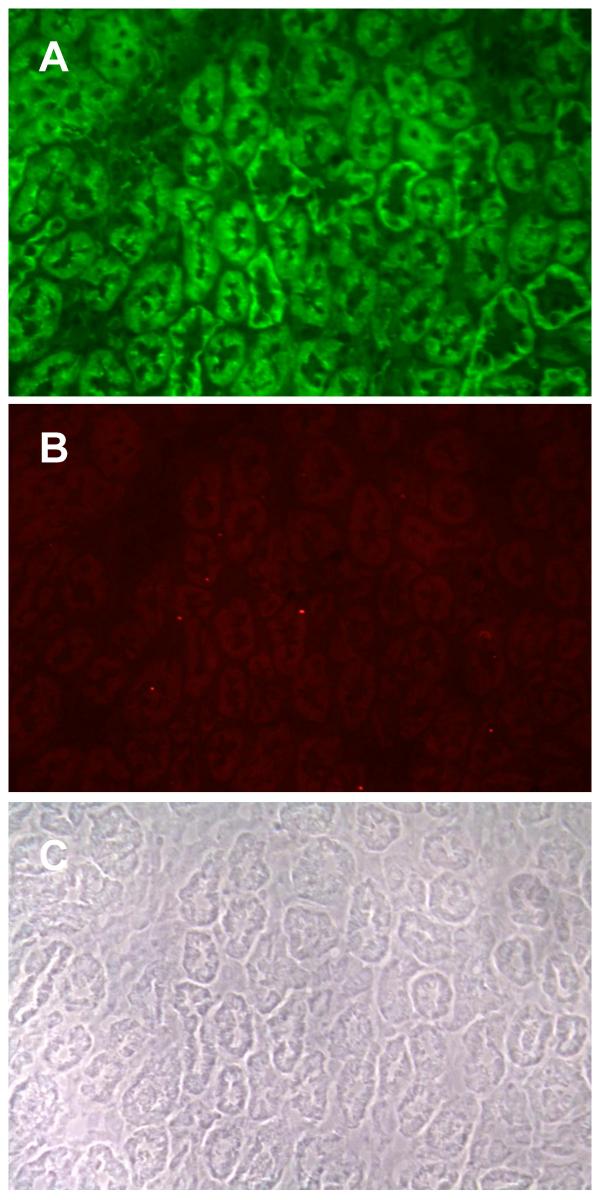
Figure 3 shows the dual labeling of E- and N-cadherin in a typical field from the renal medulla. Note that most of the tubular structures show intense E-cadherin labeling that is concentrated at the lateral cell-cell contacts. However, no N-cadherin labeling is evident in the medulla.
Figure 3.
Dual labeling of E-cadherin and N-cadherin in the renal medulla. Samples were processed for the dual labeling of E-cadherin and N-cadherin as described in the Methods and then viewed using a 40× objective. A: E-cadherin; B: N-cadherin; C: Phase contrast image of the same field. Original magnification = 174×.
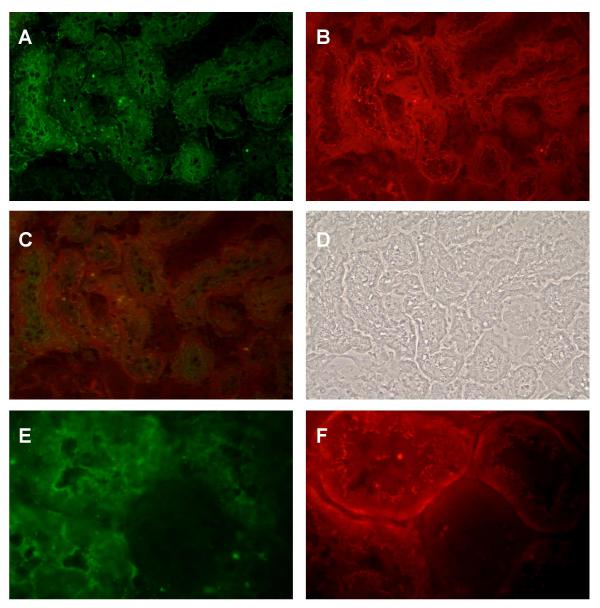
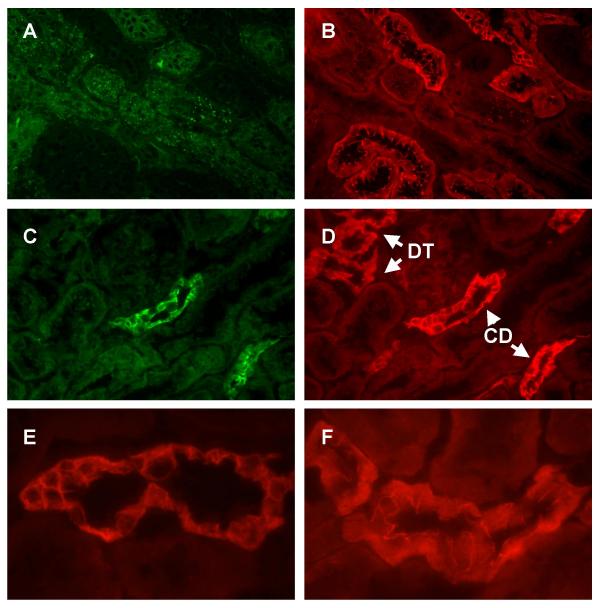
These studies examining the dual labeling of E- and N-cadherin indicated that E-cadherin is the predominant cadherin in most segments of the rat nephron, but that N-cadherin is the predominant cadherin in a subpopulation of tubules that were tentatively identified as proximal tubules. To verify this observation, we employed another dual labeling procedure to visualize N-cadherin and the proximal tubule marker protein aquaporin 1 [42-45] in the same samples. Panels A-B in Figure 4 show the dual labeling of N-cadherin and aquaporin 1 in a field from the outer cortex. Note that diffuse aquaporin 1 labeling is evident on the surface of the cells in some tubules, but not others (Panel A). The same tubules that exhibit the aquaporin 1 labeling are the ones that also exhibit N-cadherin labeling (Panel B and image overlay C). Panels E and F show a similar field viewed at higher magnification. This higher magnification gives an excellent image of the typical pattern of N-cadherin labeling in the outer cortex. Note the fine, thread-like pattern of labeling at the apical cell-cell contacts and the more diffuse labeling along the basement membrane and basal cell surface. In light of their location, general morphology and expression of aquaporin 1, these N-cadherin labeled structures in the outer cortex most likely are the S1/S2 segments of the proximal tubule (proximal convoluted tubule).
Figure 4.
Dual labeling of aquaporin 1 and N-cadherin in the outer cortex. Samples were processed for the dual labeling of the proximal tubule marker aquaporin 1 and N-cadherin as described in the Methods and then viewed using a 40× or a 100× objective. Panels A-D show lower power images of the same field. A: Aquaporin 1; B: N-cadherin; C: Overlay of images A and B; D: Phase contrast image of the same field. Panels E and F show the dual labeling in a different field viewed at higher magnification. E: Aquaporin 1; F: N-cadherin. Original magnification = 174× (Panels A-D) and 435× (Panels E and F).
Figure 5 shows the dual labeling of N-cadherin and aquaporin 1 in a field from the inner cortex. As may be seen in Panel A, aquaporin 1 labeling is evident on the surface of the cells in some, but not all, tubules. These aquaporin 1-labeled tubules are the same tubules that show intense N-cadherin labeling (Panel B and image overlay C), and they almost certainly represent the S3 segments of the proximal tubule (proximal straight tubule). Panels E and F show the aquaporin 1 and N-cadherin labeling in a similar field at higher magnification. Note that the pattern of N-cadherin labeling in this segment is quite different form that in the S1/S2 segments (compare with Figure 4B). Here, the labeling is concentrated at the lateral cell-cell contacts, and there is no labeling on the apical cell surface.
Figure 5.
Dual labeling of Aquaporin 1 and N-cadherin in the inner cortex. Samples were processed for the dual labeling of the proximal tubule marker aquaporin 1 and N-cadherin as described in the Methods and then viewed using a 40× or a 100× objective. Panels A-D show lower images of the same field. A: Aquaporin 1; B: N-cadherin; C: Overlay of images A and B; D: Phase contrast image of the same field. Panels E and F show the dual labeling in a different field viewed at higher magnification. E: Aquaporin 1; F: N-cadherin. Original magnification = 174× (Panels A-D) and 435× (Panels E and F).
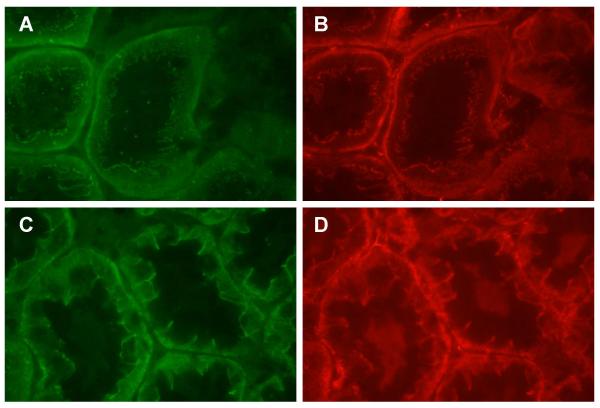
In order to further identify the cortical structures that express high levels of E-cadherin, we employed another dual labeling procedure to visualize E-cadherin and either aquaporin 1 or aquaporin 2 in the same samples. The latter molecule is a vasopressin-sensitive water channel protein that is found at high levels in the principal cells of the collecting duct, but is essentially absent in other nephron segments [44,45]. Panels A and B in Figure 6 show the dual labeling of proximal tubule marker aquaporin 1 and E-cadherin in a field from the outer cortex. As may be seen, the tubules that exhibit the most intense aquaporin 1 labeling (Panel A) show only weak E-cadherin labeling (Panel B). Conversely, the tubules that show the most intense E-cadherin labeling show no labeling for aquaporin 1, indicating that the E-cadherin labeled structures are not proximal tubules. Panels C and D show the dual labeling of the collecting duct marker aquaporin 2 (C) and E-cadherin (D) in a similar field from the outer cortex. As may be seen in panel C, the aquaporin 2 labeling is confined to a few, smaller, but well defined tubular structures in the center and lower right regions of the field. These tubules, which are most likely collecting ducts (CD), also exhibit prominent E-cadherin labeling at the lateral cell-cell contacts (Panel D). In addition, intense E-cadherin labeling is present in other tubules in the upper right region of the field that do not express aquaporin 2. Based on their location and morphology, these are most likely distal tubules (DT). Panels E and F show higher magnification images of the patterns of E-cadherin labeling in a collecting duct and distal tubule respectively.
Figure 6.
Dual labeling of E-cadherin and aquaporin 1 or aquaporin 2 in the outer cortex. Samples were processed for the dual labeling of E-cadherin and either aquaporin 1 or aquaporin 2 as described in the Methods and viewed using either a 40× or a 100× objective. Panels A and B show dual labeling of aquaporin 1 (A) and E-cadherin (B) in the same field. Panels C and D show the dual labeling of aquaporin 2 (C) and E-cadherin (D). CD denotes collecting ducts and DT denotes distal tubules. (E) E-cadherin labeling in a collecting duct. (F) E-cadherin labeling in a distal tubule. Original magnification = 174× (Panels A-D) and 435× (Panels E and F).
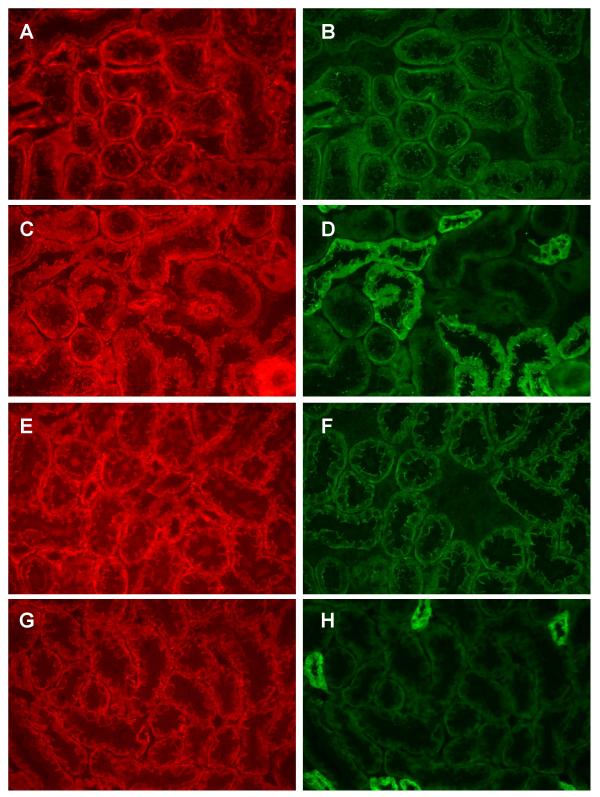
In an additional component of this study, we examined the localization of the cadherin-binding and nuclear signaling protein β-catenin in relation to that of E-cadherin and N-cadherin. Panels A and B in figure 7 show the dual labeling of β-catenin (A) and N-cadherin (B), and panels C and D show the dual labeling of β-catenin (C) and E-cadherin (D) in fields from the outer cortex. As may be seen, β-catenin labeling is evident in essentially all of the tubules (A and C) and is colocalized with both N-cadherin (B) and E-cadherin (D). Panels E-H show these same molecules in fields from the inner cortex. Again, β-catenin labeling is present in all tubules (E and G) where it is colocalized with both N-cadherin (F) and E-cadherin (H). Interestingly, the β-catenin labeling in the various segments of the proximal tubule exhibits the same patterns of labeling as N-cadherin. This is especially evident in the photos in Figure 8, which show higher magnification images of the dual labeling of N-cadherin and β-catenin in the S1/S2 segments and the S3 segment of the proximal tubule. In the S1/S2 segments (Panels A and B), both molecules show diffuse labeling along the basement membrane and the fine, thread-like labeling near the apical cell surface. In the S3 segment (Panels E and F) both molecules show intense labeling at the basolateral cell surface and the lateral cell-cell contacts.
Figure 7.
β-catenin is colocalized with both N-cadherin and E-cadherin. Samples were processed for the dual labeling of β-catenin and either N- or E-cadherin as described in the methods and viewed using a 40× objective. Panels A and B show the dual labeling of β-catenin (A) and N-cadherin (B) in a field from the outer cortex whereas panels C and D show the dual labeling of β-catenin (C) and E-cadherin (D) in a similar field. Panels E-F show the same molecules in fields from the inner cortex. E and F: dual labeling of β-catenin (E) and N-cadherin (F); G and H dual labeling of β-catenin (G) and E-cadherin (H). Original magnification = 174×.
Figure 8.
Dual labeling of N-cadherin and β-catenin in the proximal tubule. Panels A and B show that dual labeling of N-cadherin (A) and β-catenin (B) in the S1/S2 segment of the proximal tubule, whereas panels C and D show the dual labeling of N-cadherin (C) and β-catenin (D) in the S3 segment of the proximal tubule. Original magnification = 435×.
To the best of our knowledge, the apical thread-like pattern of N-cadherin labeling that we observed in the S1/S2 segments of the proximal tubule has not been described previously in rat kidney. To further characterize this unusual pattern of labeling, we utilized a deconvolution technique to examine the labeling in a series of optical planes through a single section. The images were then used to construct a video clip showing the N-cadherin labeling as the plane of focus was moved through the section. The serial images in the video clip can be viewed as an additional data file. Note that as the plane of focus moves through the sample, the fine thread-like labeling can be seen to be especially concentrated at the lateral contacts between the epithelial cells just below the apical surface.
Discussion
Previous studies have shown that a variety of cadherins are present in the kidney, where they serve critical roles in establishing epithelial polarity and regulating barrier function [1,28,46]. In addition, recent studies suggest that alternations in the expression and function of renal cadherins and/or β-catenin may be associated with a variety of pathologic conditions including: glomerulonephritis [47], polycystic kidney disease [48-52], renal ischemic injury [53,54], renal carcinogenesis [55,56] and metal nephrotoxicity [34,36,40,57]. Thus, an understanding of the specific distribution and function of cadherins in the kidney may provide new insights into renal function and pathophysiology. While numerous studies in the literature have shown that various cadherins are differentially expressed along the nephron, the interpretation of the findings has been complicated by the fact that the various studies focused on different panels of cadherins and utilized different species. Moreover, since only a few of the previous studies focused on the rat, information regarding the expression and localization of renal cadherins in this important species is lacking.
The results of the present study show that E-cadherin and N-cadherin are differentially expressed along the adult rat nephron. E-cadherin is abundant in the distal tubule, collecting duct and most other nephron segments. By contrast, N-cadherin is abundant in the proximal tubule but is not expressed in other nephron segments. Not surprisingly, the distribution of the cadherin-binding protein β-catenin closely parallels that of both E- and N-cadherin.
The findings that N-cadherin is present at much higher levels than E-cadherin in the proximal tubule of the rat kidney was somewhat surprising in light of the elegant studies by Piepenhagen et al. [24] and Cho et al. [29] showing that E-cadherin is abundant in the proximal tubule of mouse kidney, and the fact that proximal tubule derived epithelial cell lines from a variety of species primarily express E-cadherin. However, our findings are consistent with the recent reports by Leussink et al. [34] and Parrish et al. [35] showing that N-cadherin is expressed in the proximal tubule of rat kidney.
One of the more remarkable findings in the present study is that the pattern of N-cadherin localization varies markedly along the different segments of the proximal tubule. The S1/S2 segments of the proximal tubule exhibit a diffuse pattern of labeling along the basolateral cell surface and a fine-thread-like pattern of labeling at the cell contacts near the apical cell surface. By contrast, the N-cadherin labeling in the S3 segment is highly concentrated at the basolateral cell surface and the lateral cell-cell contacts. The pattern of N-cadherin labeling that we observed in the S3 segment is similar to that reported for the localization of other classical cadherins in renal tubules [24,29,34]. The apical, thread-like labeling, the pattern of N-cadherin that we observed in the S1/S2 segments of the proximal tubule is in many ways similar to the pattern reported by Nouwen, et al. [23] in their study of human kidney. It is also noteworthy that in our study, the pattern of β-catenin labeling in the proximal tubule closely paralleled that of N-cadherin. In S1/S2 segments both molecules exhibited the fine thread-like labeling near apical cell surface and in the S3 segment the labeling of both molecules was concentrated at the lateral cell-cell contacts. Interestingly, these different patterns of N-cadherin and β-catenin labeling that we observed in the various segments of the rat proximal tubule resemble in many respects the patterns of β-catenin labeling in the mouse proximal tubule described by Piepenhagen and Nelson [58].
At present, the significance of these differences in the patterns of N-cadherin localization in the various segments of the proximal tubule is unclear. However, since the classical cadherins such as N-cadherin are usually localized at the adherens junction of epithelial cells, the different patterns of N-cadherin labeling may reflect the differences in cell-cell interdigitations and the ultrastructure of the adherens junctions in various segments of the proximal tubule. Such cell-cell interdigitations are much more abundant in the S1 and S2 segments of the proximal tubule than in the S3 segment M [59,60].
In light of the recent reports that alterations in renal cadherins may be associated with nephrotoxic injury, the finding that the levels and patterns of cadherin expression vary markedly along the nephron could also have important implications regarding the susceptibility of the various nephron segments to toxic injury. For example, the proximal tubule which expresses moderate levels of N-cadherin is especially sensitive to injury by nephrotoxic metals such as Cd2+, Hg2+ and Bi2+. Several recent reports indicate that the nephrotoxic effects of these metals are associated with alterations in the localization of N-cadherin in the proximal tubule [34,36,40]. By contrast, other segments of the nephron that express higher levels of E-cadherin are relatively resistant to injury by these metals. While the specific relationship between these metal-induced changes in cadherin localization and alterations in renal function are unclear, this would seem to be an interesting area for further research.
Conclusions
The results of the present studies show that N-cadherin and E-cadherin are differentially expressed in proximal and distal segments of the rat nephron. N-cadherin is the predominant cadherin in the proximal tubule, but is essentially absent in other nephron segments. By contrast, E-cadherin is abundant in the distal tubule, collecting duct and most medullary segments, but is present only at very low levels in the proximal tubule. In addition, the various segments of the proximal tubule exhibit different patterns of N-cadherin labeling. The S1 and S2 segments exhibit a fine threadlike pattern of labeling at the apical cell surface, whereas the S3 segments show intense labeling at the lateral cell-cell contacts. These findings raise the possibility that differences in cadherin expression and localization may contribute to the differences in the susceptibility of various nephron segments to renal pathology or nephrotoxic injury.
Methods
Animals and Processing of Tissue Samples
Male Sprague-Dawley rats weighing 250–300 grams were deeply anesthetized with a mixture of ketamine/xylazine (67/7 mg/kg IP). The abdominal cavity was opened, and the kidneys were removed and cut sagitally into 3 segments. The segments were immediately frozen in Cryomatrix (Thermo Shandon, Pittsburgh, PA) in a bath of liquid N2 and then stored at -80°C until sectioned. The frozen samples were sagittally sectioned at a thickness of 5 μ and the sections were transferred to Superfrost Plus glass slides (Fisher Scientific, Pittsburgh, PA). The sections were then stored at -80°C until they were processed for the visualization of the molecules of interest.
Visualization of Cadherins and β-Catenin
N-Cadherin, E-cadherin and β-catenin were visualized by indirect immunofluorescence techniques. In the first series of experiments, a dual labeling procedure was used to visualize E-cadherin and N-cadherin in the same sections. Cryosections were permeabilized in -20°C methanol for 10 minutes and blocked in 3% goat serum for 15 minutes. They were then incubated for 1 hour with the primary antibodies, a mouse anti-human E-cadherin (B-D Transduction Labs #C20820, San Diego, CA) and a rabbit antihuman N-cadherin (Calbiochem #205606, LaJolla, CA). The samples were washed in phosphate buffered saline (PBS) and incubated for 40 minutes in the secondary antibodies, a FITC-conjugated goat anti-mouse IgG (Sigma #F0257, St. Louis, MO) and a TRITC-conjugated goat anti-rabbit IgG (Sigma #T5268, St. Louis, MO). The samples were then washed in deionized water, covered with glass coverslips over AquaPolymount (Polysciences, Inc., Warrington, PA) and viewed with a Nikon Eclipse 400 fluorescence microscope using either 40× or 100× objectives.
In another series of experiments, a dual-labeling procedure was used to visualize β-catenin and either E-or N-cadherin in the same sections. Samples were fixed and permeabilized for 10 minutes in -20°C methanol and blocked in 3% goat serum for 15 minutes. The samples were then incubated for 1 hour in the primary antibodies, a rabbit polyclonal anti-β-catenin (Zymed #71-2700, South San Francisco, CA) and either a mouse anti-human E-cadherin or a mouse anti-human N-cadherin (BD Transduction Labs #C70320). The samples were washed in PBS and incubated for 40 minutes in the secondary antibodies, a TRITC-conjugated goat anti-rabbit IgG and a FITC-conjugated goat anti-mouse IgG. Samples were then washed in deionized water, mounted and viewed as previously described.
In the last series of experiments, dual labeling procedures were used to visualize either E-cadherin or N-cadherin and specific markers for proximal and distal nephron segments. The specific markers that were labeled included aquaporin 1, which is normally expressed in the proximal tubule, and to a lesser extent in the descending thin limb of the loop of Henle [42,43], and aquaporin 2, which is normally expressed in the collecting duct [44,45]. Samples were fixed and permeabilized in -20°C methanol for 10 minutes and blocked in 3% horse serum for 15 minutes. The samples were then incubated for 1 hour in the primary antibodies, either a mouse anti-human N-cadherin (B-D Transduction Labs #C70320) or a mouse anti-human E-cadherin (B-D Transduction Labs #C20820) and either a rabbit anti-rat aquaporin 1 (Alpha Diagnostics International, San Antonio, TX #AQP11-5) or aquaporin 2 (Alpha Diagnostics International #AQP21-5). Samples were rinsed in PBS and incubated for 45 minutes in the secondary antibodies, a TRITC-conjugated goat anti-mouse IgG (Sigma #T5393) and a FITC-conjugated goat anti-rabbit IgG (Sigma #F0382). Samples were then rinsed, mounted and viewed as described previously.
For most of the studies, digital images were captured with a Spot digital camera (Diagnostic Instruments, Sterling Heights, MI) using automated exposure times and gain settings for the bright-field images. The dark-field fluorescence images of E-cadherin, N-cadherin and β-catenin labeling were captured using a gain setting of 16 for FITC and 8 for TRITC, and exposure times of 3 s for green and 1 s for red and blue. The digital images were processed using the Image-Pro Plus image analysis software package (Media Cybernetics, Silver Springs, MD). To further characterize the patterns of cadherin labeling in specific nephron segments, some of the samples were also examined using an automated focusing system and deconvolution technique to obtain images of a series of optical planes through the section. For these studies, the sections were viewed with a Nikon E800 microscope fitted with a Spot RT SE6 camera (Diagnostic Instruments) and a Z-axis controller interfaced with the Image Pro Plus 4.5 software package (Media Cybernetics) on a Dell 8200 computer. A stack of 20 images was acquired in 0.3 μ increments using a 100× objective. To remove out-of-focus information from each image in the stack, the images were subjected to a blind deconvolution algorithm using AutoDeblur software (AutoQuant Imaging, Inc., Watervliet, NY) to yield a frame-by-frame reconstruction of the specimen.
Negative controls for all labeling studies consisted of kidney sections that were incubated without the primary antibodies, as well as sections that were incubated with non-diluted, non-immune serum from the same species in which the primary antibodies had been generated. To rule out the possibility that any apparent labeling may have resulted from fluorescence "spill over" when the FITC labeled samples were viewed with the TRITC filter configuration and vice versa, all experiments also included a group of samples that were labeled with each of the TRITC- or FITC-tagged antibodies individually. When the TRITC-labeled samples were viewed with the FITC filter configuration and the FITC-labeled samples were viewed with TRITC filters, no labeling was evident in any of the samples. All labeling experiments were repeated at least three times and appeared to be highly reproducible.
List of Abbreviations
FITC fluorescein isothiocyanate
TRITC tetramethylrhodamine isothiocyanate
Authors Contributions
W.C.P. designed and directed the study and did most of the photography and morphologic analyses. P.C.L. prepared all cryosections and did most of the immunofluorescence labeling. D.M.A. performed the deconvolution analyses. All authors read and approved the final manuscript.
Supplementary Material
The data file shows images of the N-cadherin labeling in a series of optical planes through a typical field of the outer cortex. These images were generated using the automated focusing system and deconvolution technique described in the Methods. The threadlike pattern of N-cadherin labeling near the apical cell surface of the cells in the S1/S2 segment of the proximal tubule can best be seen by clicking on the small black triangular cursor to the middle region of the scale shown just below the viewing screen.
Acknowledgments
Acknowledgements
This work was supported by Grant R01 ES006478 from the National Institute of Environmental Health Sciences. The authors gratefully thank Victoria Sears for her assistance in processing the digital photos and for her help in preparing the manuscript.
Contributor Information
Walter C Prozialeck, Email: wprozi@midwestern.edu.
Peter C Lamar, Email: plamar@midwestern.edu.
Denah M Appelt, Email: DenahA@pcom.edu.
References
- Matlin KS, Caplan MJ. Epithelial cell structure and polarity. In: SeldinDW and GiebischG, editor. The Kidney: Physiology and Pathophysiology. Philadelphia, Lippincott, Williams and Wilkins; 2000. pp. 533–567. [Google Scholar]
- Cereijido M, Gonzalez-Mariscal L, Avila G, Contreras RG. Tight Junctions. Crit Rev Anat Sci. 1988;1:171–191. doi: 10.1164/ajrccm/138.6_Pt_2.S17. [DOI] [PubMed] [Google Scholar]
- Boyer B, Thiery JP. Epithelial cell adhesion mechanisms. J Membr Biol. 1989;112:97–108. doi: 10.1007/BF01871271. [DOI] [PubMed] [Google Scholar]
- Hirsch M, Noske W. The tight junction: structure and function. Micron. 1993;24:325–352. doi: 10.1016/0968-4328(93)90057-8. [DOI] [Google Scholar]
- Fanning AS, Mitic LL, Anderson JM. Transmembrane proteins in the tight junction barrier. J Am Soc Nephrol. 1999;10:1337–1345. doi: 10.1681/ASN.V1061337. [DOI] [PubMed] [Google Scholar]
- Wagner MC, Molitoris BA. Renal epithelial polarity in health and disease. Pediatr Nephrol. 1999;13:163–170. doi: 10.1007/s004670050586. [DOI] [PubMed] [Google Scholar]
- Geiger B. Membrane-cytoskeleton interaction. Biochim Biophys Acta. 1983;737:305–341. doi: 10.1016/0304-4157(83)90005-9. [DOI] [PubMed] [Google Scholar]
- Lutz KL, Siahaan TJ. Molecular structure of the apical junction complex and its contribution to the paracellular barrier. J Pharm Sci. 1997;86:977–984. doi: 10.1021/js970134j. [DOI] [PubMed] [Google Scholar]
- Petruzzelli L, Takami M, Humes HD. Structure and function of cell adhesion molecules. Am J Med. 1999;106:467–476. doi: 10.1016/S0002-9343(99)00058-3. [DOI] [PubMed] [Google Scholar]
- Gallin WJ. Evolution of the "classical" cadherin family of cell adhesion molecules in vertebrates. Mol Biol Evol. 1998;15:1099–1107. doi: 10.1093/oxfordjournals.molbev.a026017. [DOI] [PubMed] [Google Scholar]
- Nollet F, Kools P, van Roy F. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J Mol Biol. 2000;299:551–572. doi: 10.1006/jmbi.2000.3777. [DOI] [PubMed] [Google Scholar]
- Boller K, Vestweber D, Kemler R. Cell-adhesion molecule uvomorulin is localized in the intermediate junctions of adult intestinal epithelial cells. J Cell Biol. 1985;100:327–332. doi: 10.1083/jcb.100.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays RW, Nelson WJ, Marrs JA. Generation of epithelial cell polarity: roles for protein trafficking, membrane-cytoskeleton, and E-cadherin-mediated cell adhesion. Cold Spring Harb Symp Quant Biol. 1995;60:763–773. doi: 10.1101/sqb.1995.060.01.082. [DOI] [PubMed] [Google Scholar]
- Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Kemler R. Molecular organization of the uvomorulin-catenin complex. J Cell Biol. 1992;116:989–996. doi: 10.1083/jcb.116.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou TS, Stewart DB, Stappert J, Nelson WJ, Marrs JA. Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex. Proc Natl Acad Sci U S A. 1995;92:5067–5071. doi: 10.1073/pnas.92.11.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams CL, Nelson WJ. Cytomechanics of cadherin-mediated cell-cell adhesion. Curr Opin Cell Biol. 1998;10:572–577. doi: 10.1016/S0955-0674(98)80031-8. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Behrens J. Cadherins and catenins: role in signal transduction and tumor progression. Cancer Metastasis Rev. 1999;18:15–30. doi: 10.1023/A:1006200102166. [DOI] [PubMed] [Google Scholar]
- Beavon IR. The E-cadherin-catenin complex in tumour metastasis: structure, function and regulation. Eur J Cancer. 2000;36:1607–1620. doi: 10.1016/S0959-8049(00)00158-1. [DOI] [PubMed] [Google Scholar]
- Ben Ze'ev A, Shtutman M, Zhurinsky J. The integration of cell adhesion with gene expression: the role of beta-catenin. Exp Cell Res. 2000;261:75–82. doi: 10.1006/excr.2000.5045. [DOI] [PubMed] [Google Scholar]
- Vestweber D, Kemler R, Ekblom P. Cell-adhesion molecule uvomorulin during kidney development. Dev Biol. 1985;112:213–221. doi: 10.1016/0012-1606(85)90135-6. [DOI] [PubMed] [Google Scholar]
- Nouwen EJ, Dauwe S, van der Biest,I, De Broe ME. Stage- and segment-specific expression of cell-adhesion molecules N-CAM, A-CAM, and L-CAM in the kidney. Kidney Int. 1993;44:147–158. doi: 10.1038/ki.1993.225. [DOI] [PubMed] [Google Scholar]
- Piepenhagen PA, Peters LL, Lux SE, Nelson WJ. Differential expression of Na(+)-K(+)-ATPase, ankyrin, fodrin, and E-cadherin along the kidney nephron. Am J Physiol. 1995;269:C1417–C1432. doi: 10.1152/ajpcell.1995.269.6.C1417. [DOI] [PubMed] [Google Scholar]
- Tani T, Laitinen L, Kangas L, Lehto VP, Virtanen I. Expression of E- and N-cadherin in renal cell carcinomas, in renal cell carcinoma cell lines in vitro and in their xenografts. Int J Cancer. 1995;64:407–414. doi: 10.1002/ijc.2910640610. [DOI] [PubMed] [Google Scholar]
- Thomson RB, Igarashi P, Biemesderfer D, Kim R, Abu-Alfa A, Soleimani M, Aronson PS. Isolation and cDNA cloning of Ksp-cadherin, a novel kidney-specific member of the cadherin multigene family. J Biol Chem. 1995;270:17594–17601. doi: 10.1074/jbc.270.29.17594. [DOI] [PubMed] [Google Scholar]
- Paul R, Ewing CM, Robinson JC, Marshall FF, Johnson KR, Wheelock MJ, Isaacs WB. Cadherin-6, a cell adhesion molecule specifically expressed in the proximal renal tubule and renal cell carcinoma. Cancer Res. 1997;57:2741–2748. [PubMed] [Google Scholar]
- Perantoni AO. Cell adhesion molecules in the kidney: from embryo to adult. Exp Nephrol. 1999;7:80–102. doi: 10.1159/000020590. [DOI] [PubMed] [Google Scholar]
- Cho EA, Patterson LT, Brookhiser WT, Mah S, Kintner C, Dressler GR. Differential expression and function of cadherin-6 during renal epithelium development. Development. 1998;125:803–812. doi: 10.1242/dev.125.5.803. [DOI] [PubMed] [Google Scholar]
- Shimazui T, Oosterwijk-Wakka J, Akaza H, Bringuier PP, Ruijter E, Debruyne FM, Schalken JA, Oosterwijk E. Alterations in expression of cadherin-6 and E-cadherin during kidney development and in renal cell carcinoma. Eur Urol. 2000;38:331–338. doi: 10.1159/000020302. [DOI] [PubMed] [Google Scholar]
- Dahl U, Sjodin A, Larue L, Radice GL, Cajander S, Takeichi M, Kemler R, Semb H. Genetic dissection of cadherin function during nephrogenesis. Mol Cell Biol. 2002;22:1474–1487. doi: 10.1128/MCB.22.5.1474-1487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton TA, Mang HE, Campos SB, Sandoval RM, Yoder MC, Molitoris BA. Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol Renal Physiol. 2003;285:F191–F198. doi: 10.1152/ajprenal.00042.2003. [DOI] [PubMed] [Google Scholar]
- Simonneau L, Kitagawa M, Suzuki S, Thiery JP. Cadherin 11 expression marks the mesenchymal phenotype: towards new functions for cadherins? Cell Adhes Commun. 1995;3:115–130. doi: 10.3109/15419069509081281. [DOI] [PubMed] [Google Scholar]
- Leussink BT, Litvinov SV, de Heer E, Slikkerveer A, van der Voet GB, Bruijn JA, de Wolff FA. Loss of homotypic epithelial cell adhesion by selective N-cadherin displacement in bismuth nephrotoxicity. Toxicol Appl Pharmacol. 2001;175:54–59. doi: 10.1006/taap.2001.9228. [DOI] [PubMed] [Google Scholar]
- Parrish AR, Jiang JJ, Dean D, Burghardt RC. Determination of the spatial pattern of cadherin and catenin expression along nephron in renal pathophysiology. FASEB J. 2003;17 [Google Scholar]
- Prozialeck WC, Lamar PC, Lynch SM. Cadmium alters the localization of N-cadherin, E-cadherin, and beta-catenin in the proximal tubule epithelium. Toxicol Appl Pharmacol. 2003;189:180–195. doi: 10.1016/S0041-008X(03)00130-3. [DOI] [PubMed] [Google Scholar]
- Thomson RB, Aronson PS. Immunolocalization of Ksp-cadherin in the adult and developing rabbit kidney. Am J Physiol. 1999;277:F146–F156. doi: 10.1152/ajprenal.1999.277.1.F146. [DOI] [PubMed] [Google Scholar]
- Whyte DA, Li C, Thomson RB, Nix SL, Zanjani R, Karp SL, Aronson PS, Igarashi P. Ksp-cadherin gene promoter. I. Characterization and renal epithelial cell-specific activity. Am J Physiol. 1999;277:F587–F598. doi: 10.1152/ajprenal.1999.277.4.F587. [DOI] [PubMed] [Google Scholar]
- Igarashi P, Shashikant CS, Thomson RB, Whyte DA, Liu-Chen S, Ruddle FH, Aronson PS. Ksp-cadherin gene promoter. II. Kidney-specific activity in transgenic mice. Am J Physiol. 1999;277:F599–F610. doi: 10.1152/ajprenal.1999.277.4.F599. [DOI] [PubMed] [Google Scholar]
- Jiang J, Burghardt RC, Parrish AR. Disruption of N- and Ksp-cadherin localization and expression is associated with mercury-induced acute renal failure. Toxicological Sciences. 2004;66 [Google Scholar]
- Gaylor S, Jiang J, Burghardt RC. Selective loss of N-cadherin expressin in the kidneys of aged Fisher 344 rats, but not SHR rats. Toxicological Sciences. 2002;66 [Google Scholar]
- Sabolic I, Valenti G, Verbavatz JM, Van Hoek AN, Verkman AS, Ausiello DA, Brown D. Localization of the CHIP28 water channel in rat kidney. Am J Physiol. 1992;263:C1225–C1233. doi: 10.1152/ajpcell.1992.263.6.C1225. [DOI] [PubMed] [Google Scholar]
- Maunsbach AB, Marples D, Chin E, Ning G, Bondy C, Agre P, Nielsen S. Aquaporin-1 water channel expression in human kidney. J Am Soc Nephrol. 1997;8:1–14. doi: 10.1681/ASN.V811. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Sasaki S. Aquaporins in the kidney: emerging new aspects. Kidney Int. 1998;54:1041–1051. doi: 10.1046/j.1523-1755.1998.00123.x. [DOI] [PubMed] [Google Scholar]
- Agre P, Nielson S, Knepper MA. Aquaporin water chanels in mammalian kidney. In: SeldinDW and GiebischG, editor. The Kidney: Physiology and Pathophysiology. Philadelphia, Luppincott, Williams and Wilkins; 2000. pp. 363–377. [Google Scholar]
- Piepenhagen PA, Nelson WJ. Biogenesis of polarized epithelial cells during kidney development in situ: roles of E-cadherin-mediated cell-cell adhesion and membrane cytoskeleton organization. Mol Biol Cell. 1998;9:3161–3177. doi: 10.1091/mbc.9.11.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakopoulou L, Lazaris AC, Boletis IN, Michail S, Giannopoulou I, Zeis PM, Stathakis CP, Davaris PS. Evaluation of E-cadherin/catenin complex in primary and secondary glomerulonephritis. Am J Kidney Dis. 2002;39:469–474. doi: 10.1053/ajkd.2002.31390. [DOI] [PubMed] [Google Scholar]
- Rocco MV, Neilson EG, Hoyer JR, Ziyadeh FN. Attenuated expression of epithelial cell adhesion molecules in murine polycystic kidney disease. Am J Physiol. 1992;262:F679–F686. doi: 10.1152/ajprenal.1992.262.4.F679. [DOI] [PubMed] [Google Scholar]
- Barisoni L, Trudel M, Chretien N, Ward L, van Adelsberg J, D'Agati V. Analysis of the role of membrane polarity in polycystic kidney disease of transgenic SBM mice. Am J Pathol. 1995;147:1728–1735. [PMC free article] [PubMed] [Google Scholar]
- Sorenson CM. Nuclear localization of beta-catenin and loss of apical brush border actin in cystic tubules of bcl-2 -/- mice. Am J Physiol. 1999;276:F210–F217. doi: 10.1152/ajprenal.1999.276.2.F210. [DOI] [PubMed] [Google Scholar]
- Huan Y, van Adelsberg J. Polycystin-1, the PKD1 gene product, is in a complex containing E-cadherin and the catenins. J Clin Invest. 1999;104:1459–1468. doi: 10.1172/JCI5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitbak T, Ward CJ, Harris PC, Bacallao R, Ness SA, Wandinger-Ness A. A polycystin-1 multiprotein complex is disrupted in polycystic kidney disease cells. Mol Biol Cell. 2004;15:1334–1346. doi: 10.1091/mbc.E03-05-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitoris BA, Marrs J. The role of cell adhesion molecules in ischemic acute renal failure. Am J Med. 1999;106:583–592. doi: 10.1016/S0002-9343(99)00061-3. [DOI] [PubMed] [Google Scholar]
- Bush KT, Tsukamoto T, Nigam SK. Selective degradation of E-cadherin and dissolution of E-cadherin-catenin complexes in epithelial ischemia. Am J Physiol Renal Physiol. 2000;278:F847–F852. doi: 10.1152/ajprenal.2000.278.5.F847. [DOI] [PubMed] [Google Scholar]
- Heicappell R. Cadherins in renal cell carcinoma. Anticancer Res. 1999;19:1501–1504. [PubMed] [Google Scholar]
- Giroldi LA, Shimazui T, Schalken JA, Yamasaki H, Bringuier PP. Classical cadherins in urological cancers. Morphologie. 2000;84:31–38. [PubMed] [Google Scholar]
- Prozialeck WC. Evidence that E-cadherin may be a target for cadmium toxicity in epithelial cells. Toxicol Appl Pharmacol. 2000;164:231–249. doi: 10.1006/taap.2000.8905. [DOI] [PubMed] [Google Scholar]
- Piepenhagen PA, Nelson WJ. Differential expression of cell-cell and cell-substratum adhesion proteins along the kidney nephron. Am J Physiol. 1995;269:C1433–C1449. doi: 10.1152/ajpcell.1995.269.6.C1433. [DOI] [PubMed] [Google Scholar]
- Maunsbach AB. Functional ultrastructure of the proximal tubule. In: WindhagenEE, editor. Handbook of Physiology. New York, Oxford University Press; 1992. pp. 41–108. [Google Scholar]
- Kriz W, Kaissling B. Structural organization of the mammalian kidney. In: SeldinDW and GiebischG, editor. The Kidney: Physiology and Pathophysiology. Philadelphia, Lippincott, Williams and Wilkins; 2000. pp. 587–654. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The data file shows images of the N-cadherin labeling in a series of optical planes through a typical field of the outer cortex. These images were generated using the automated focusing system and deconvolution technique described in the Methods. The threadlike pattern of N-cadherin labeling near the apical cell surface of the cells in the S1/S2 segment of the proximal tubule can best be seen by clicking on the small black triangular cursor to the middle region of the scale shown just below the viewing screen.